Abstract
Background
Nerve growth factor (NGF) is a neurotrophin that supports the survival and differentiation of sympathetic neurons, and its increased expression after myocardial infarct was correlated with cardiac sympathetic hyperinnervation and arrhythmias. However, it is unclear whether NGF protects the heart during infarct. In this study, we sought to address this issue in rat heart exposed to ischemia/reperfusion injury (IRI).
Methods
NGF was administered intravenously (IV), 15 min before ischemia, at different concentrations in the absence or presence of inhibitors of phosphatidylinositol-3 kinase (PI3K) or nitric oxide synthase (NOS) in different groups of rats (n = 6) with left coronary occlusion for 30 min followed by 120-min reperfusion. The area at risk and infarct to risk ratios were determined from sections stained with 1 % 2,3,5-triphenylterazolium chloride.
Results
NGF treatment at doses of 0.015–15 μg/kg, with an optimal dose of 0.15 μg/kg given IV before ischemia, reduced the infarct size from about 60% at the area of risk to about 25%, indicating cardioprotection by about 60%. The infarct-sparing effects of NGF were partially abolished by the inhibition of PI3K and NOS using wortmannin and N(G)-monomethyl-l-arginine, respectively.
Conclusions
We have demonstrated for the first time that NGF attenuates myocardial infarct damage in an in vivo rat model of myocardial regional IRI. This cardioprotective effect is proposed to be related to the activities of PI3K and NOS. This suggests that NGF has a potential therapeutic role in the treatment of IRI.
Keywords: cardioprotection, infarct, myocardial ischemia/reperfusion, NGF
Nerve growth factor (NGF), a member of the neurotrophin family of growth factors, plays a crucial function in the nervous system [1] and is very important in the cross talk between the nervous and cardiovascular systems [2]. Growing evidence has suggested a critical role for NGF during cardiovascular development. NGF is an essential factor in the formation of the heart and a critical regulator of vascular development. Postnatally, NGF controls the survival of blood vessel endothelial cells, vascular smooth muscle cells, and heart cardiomyocytes, and regulates angiogenesis and vasculogenesis by autocrine and paracrine mechanisms [3]. While many studies have established the role of NGF in cardiac pathophysiology [4], the hypothesis that NGF plays an important cardioprotective role in myocardial ischemia/reperfusion injury (IRI) has not yet been explored.
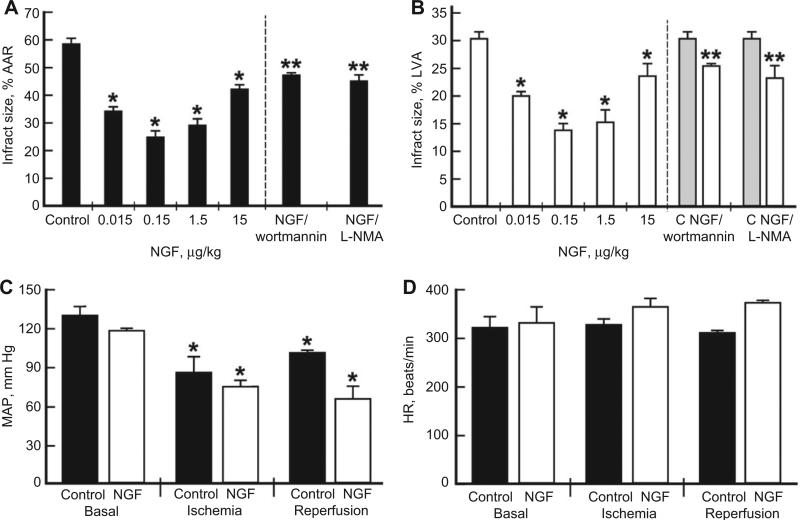
To determine the effects of NGF on myocardial IRI, NGF was administered intravenously (IV) 15 min before ischemia to rats, in a series of experiments (n=6 per group) for finding the dose-dependent effect (0.015–15 μg/kg) and for the assessment of the optimal dose. To determine the mechanisms involved, the rats were injected with inhibitors of phosphatidylinositol-3 kinase (PI3K) (wortmannin, 15 μg/kg) or nitric oxide synthase (NOS) [N(G)-monomethyl-l-arginine (L-NMA), 15 mg/kg] 15 min before the injection of NGF (0.15 μg/kg) – conditions previously reported by us to inhibit these signaling pathways in this rat model [5, 6]. The principal end point was infarct size expressed either as a percentage of the area at risk (AAR) or the left ventricular area (LVA). The infarct size was 60%±3% of the AAR (Figure 1A) and 30%±2% of the LVA (Figure 1B). A bell-shaped dose-response reduction in infarct size was seen with NGF treatment, 0.15 μg/kg being the optimally effective dose. The hearts of treated rats had an infarct size of 24.9%±1.8% of the AAR and 13.8%±1% of the LVA, which are approximately 58.5% and 54% reduction in infarct size compared with the control, respectively (Figure 1A,B). Heart rate and blood pressure were monitored throughout the procedure, and there were no significant differences in basal hemodynamic parameters between groups. Mean arterial pressures decreased during ischemia and reperfusion by 35%–45% in all groups [7]; however, there was no significant difference between the groups (Figure 1C,D).
Figure 1. NGF-induced cardioprotection (A, B) and hemodynamic values of mean arterial pressure (C) and heart rate (D) in a rat myocardial ischemia/reperfusion model.
An in vivo anesthetized rat model was used for these experiments, with the general surgical protocol and determination of infarct size as described previously [7]. Briefly, thiobutabarbital sodium (inactin, 100 mg/kg, IP) was used for anesthesia, followed by a tracheotomy and artificial ventilation. Male Sprague-Dawley rats at 8 weeks of age (received humane care in compliance with the Guide for the Care and Use of Laboratory Animals; experiments were approved by the Ethical Committee of Medical College of Wisconsin) were ventilated with room air at 35–45 breaths/min supplemented with O2. Arterial pH, pCO2, and pO2 were monitored and maintained throughout the experiment using an ABL 80 Flex pH/Blood Gas Analyzer. Body temperature was maintained at 37°C using a heating pad. The left common carotid artery was cannulated for blood pressure, heart rate, and blood gas measurements. A thoracotomy was performed at the fifth intercostal space, the pericardium was excised, and a ligature was placed around the left anterior descending coronary artery. Following surgical intervention and stabilization, rats were separated into groups and subjected to 30 min of ischemia and 120 min of reperfusion. The myocardium was made ischemic by placing the two ends of the ligature around the left anterior descending coronary artery through a polypropylene tube and fixing the tube to the epicardial surface with a hemostat. Removal of the hemostat allowed for reperfusion of the AAR. After 2 h of reperfusion, the ligature was again re-occluded and 0.5% Evans’ blue was perfused into the aortic cannula. The AAR was determined by the blue negative staining. The left ventricle was excised and cross-sectioned into four to five slices and further separated into the normal zone (LVA) and AAR. Slices were incubated in 1% 2,3,5-triphenylterazolium chloride to determine the infarct size. The heart was incubated overnight in 10% formaldehyde, and the infarcted tissue was dissected from the AAR and measured gravimetrically. Infarct size was expressed as a percentage of the AAR (part A) or the LVA (part B). Rats were treated IV with either saline or increasing doses of βNGF (Alomone Labs, Jerusalem, Israel) administered as an IV bolus, 15 min before 30-min ischemia followed by 120-min reperfusion. The inhibitors were injected 15 min before NGF treatment. Data are mean±SD of infarct size, n=6/group; *p<0.05 vs. control; **p<0.05 vs. 0.15 μg/kg NGF.
PI3K is an important mediator of cardioprotection [8]. To determine whether NGF-induced cardioprotection is mediated by this pathway, we injected IV, before IRI, wortmannin, a PI3K inhibitor, alone or in combination with NGF. Wortmannin abolished the cardioprotective effect of NGF by 50% but had no effect when given alone.
Increased NO produced by activating NOS protects the heart against IRI [9]. To determine whether NGF-induced cardioprotection is mediated by this pathway, we injected IV, before IRI, L-NMA, a general NOS inhibitor. L-NMA abolished the cardioprotective effect of NGF by 50% but had no effect when given alone.
The major finding of this study is that a single treatment with NGF before ischemia significantly reduced the heart infarct size associated with IRI. In addition, we found that inhibition of the signaling molecules PI3K and NOS significantly abrogated the NGF-induced cardioprotective effect, proposing their involvement in the mechanism of action of NGF. It is known that heart cells secrete NGF and express its receptors p75NTR and trkA. The activation of these receptors in cardiomyocytes triggers a prosurvival effect involving the PI3K/Akt pathway [10]. It is also established that NGF, by activation of its trkA receptors, activates the Ras-Erk pathway responsible for the induction of NOS in sympathetic neurons [11] involved in NGF-induced neuroprotective effects [12]. Therefore, it is reasonable to propose that the NGF-induced cardio-protective effect in the rat heart exposed to IRI is mediated by trkA activation of PI3K/NOS. We recognize that the contributions of these signaling pathways were primarily examined with kinase inhibitors, which is not without problems. However, by showing that the Akt phosphorylation and endothelial NOS (eNOS) regulation of cGMP production are inhibited by wortmannin and L-NMA [5, 6], we believe that this supports the circumstantial evidence that PI3K/Akt and eNOS are involved in NGF cardioprotection, primarily suggesting that NGF modulates cardiomyocyte ischemic tolerance: PI3K and NOS are the major modulators of cardiomyocyte ischemic tolerance [13, 14].
NGF is present in infarcted human heart, and mice with infarcted heart treated with an antibody against NGF showed cardiomyocyte apoptosis and worsened cardiac function [15], further stressing the important cardioprotective role of NGF in the heart. Following myocardial infarction (MI), NGF levels initially increase and thereafter decline, emphasizing its cardioprotective, neurotrophic, and neurotropic sympathetic pleiotropic effects in the heart [4]. The mechanism behind the ability of NGF to upregulate the cardioprotective pathways is currently unknown. We do not know if the cardioprotective effect of NGF is a reflection of its direct prosurvival effect on heart cardiomyocytes [10], indirectly mediated by remote IRI preconditioning [16] due to sympathetic and/or sensory nerves and/or induced by myocardium microcapillary endothelial [17] and/or smooth muscle cell activation. Future studies should address the role of endogenous NGF on ischemic tolerance and specifically its role in the different forms of preconditioning.
Whatever the mechanism, the present results are the first to demonstrate that a single treatment with NGF confers immediate protection against injury caused by ischemia/reperfusion in the heart, proposing a potential therapeutic role for NGF in MI. The potential impact of the modulation of cardiac ischemic tolerance by NGF can be manifold: increasing ischemic tolerance, and particularly tolerance to reperfusion, can serve as an important therapeutic target. Furthermore, interactions with NGF of existing drugs and endogenous agents should be studied: if exogenous drugs, such as noradrenaline infusion, reduce pericardial NGF or its effect [18], then they may reduce ischemic tolerance, thereby increasing the risk of ischemic events, leading to occult cardiotoxicity [19].
Footnotes
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: PL holds The Jacob Gitlin Chair in Physiology and is affiliated and partially supported by the David R. Bloom Center for Pharmacy and the Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem, Israel.
Contributor Information
Jennifer L. Strande, Division of Cardiovascular Medicine , Medical College of Wisconsin, Milwaukee, WI , USA
Kasi V. Routhu, Division of Cardiovascular Medicine , Medical College of Wisconsin, Milwaukee, WI , USA
Shimon Lecht, Department of Bioengineering and Temple Institute for Regenerative Medicine and Engineering , Temple University, Philadelphia, PA , USA.
Philip Lazarovici, Institute for Drug Research, School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem, POB 12065, Jerusalem 91120, Israel.
References
- 1.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF) – implications in drug development. Curr Pharm Des. 2006;12:2609–22. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- 3.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govoni S, Pascale A, Amadio M, Calvillo L, D'Elia E, Cereda C, et al. NGF and heart: is there a role in heart disease ? Pharmacol Res. 2011;63:266–77. doi: 10.1016/j.phrs.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Routhu KV, Tsopanoglou NE, Strande JL. Parstatin(1–26): the putative signal peptide of protease-activated receptor 1 confers potent protection from myocardial ischemia-reperfusion injury. J Pharmacol Exp Ther. 2010;332:898–905. doi: 10.1124/jpet.109.162602. [DOI] [PubMed] [Google Scholar]
- 6.Strande JL, Widlansky ME, Tsopanoglou NE, Su J, Wang J, Hsu A, et al. Parstatin: a cryptic peptide involved in cardioprotection after ischaemia and reperfusion injury. Cardiovasc Res. 2009;83:325–34. doi: 10.1093/cvr/cvp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. Inhibiting protease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther. 2008;324:1045–54. doi: 10.1124/jpet.107.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–3. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Yang XP, Liu YH, Carretero OA, LaPointe MC. Reduction of myocardial infarct size by inhibition of inducible nitric oxide synthase. Am J Hypertens. 1999;12:174–82. doi: 10.1016/s0895-7061(98)00235-0. [DOI] [PubMed] [Google Scholar]
- 10.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonhoff CM, Bulseco DA, Brancho DM, Parada LF, Ross AH. The Ras-ERK pathway is required for the induction of neuronal nitric oxide synthase in differentiating PC12 cells. J Neurochem. 2001;78:631–9. doi: 10.1046/j.1471-4159.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res Dev Brain Res. 2003;140:15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- 13.Ravingerova T. Intrinsic defensive mechanisms in the heart: a potential novel approach to cardiac protection against ischemic injury. Gen Physiol Biophys. 2007;26:3–13. [PubMed] [Google Scholar]
- 14.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 15.Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, et al. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–84. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjoquist PO, et al. Remote ischaemic pre- and delayed postconditioning – similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012;97:908–17. doi: 10.1113/expphysiol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lecht S, Foerster C, Arien-Zakay H, Marcinkiewicz C, Lazarovici P, Lelkes PI. Cardiac microvascular endothelial cells express and release nerve growth factor but not fibroblast growth factor-2. In Vitro Cell Dev Biol Anim. 2010;46:469–76. doi: 10.1007/s11626-009-9267-5. [DOI] [PubMed] [Google Scholar]
- 18.Qin F, Vulapalli RS, Stevens SY, Liang CS. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am J Physiol Heart Circ Physiol. 2002;282:H363–71. doi: 10.1152/ajpheart.00319.2001. [DOI] [PubMed] [Google Scholar]
- 19.Golomb E, Nyska A, Schwalb H. Occult cardiotoxicity – toxic effects on cardiac ischemic tolerance. Toxicol Pathol. 2009;37:572–93. doi: 10.1177/0192623309339503. [DOI] [PubMed] [Google Scholar]