Abstract
Two dietary carotenoids, lutein and zeaxanthin, are specifically delivered to the human macula at the highest concentration anywhere in the body. Whenever a tissue exhibits highly selective uptake of a compound, it is likely that one or more specific binding proteins are involved in the process. Over the past decade, our laboratory has identified and characterized several carotenoid-binding proteins from human retina including a pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein, a member of the steroidogenic acute regulatory domain (StARD) family as a lutein-binding protein, and tubulin as a less specific, but higher capacity site for carotenoid deposition. In this article, we review the purification and characterization of these carotenoid-binding proteins, and we relate these ocular carotenoid-binding proteins to the transport and uptake role of serum lipoproteins and scavenger receptor proteins in a proposed pathway for macular pigment carotenoid delivery to the human retina.
Introduction
The first report of the macula lutea or “yellow spot” in postmortem human retina was published by Home in 1798,1 but its physiological significance, of course, was not understood at the time. After examining the absorption spectrum of the macular pigment (MP), Wald concluded in 1945 that the macula lutea's pigment was likely to be related to the xanthophyll carotenoids found in green leaves,2 but it was not until 1985 and 1993 that Bone and Landrum chemically identified the macular pigment as a 1 : 1 : 1 mixture of two dietary carotenoids, (3R,3′R,6′R)-lutein and (3R,3′R)-zeaxanthin, and a non-dietary metabolite, (3R,3′S-meso)-zeaxanthin (Fig. 1).3, 4
Fig. 1.
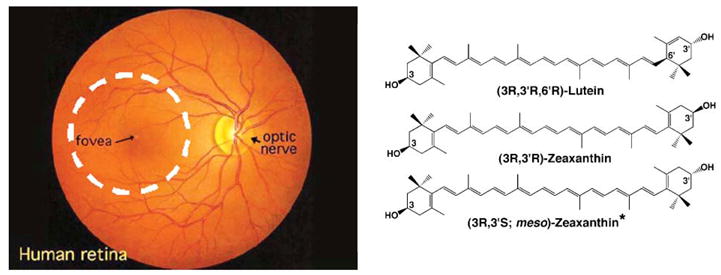
Ophthalmoscopic view of a human retina (left) showing the boundaries of the human macula as a 5 mm diameter dashed white circle centered on the fovea. The macular carotenoid pigment is concentrated in the central 500 microns of the macula at the fovea. The chemical structures of the major macular pigment carotenoids are shown on the right.
Based on their known functions and chemical properties, two major biological roles of macular pigment carotenoids have been proposed: (1) enhancement of visual acuity by decreasing glare and chromatic aberrations; and (2) protection against light-induced oxidative damage by filtering blue light incident on the retina and by scavenging reactive oxygen species generated in retinal tissues. These latter mechanisms are particularly relevant with respect to protection against visual loss from age-related macular degeneration (AMD).5–10 An autopsy study has shown that MP is ∼30% lower in AMD donor eyes relative to age-matched control eyes,11 and a clinic-based case-control study using non-invasive resonance Raman measurements came to a similar conclusion.12 Epidemiologically, it was reported that high carotenoid intake and serum levels are associated with a 43% risk reduction for exudative AMD,13–16 and the AREDS II clinical study is testing the value of long-term lutein and zeaxanthin supplementation in high-risk AMD patients in a randomized, placebo-controlled manner. In support for this mechanism, Thomson et al. reported that when quail exposed to bright light were fed zeaxanthin-supplemented diets, they had significantly less light-induced photoreceptor apoptosis than those fed with carotenoid-deficient diets.17 Moreover, several oxidation products of lutein and zeaxanthin are enriched in human retina, further supporting the antioxidant ability of MP.18 Zeaxanthin shows a higher ability to protect model lipid membranes from oxidation when bound to its binding protein,19 and in vitro experiments have shown that carotenoids can scavenge most reactive oxygen species,20, 21 suggesting that MP carotenoids can quench free radicals generated in human retina.
The macular carotenoids are deposited at the foveal center at a concentration estimated to be greater than 1 mM in many humans, and cross-sectionally the majority is concentrated in the outer and inner plexiform layers (Fig. 2a).22–26 Their inner retinal localization is ideal for them to act as light filters for incoming blue light, the most energetic light routinely incident on the retina. Macular pigment concentration is much lower in the photoreceptor outer segments,26 a region rich in polyunsaturated lipids that are very susceptible to oxidation, but even micromolar concentrations of MP carotenoids could play an important physiological antioxidant role.
Fig. 2.
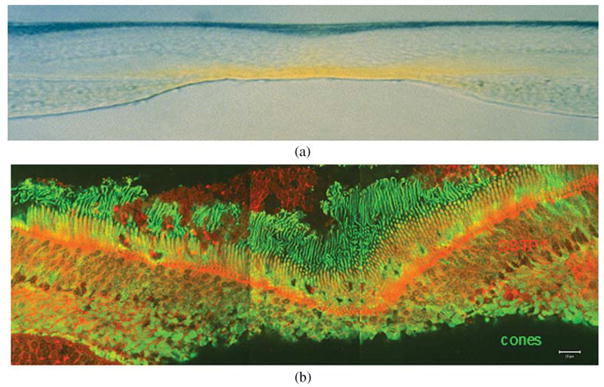
(a) Vertical section (vitreous side down) through a monkey fovea showing the distribution of the yellow macular carotenoids. Image courtesy of D. Max Snodderly, PhD. (b) GSTP1 labeling of foveal cones in the macula of a 3-year-old monkey. The orientation of the section is the same as in (a). This montage shows strongest labeling by antibody against GSTP1 (red) over the myoid and ellipsoid regions of cones identified by monoclonal antibody 7G6 (green).
There are over 600 carotenoids in nature, 30–50 of which are found in the typical human diet, but only 10–15 are usually measurable in human serum, and of these only two, lutein and zeaxanthin, are detectable in the human retina.27–29 Within the retina there is additional specificity, as the carotenoid concentration at the foveal center exceeds other areas of the retina just a few millimetres away by a factor of 100, and the lutein : zeaxanthin : meso-zeaxanthin ratio changes progressively from 1 : 1 : 1 to a ratio approaching 3 : 1 : 0.25, 30 Whenever a tissue exhibits highly selective uptake for a compound, it is likely that one or more specific binding proteins are involved in the process. For instance, retinoids are highly concentrated in the retina as a result of the combined actions of retinoid-binding proteins and a membrane receptor recognizing plasma retinol-binding protein on the basal surface of the retinal pigment epithelium (RPE).31 Among mammals, this selective uptake of high concentrations of xanthophyll carotenoids in specific regions of the retina is unique to primates, while birds, amphibians, reptiles, and fish employ an alternative strategy to concentrate carotenoids in ocular tissues that involves esterification to fatty acids and deposition into oil droplets.32, 33 Our laboratory has devoted considerable effort to the delineation of the physiological processes underlying the selective uptake of lutein, zeaxanthin, and their metabolites in the primate macula for more than a decade in order to understand further the role of these macular carotenoids in the maintenance of ocular health with special emphasis on the prevention and treatment of age-related macular degeneration. Here, we review the progress on the identification and characterization of the proteins related to the transport and uptake of MP carotenoids into the human retina.
Tubulin
Our initial attempts to identify the first vertebrate carotenoid-binding protein utilized bovine retinal tissue because this animal, like humans, takes up lutein and zeaxanthin selectively into its retinal tissue, albeit at much lower concentrations and without spatial specificity, and the tissue is readily available in large quantities.34 As reported in 1997, soluble bovine retinal proteins were incubated for an hour in the dark with a photoactivatable 14C-labeled carotenoid, canthaxanthin, which is known to accumulate in retinal tissues after excessive dietary intake.35 After removal of unbound ligands by passing through a hydrophobic column in the dark, the retinal protein solution was purified on an anion exchange column, and the fractions containing the most radioactive label were exposed to intense light to covalently link the bound ligand to associated protein. Photoaffinity-labeled fractions were passed through a gel-filtration column, and the labeled 55 kDa protein was identified to be beta-tubulin by autoradiography and mass spectroscopy. Based on these encouraging results, we subsequently switched to soluble protein extracts of human macula where the endogenous carotenoid content is around 5–40 ng per macula. Therefore, exogenous carotenoids were not required during the purification process, lessening the potential to generate artifactual carotenoid-protein interactions. We demonstrated that lutein and zeaxanthin co-purified with human macular tubulin; however, despite molecular modeling evidence that lutein is likely to bind at tubulin's paclitaxel binding site,36 subsequent binding affinity and specificity studies in our laboratory have shown that many carotenoids besides lutein and zeaxanthin can bind to tubulin,37 indicating tubulin was unlikely to be the mediator of the specific uptake of the macular carotenoids. More likely, tubulin is a site for high capacity deposition of carotenoids after specific uptake by other more specific binding proteins especially since it is found in abundance in the photoreceptor axon layers of the fovea (Henle fiber layer), the same of site of maximal carotenoid concentration in the primate retina.23
Glutathione S-transferase P1 (GSTP1)
Many carotenoid-binding proteins of plants and invertebrates are membrane-associated proteins, so it made sense to turn our attention to potential membrane-associated carotenoid-binding proteins in human macula. After first confirming that a substantial proportion of macular lutein and zeaxanthin was associated with membrane fractions, a protocol for purification of membrane-associated carotenoid-binding proteins was developed.37 Yellow, carotenoid-enriched macular membranes were initially prepared by differential centrifugation and subsequently solubilized by a zwitterionic detergent. Further purification was achieved using two ion-exchange chromatography steps and a gel-filtration chromatography step coupled with continuous photodiode-array monitoring to identify fractions enriched in endogenously associated macular carotenoids. We obtained a highly purified preparation containing one major protein band at 23 kDa that consistently co-eluted with endogenous lutein and zeaxanthin in a 1 : 2.5 ratio. Four protein spots with molecular weight of around 23 kDa were present on 2-D gels, and the most prominent spot with an isoelectric point of 5.7 was identified as a pi isoform of human glutathione S-transferase (GSTP1). Recombinant human GSTP1 exhibited high affinity for macular zeaxanthins with an equilibrium two-site average KD of 0.33 μM for (3R,3′R)-zeaxanthin and 0.52 μM for (3R,3′S-meso)-zeaxanthin and only low-affinity interactions with lutein. When closely related human GST proteins were tested, GSTM1 and GSTA1 exhibited no appreciable affinity for lutein or zeaxanthin, further confirming the specificity of interaction between GSTP1 and macular zeaxanthin. Immunolocalization of GSTP1 in the human and monkey retina revealed that GSTP1 was concentrated in the outer and inner plexiform layers of the fovea and in the photoreceptor inner segment ellipsoid region (Fig. 2b).37
Glutathione-S-transferases (GSTs) are members of a family of Phase II detoxification enzymes that catalyzes the conjugation of glutathione (GSH) to a wide variety of endogenous and exogenous electrophilic compounds. GSTs are classified into two superfamilies: the membrane-bound microsomal family and the cytosolic family. Human cytosolic GSTs can be divided into six classes: α, μ, ω, π, θ and ζ.38 Only the pi isoform of GST, GSTP1 (formerly GST π) is known to be expressed abundantly in human tissue, and its gene is localized to chromosomal locus 11q13-qter.39 GSTP1 is widely expressed in human epithelial tissue and is composed of two identical 23 kDa subunits.40 GSTP1 cDNA contains an open reading frame of 630 nucleotides, encoding 210 amino acids.41 The first 77 amino acids are thought to be the conserved glutathione-binding domain.42 It has been reported that GSTP1 can act as a retinoic acid cis-trans isomerase in a glutathione-independent manner.43 The GSTP1 gene is up-regulated during the early stages of oncogenesis, and it is the most significantly overexpressed GST gene in many human tumors.44 Recently, GSTP1 was identified as a nitric oxide scavenger protein, and it also can serve as a regulator of protein S-glutathionylation caused by reactive oxygen and nitrogen species.45, 46 Our identification of GSTP1 as a zeaxanthin-binding protein in the macula of human eye37 and our subsequent finding that they can synergistically protect lipid membranes from oxidation19 assign additional important roles to this well known protein.
Three polymorphic GSTP1 genes have been cloned from malignant glioma cells.41 More recently, it has been suggested that certain gene polymorphisms of glutathione S-transferases including GSTP1 may be associated with the subsequent developments of neovascular AMD47 and cortical cataracts.48
Steroidogenic acute regulatory domain (StARD) proteins
During the purification processes of GSTP1 from human macular membranes, we noted the presence of another potential xanthophyll-binding protein also with a three-peak absorbance pattern typical of many xanthophyll carotenoids. In contrast to the macular zeaxanthin-binding protein, this membrane-associated protein bound to the cation-exchange column and exhibited a much larger bathochromic shift (90 nm versus 10 nm).49 HPLC analysis demonstrated that lutein was the dominant carotenoid in this fraction, suggesting that this fraction probably contains a lutein-binding protein. 2-D gels of the purified lutein-rich fraction revealed that it was composed of two major protein spots at ∼55 kDa and at ∼29 kDa. Mass spectral identification of these proteins proved unrevealing, so we tested for immunoreactivity with a series of antibodies against plant and invertebrate-carotenoid binding proteins. We observed immunoreactivity of the purified lutein-binding protein fraction with an antibody raised against the silkworm lutein-binding protein (CBP) identified by Tsuchida's laboratory in Japan,50, 51 and this same antibody specifically labeled the mitochondrion-rich ellipsoid region of monkey photoreceptors, a region of the retina subjected to considerable oxidative stress. Silkworm CBP belongs to the steroidogenic acute regulatory domain (StARD) protein family50 StARD proteins have conserved sequences and structures in a variety of organisms ranging from plants to higher animals.52, 53 They all share a domain of ∼210 amino acid residues which can bind numerous small hydrophobic molecules including sterols, so it is not unreasonable to hypothesize that human homologs of CBP could also bind carotenoids. The human genome has 15 genes encoding StARD proteins that can be divided into six subfamilies.53 Silkworm CBP exhibits 29% sequence identity to StARD3, its closest human homolog.50 Definitive identification of which of the 15 human StARD proteins is the human retinal lutein-binding protein is still in progress in our laboratory.
Plasma lipoproteins, scavenger receptor proteins, metabolic enzymes
Plasma lipids such as cholesterol, triglycerides, and phospholipids along with low levels of other hydrophobic compounds such as vitamin E and retinoids, are carried in the blood by water-soluble lipoproteins, and the same holds true for the various carotenoids.10, 54 Lipoproteins have a polar outer shell of protein and phospholipid and inner core of neutral lipid. Lipoproteins can be divided into six groups: chylomicrons, chylomicron remnants, very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL).55 HDL is the smallest and densest of all plasma lipoproteins. It plays a critical function in cholesterol metabolism with an important role in removing cholesterol from peripheral tissues, a process known as “reverse cholesterol transport,” and in directly delivering cholesteryl esters to other lipoproteins and to tissues.56 HDLs are a group of lipoprotein particles containing nearly equal amounts of lipid and protein. The major apolipoproteins of HDL are apoA-I and apoA-II. It has been observed in many large-scale prospective studies that there is an inverse relationship between HDL levels and premature cardiovascular disease.57 HDL can inhibit the oxidation of LDL, promote endothelial repair, and improve endothelial functions.58 HDL also plays a role in the promotion of lesion regression in animals.59 In the bloodstream, all carotenoids are detectable in all lipoprotein classes to varying degrees, but lutein and zeaxanthin are primarily associated with high-density lipoprotein HDL,60 consistent with their less hydrophobic nature relative to the carotenes. The specific components of HDL responsible for carotenoid binding remain to be identified, however. The Wisconsin hypoalpha mutant (WHAM) chicken has very low levels of HDL due to a mutation in the ABCA1 transporter gene.61 When these chickens are fed a high-lutein diet, lutein levels increase in plasma, heart, and liver, but not in retina, suggesting that HDL is critical for delivery of carotenoids to retinal tissue.
SR-BI, a cell surface glycoprotein that binds HDL, mediates selective cholesteryl ester uptake from lipoprotein into liver and steroidogenic tissues as well as chlolesterol efflux from macrophages.62, 63 SR-BI is a member of CD36 superfamily.64 The SR-BI gene is located on chromosome 12q24, encoding a 509-amino-acid protein.54 SR-BI contains two trans-membrane domains on both the N- and the C-terminals and a large extracellular domain.65 The predicted size of SR-BI is 57 kD, but it usually behaves as an 82 kD membrane protein in vivo, as it is heavily N-glycosylated.66 SR-BI is highly expressed in liver, adrenals, and ovaries, with the highest amounts in the liver.67 Female SR-BI knockout mice are infertile, suggesting SR-BI may play an important role in normal ovarian function.68 As SR-BI is involved in the initial step of reverse cholesterol transport, it could influence atherosclerosis and coronary artery disease.69 It has also been shown that SR-BI participates in intestinal cholesterol absorption, embryogenesis, and vitamin E transport.70
Recently, there have been several reports that SR-BI is involved in the process of carotenoid uptake and transport to human and fly retina. During et al. demonstrated that macular carotenoids lutein and zeaxanthin, especially zeaxanthin, can be better taken up by RPE cells than beta-carotene through an SR-BI-dependent mechanism.71 When macular carotenoids or beta-carotene were incubated with fully differentiated ARPE-19 cells, the quantity of the macular carotenoids taken up by the cells was two times higher than beta-carotene. Blocking SR-BI by its antibody or knocking down SR-BI expression by small interfering RNA reduced the absorption of carotenoids by RPE cells, especially for zeaxanthin. Similarly, Kiefer et al. showed that the molecular basis for the blindness of a Drosophila mutant, NinaD, is a defect in the celluar uptake of carotenoids caused by a mutation in the NinaD gene which has high similarity to mammalian SR-BI.72 While it seems likely that SR-BI is important for carotenoid uptake into the RPE in living humans, its role in the retinal uptake of the macular carotenoids is less clear, as its immunolocalization in the primate retina does not match lutein's or zeaxanthin's distribution in the retina.73 Its scavenger receptor relative, CD36, is a better match73 Interestingly, Cameo2, a CD36 homolog in silkworms, is required for uptake of lutein into the silk gland.74
Enzymes that metabolize ocular carotenoids clearly must bind their carotenoid substrates, but relatively few of these enzymes have been identified. Two carotenoid cleavage enzymes have been immunolocalized to human RPE: BCO1 cleaves carotenes symmetrically, an essential step for generation of vitamin A, while BCO2 catalyzes eccentric cleavage of carotenes and xanthophylls.75 The mechanism underlying in vivo production of meso-zea.xa.nthm from lutein in the primate retina is still unclear. Despite considerable effort, no such enzyme has yet been identified.76
Discussion
The human macula may be considered as a “carotenoid sink,” as MP carotenoids are concentrated in the human macula at the highest concentration anywhere in the human body. Humans are not able to synthesize carotenoids and must obtain them from dietary sources such as fruits and vegetables. Therefore, the human diet is the “carotenoid source” for the human body, especially the retina. Although our understanding of the transport processes of carotenoids from the source to the sink is still incomplete, the currently available data suggest that the uptake of carotenoids probably shares the transport pathway with cholesterol, and that HDL and the receptors of HDL such as SR-BI may be involved in this process for delivery to the RPE, a tissue with a moderate concentration of a diverse range of carotenoids.30 Although not yet proven, we speculate that carotenoids are delivered from the RPE to the retina by a pathway analogous to the one used for retinoid transport that employs interphotoreceptor retinoid-binding protein (IRBP) to facilitate transport of hydrophobic ligands across the interphotoreceptor space. Within the retina, specific deposition of lutein and zeaxanthin may occur in a manner analogous to the mechanism employed by silkworms to deliver lutein to the silk gland where both a specific cell-surface uptake protein, Cameo2, and a specific binding protein, CBP, are obligatory.74 In the human macula, the corresponding process appears to involve specific binding proteins for zeaxanthin (GSTP1) and lutein (a StARD family protein) and CD36 as a possible transport protein. Tubulin may serve as a secondary high capacity deposition site for carotenoids within the Henle fiber layer.
In Fig. 3 we provide a brief schematic to describe our current understanding of the whole process of uptake, transport, and accumulation of macular pigment carotenoids in the human retina. Dietary carotenoids are released from ingested foods after ester saponification if necessary and incorporated into lipid micelles. SR-BI located on the surface of intestine cell then facilitates uptake and transport to the portal circulation in the chylomicron fraction.77, 78 Although it is still not known if carotenoids are modified in the liver before release into the bloodstream, supplying carotenoids to animals can increase their content in the liver.79, 80 Most hydrophobic carotenoids such as lycopene and beta-carotene are transported on LDL, whereas the more hydrophilic xanthophyll carotenoids, such as lutein and zeaxanthin, are primarily carried by HDL.81 RPE SR-BI facilitates uptake of lutein, zeaxanthin, and other carotenoids into the cell. IRBP may facilitate transport of lutein and zeaxanthin to the retinal cells via CD36, but specificity and uptake are ultimately driven by selective binding proteins such as GSTP1 and a StARD family protein, possibly in conjunction with tubulin. The process by which carotenoids and their oxidation and degradation products are removed from the retina is much less clear, but it is known that macular carotenoid levels are remarkably stable even in the face of large fluctuations in plasma levels that might occur during supplementation. Moreover, non-human primates placed on carotenoid-deficient diets typically take several years to achieve non-detectable macular pigment.82
Fig. 3.
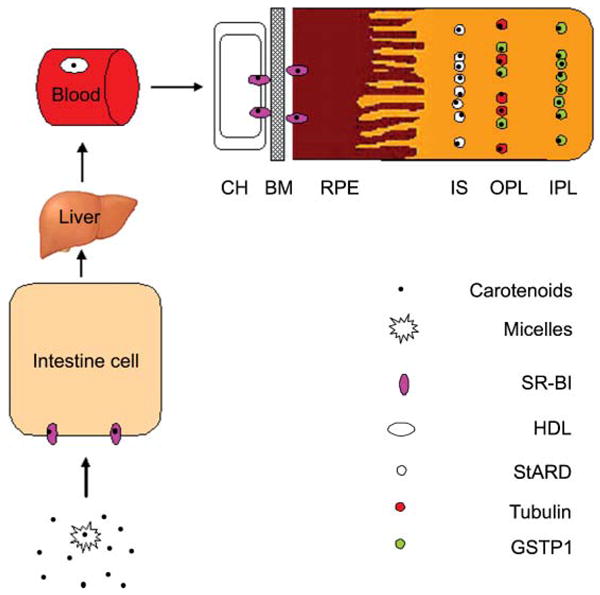
Possible pathway for MP carotenoid uptake, transport, and accumulation in the human retina. Choroicapillaris (CH); Bruch's membrane (BM); retinal pigment epithelium (RPE); inner segments (IS); outer plexiform layer (OPL); inner plexiform layer (IPL).
It will be a very important task to explore the potential role of MP carotenoid proteins in AMD and other eye diseases. The association between polymorphisms of glutathione S-transferase (GST) M1, T1, and P1 and risk of AMD has been investigated, and the primary results showed that the variants of GSTP1 combined with GSTPM1 (null) and GSTT1 (null) are associated with exudative AMD.47 SR-BI has been found to be a potential new AMD genetic factor because genotyping results have shown an association between variants of the SR-BI gene and AMD.83 It has also been demonstrated that zeaxanthin associated with its binding protein shows a higher ability to prevent lipid oxidation.19 All of these findings together indicate that there is a high possibility that human variation in ocular carotenoid-binding protein expression and function may be risk factors for AMD. Based on the inverse relationship between MP levels and risk of AMD, we speculate that the expression of the ocular carotenoid-binding proteins, GSTP1, StARD, and tubulin probably is lower in AMD than normal subjects. In addition, Issa and colleagues more recently reported that acquired deficiencies and abnormal distributions of macular pigments are prominent features of macular telangiectasia (Mac-Tel), an eye disease which can result in loss of visual acuity, reading difficulties and/or metamorphopsia.84 This suggests that further investigations on human ocular carotenoid-binding proteins will also help to better understand the pathophysiological processes of other eye diseases as well.
In the future, in addition to further investigation of the role of the known ocular carotenoid-binding proteins in ocular health and diseases, efforts should be continued to identify additional ocular carotenoid-binding proteins, carotenoid receptors, metabolizing enzymes, and transporters until the pathways for uptake and accumulation of MP carotenoids are completely understood. To identify new MP carotenoid-related proteins, molecular biological, cell biological, and proteomic methods also should be employed to complement traditional biochemistry approaches. For example, through comparison of gene and protein expression patterns of human fovea and peripheral retina, fovea-enriched genes and proteins may be identified that could be candidates to be new ocular carotenoid-binding proteins. We also can test if additional families of proteins which are known to interact with carotenoids in other species are present in human ocular tissues, as we have successfully demonstrated with StARD family proteins.49 In the past, we have assessed binding interactions between potential carotenoid-binding proteins and their ligands through overnight equilibrium binding studies that are cumbersome and consume large amounts of valuable proteins. More recently, we have optimized surface plasmon resonance (SPR) methods that allow real-time assessment of on- and off-rates of ligands with proteins immobilized on gold surfaces. This facilitates higher throughput screening of a variety of ligands in shorter amounts of time in a reproducible and sensitive manner. It is anticipated that these newer techniques will accelerate elucidation of the role of the macular pigment carotenoids in maintaining macular function in health and disease.
Acknowledgments
This work was supported by a grant from the National Institute of Health (EY-11600) and by an unrestricted departmental grant from Research to Prevent Blindness, Inc. (New York, NY).
Biographies

Binxing Li: Binxing Li received his Bachelor's degree from Agricultural University of Hebei, Baoding, P.R. China, in 1998. He received his PhD from Institute of Botany, Chinese Academy of Sciences, Beijing, P.R. China, in 2005, where his research emphasized structure and function of pho-tosynthetic membrane pigment–protein complexes. He then began his post-doctoral work with Professor Paul S. Bernstein at the Moran Eye Center, University of Utah, where he is studying carotenoid-binding proteins in the human retina. His work has resulted in around 20 publications.

Preejith Vachali: Preejith Vachali received his PhD in the field of Biosensors from Nanyang Technological University, Singapore, in 2007. Currently, he is a post-doctoral Fellow at the Moran Eye Centre, University of Utah, USA. His research interests include the development of novel biosensor techniques to investigate protein–carotenoid interaction related to age related macular degeneration (AMD), microbial carotenoid production and strain improvement, and nutritional biochemistry related to AMD.

Paul S. Bernstein: Paul S. Bernstein received his MD and PhD degrees from Harvard Medical School in 1988, and he is now the Mary Boesche Professor of Ophthalmology and Visual Sciences at the Moran Eye Center of the University of Utah. He is a clinician-scientist who divides his time between a busy retina practice and directing a research laboratory dedicated to the study of the biochemistry and biophysics of nutritional factors that may help to prevent the progression of blinding eye diseases such as age-related macular degeneration. He has four patents and nearly one hundred publications in the field.
Footnotes
This article is published as part of a themed issue on photosensitive visual pigments: opsins and retinoids.
References
- 1.Home E. An account of the orifice in the retina of the human eye, discovered by Professor Soemmering: to which are added proofs of this appearance being extended to the eyes of other animals. Philos Trans R Soc Land. 1798;2:332–345. [Google Scholar]
- 2.Wald G. Human vision and the spectrum. Science. 1945;101:653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- 3.Bone RA, Laudrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 4.Bone RA, Laudrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Inves Ophthalmol Vis Sci. 1993;34:2033–2040. [PubMed] [Google Scholar]
- 5.Whitehead AJ, Mares JA, Danis RP. Macular pigment. Arch Ophthalmol. 2006;124:1038–1045. doi: 10.1001/archopht.124.7.1038. [DOI] [PubMed] [Google Scholar]
- 6.Hammond BR, Jr, Wooten BR, Curran-Celentano J. Carotenoids in the retina and lens: possible acute and chronic effects on human visual performance. Arch Biochem Biophys. 2001;385:41–46. doi: 10.1006/abbi.2000.2184. [DOI] [PubMed] [Google Scholar]
- 7.Reading VM, Weale RA. Macular pigment and chromatic aberration. J Opt Soc Am. 1974;64:231–234. doi: 10.1364/josa.64.000231. [DOI] [PubMed] [Google Scholar]
- 8.Beatty S, Boulton M, Henson D, Kon HH, Murray IJ. Macular pigment and age related macular degeneration. Br J Ophthalmol. 1999;83:867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handelman GJ, Draz EA, Reay CC, Kuijk FJGMv. Carotenoids in the human macula and whole retina. Invest Ophthalmol Visual Sci. 1988;29:850–855. [PubMed] [Google Scholar]
- 10.Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 12.Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–1787. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EDS Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 14.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA, J Am Med Assoc. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 15.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 16.Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995;113:1518–1523. doi: 10.1001/archopht.1995.01100120048007. [DOI] [PubMed] [Google Scholar]
- 17.Thomson LR, Toyoda Y, Delori FC, Garnett KM, Wong ZY, Nichols CR, Cheng KM, Craft NE, Dorey CK. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp Eye Res. 2002;75:529–542. doi: 10.1006/exer.2002.2050. [DOI] [PubMed] [Google Scholar]
- 18.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- 19.Bhosale P, Bernstein PS. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochim Biophys Acta, Mol Basis Dis. 2005;1740:116–121. doi: 10.1016/j.bbadis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Bioleau TWM, Moore AC, Erdman JW. In: Carotenoids and Vitamin A. Papas AM, editor. CRC Press; Boca Raton: 1999. pp. 133–151. [Google Scholar]
- 21.Jorgensen K, Skibsted L. Carotenoid scavenging radicals : Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z Lebensm Unters Forsch. 1993;196:423–429. doi: 10.1007/BF01190806. [DOI] [PubMed] [Google Scholar]
- 22.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 23.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Visual Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 24.Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: a model for Haidinger's brushes. Vision Res. 1984;24:103–108. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- 25.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 26.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Visual Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- 27.Khachik F, Askin FB, Lai K. In: Phytochemical A new paradigm. Bidlack WayeR, Omaye StanleyT, Meskin MarkS, Janher D., editors. CRC press LCC; 1998. pp. 76–131. [Google Scholar]
- 28.Khachik F, Beecher GR, Goli MB. Separation, identification and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography. Pure Appl Chem. 1991;63:71–80. [Google Scholar]
- 29.Khachik F, Spangler CJ, Smith JJC, Canfield LM, Pfander ASH. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- 30.Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci. 2002;43:3383–3392. [PubMed] [Google Scholar]
- 31.Saari JC. In: The Retinoids: Biology, Chemistry, and Medicine. Sporn MB, Rorberts AB, Goodman DS, editors. Raven Press; New York, USA: 1994. pp. 351–385. [Google Scholar]
- 32.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Aram Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 33.Bowmaker JK, Kovach JK, Whitmore AV, Loew ER. Visual pigments and oil droplets in genetically manipulated and carotenoid deprived quail: A microspectro-photometric study. Vision Res. 1993;33:571. doi: 10.1016/0042-6989(93)90180-5. [DOI] [PubMed] [Google Scholar]
- 34.Bhosale P, Bernstein PS. Vertebrate and invertebrate carotenoid-binding proteins. Arch Biochem Biophys. 2007;458:121–127. doi: 10.1016/j.abb.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein PS, Balashov NA, Tsong ED, Rando RR. Retinal Tubulin binds macular carotenoids. Invest Ophthalmol Vis Sci. 1997;38:167–175. [PubMed] [Google Scholar]
- 36.Crabtree DV, Ojima I, Geng X, Adler AJ. Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg Med Chem. 2001;9:1967–1976. doi: 10.1016/s0968-0896(01)00103-1. [DOI] [PubMed] [Google Scholar]
- 37.Bhosale P, Larson AJ, Frederick J, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a pi isoform of glutathione S-transferase as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 38.Townsend DM, Tew KD. The role of glutathione-S-transferase in anticancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Ilio C, Aceto A, Bucciarelli T, Angelucci S, Felaco M, Grilli A, Federici G. Glutathione transferase isoenzymes from human prostate. Biochem J. 1990;271:481–485. doi: 10.1042/bj2710481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manoharan TH, Gulick AM, Puchalski RB, Servais AL, Fahl WE. Structural studies on human glutathione S-transferase pi. Substitution mutations to determine amino acids necessary for binding glutathione. J Biol Chem. 1992:18940–18945. [PubMed] [Google Scholar]
- 41.Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular Cloning, Characterization, and Expression in Escherichia coli of Full-length cDNAs of Three Human Glutathione S-Transferase Pi Gene Variants: Evidence for differential catalytic activity of the encoded proteinsevidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 42.Gulick AM, Goihl AL, Fahl WE. Studies on human glutathione S-transferase pi. Family of native-specific monoclonal antibodies used to block catalysis. J Biol Chem. 1992;267:18946–18952. [PubMed] [Google Scholar]
- 43.Chen H, Juchau MR. Glutathione S-transferases act as isomerases in isomerization of 13-cis-retinoic acid to all-trans-retinoic acid in vitro. Biochem J. 1997;327:721–726. doi: 10.1042/bj3270721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo HW, Ali-Osman F. Genomic Cloning of hGSTPl*C, an Allelic Human Pi Class Glutathione S-Transferase Gene Variant and Functional Characterization of Its Retinoic Acid Response Elements. J Biol Chem. 1997;272:32743–32749. doi: 10.1074/jbc.272.52.32743. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen JZ, De Maria F, Turella P, Federici G, Mattei M, Fabrini R, Dawood KF, Massimi M, Caccuri AM, Ricci G. Glutathione Transferases Sequester Toxic Dinitrosyl-Iron Complexes in Cells: A protection mechanism against excess nitric oxide. J Biol Chem. 2006;282:6364–6371. doi: 10.1074/jbc.M609905200. [DOI] [PubMed] [Google Scholar]
- 46.Townsend DM, Manevich Y, He SHL, Pazoles CJ, Tew KD. Novel Role for Glutathione S-Transferase π: Regulator of protein s-glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2008;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oz O, Aras Ates N, Tamer L, Yildirim O, Adiguzel U. Glutathione s-transferase m1, t1, and p1 gene polymorphism in exudative age-related macular degeneration: a preliminary report. Eur J Ophthalmol. 2006;16:105–110. doi: 10.5301/EJO.2008.3524. [DOI] [PubMed] [Google Scholar]
- 48.Juronen E, Tasa T, Veromann S, Parts L, Tiidla A, Pulges R, Panov A, Soovere L, Koka K, Mikelsaar AV. Polymorphic glutathione S-transferases as genetic risk factors for senile cortical cataract in Estonians. Invest Ophthalmol Visual Sci. 2000;41:2262–2267. [PubMed] [Google Scholar]
- 49.Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 50.Tabunoki H, Sugiyama H, Tanaka Y, Fujii H, Banno Y, Jouni ZE, Kobayashi M, Sato R, Maekawa H, Tsuchida K. Isolation, characterization, and cDNA sequence of a carotenoid binding protein from the silk gland of Bombyx mori larvae. J Biol Chem. 2002;277:32133–32140. doi: 10.1074/jbc.M204507200. [DOI] [PubMed] [Google Scholar]
- 51.Sakudoh T, Tsuchida K, Kataoka H. BmStart1, a novel carotenoid-binding protein isoform from Bombyx mori, is orthologous to MLN64, a mammalian cholesterol transporter. Biochem Biophys Res Commun. 2005;336:1125–1135. doi: 10.1016/j.bbrc.2005.08.241. [DOI] [PubMed] [Google Scholar]
- 52.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 53.Sierra A. Neurosteroids: the StAR protein in the brain. J Neuroendocrinal. 2004;16:787–793. doi: 10.1111/j.1365-2826.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- 54.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 55.Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984;25:1277–1294. [PubMed] [Google Scholar]
- 56.Trigatti BL, Rigotti A, Braun A. Cellular and physiological roles of SR-BI, a lipoprotein receptor which mediates selective lipid uptake. Biochim Biophys Acta, Mol Cell Biol Lipids. 2000;1529:276–286. doi: 10.1016/s1388-1981(00)00154-2. [DOI] [PubMed] [Google Scholar]
- 57.Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2008;50:S195–200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tso GMC, Fan WH, Rogers C, Rye KA, Barter PJ. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler, Thromb, Vasc Biol. 2006;26:1144–1149. doi: 10.1161/01.ATV.0000216600.37436.cf. [DOI] [PubMed] [Google Scholar]
- 59.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 60.Olson JA. Absorption, transport, and metabolism of carotenoids in humans. Pure Appl Chem. 1994;66:1011–1016. [Google Scholar]
- 61.Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Visual Sci. 2007;48:4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- 62.Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer IV, Pavelka M, Eckhardt ER, Van Der Westhuyzen DR, Schütz GJ, Stangl H. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J Biol Chem. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- 63.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 64.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 65.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 66.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu MKS. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- 67.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 68.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krieger M, Kozarsky K. Influence of the HDL receptor SR-BI on atherosclerosis. Curr Opin Lipidol. 1999;10:491–497. doi: 10.1097/00041433-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 70.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 71.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiefer C, Sumser E, Wernet MF, Lintig JV. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 74.Sakudoh T, Iizuka T, Narukawa J, Sezutsu H, Kobayashi I, Kuwazaki S, Banno Y, Kitamura A, Sugiyama H, Takada N, Fujimoto H, Kadono-Okuda K, Mita K, Tamura T, Yamamoto K, Tsuchida K. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. J Biol Chem. 2010;285:7739–7751. doi: 10.1074/jbc.M109.074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 76.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Visual Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 77.Goti D, Reicher H, Malle E, Kostner GM, Panzenboeck U, Sattler W. High-density lipoprotein (HDL3)-associated -tocopherol is taken up by HepG2 cells via the selective uptake pathway and resecreted with endogenously synthesized apo-lipoprotein B-rich lipoprotein particles. Biochem J. 1998;332:57–65. doi: 10.1042/bj3320057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rigotti A, Krieger M. Getting a handle on “good” cholesterol with the high-density lipoprotein receptor. N Engl J Med. 1999;341:2011–2013. doi: 10.1056/NEJM199912233412612. [DOI] [PubMed] [Google Scholar]
- 79.Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci. 2002;43:3538–3549. [PubMed] [Google Scholar]
- 80.Toyoda Y, Thomson LR, Langner NEA, Craft KMG, Nichols CR, Cheng KM, Dorey CK. Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail. Invest Ophthalmol Vis Sci. 2002;43:1210–1221. [PubMed] [Google Scholar]
- 81.Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr. 2007;82:762–769. doi: 10.1093/ajcn/85.3.762. [DOI] [PubMed] [Google Scholar]
- 82.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863. [PubMed] [Google Scholar]
- 83.Zerbib J, Seddon JM, Richard F, Reynolds R, Leveziel N, Benlian P, Borel P, Feingold J, Munnich A, Soubrane G, Kaplan J, Rozet JM, Souied EH. rs5888 variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS One. 2009;4:e7341. doi: 10.1371/journal.pone.0007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Issa PC, Van Der Veen RL, Stijfs A, Holz FG, Scholl HP, Berendschot TT. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Exp Eye Res. 2009;89:25–31. doi: 10.1016/j.exer.2009.02.006. [DOI] [PubMed] [Google Scholar]


