Abstract
Pluripotent embryonic stem cells (ESCs) are capable of differentiating into all mesoderm-derived cell lineages, including endothelial, hematopoietic, and cardiac cell types. Common strategies to direct mesoderm differentiation of ESCs rely on exposing the cells to a series of biochemical and biophysical cues at different stages of differentiation to promote maturation toward specific cell phenotypes. Shear forces that mimic cardiovascular physiological forces can evoke a myriad of responses in somatic and stem cell populations, and have, thus, been studied as a means to direct stem cell differentiation. However, elucidating the effects of shear pre-conditioning on the subsequent vascular differentiation and morphogenesis of ESCs has yet to be examined. In this study, ESC monolayers were subjected to physiological shear (5 dyn/cm2) or static conditions for 2 days on collagen IV-coated substrates before initiating embryoid body (EB) differentiation. Immediately after the pre-conditioning period, shear pre-conditioned and statically cultured ESCs exhibited similar morphologies and largely retained a pluripotent phenotype; however, ESCs exposed to fluid shear expressed increased levels of endothelial marker genes Flk-1 (∼3-fold), VE-cadherin (∼3-fold), and PECAM (∼2-fold), compared with statically cultured ESCs. After 7 days of EB culture, ∼70% of EBs formed from shear pre-conditioned ESCs expressed significantly higher levels of endothelial marker genes compared with EBs formed from statically cultured ESCs. Interestingly, unlike EBs formed from statically cultured ESCs, EBs formed from fluid shear stress pre-conditioned ESCs exhibited a centrally localized region of VE-cadherin+ cells that persisted for at least 10 days of differentiation. These results demonstrate that fluid shear stress pre-conditioning not only promotes ESC endothelial gene expression but also subsequently impacts the organization of endothelial cells within EBs. Together, these studies highlight a novel approach to promote in vitro morphogenesis of developmental vasculogenic models and potentially promote pre-vascularization of tissue-engineered constructs derived from pluripotent stem cells.
Introduction
Pluripotent embryonic stem cells (ESCs) are an attractive cell source for tissue engineering and regenerative medicine therapies because of the diversity of cell types ESCs can yield, including rare or difficult to isolate populations from adult tissues. However, a major challenge in directing the differentiation of ESCs remains the establishment of robust and reproducible methods to effectively promote specific cell phenotypes. Many differentiation protocols rely on the delivery of growth factors and cytokines in the presence of specific extracellular matrices at various concentrations and durations as a primary means to control stem cell differentiation in a single- or multi-stage approach.1,2 However, such protocols are often subject to technical variability among different laboratories, require significant quantities of expensive factors, and generally yield relatively low numbers of differentiated cells. To circumvent some of the challenges of using biochemical cues to control ESC differentiation, physical forces have recently been utilized as a means to promote the differentiation of ESCs and vascular progenitor cells toward a number of phenotypes, including cardiac, smooth muscle, endothelial, and hematopoietic cells.3–9 Physical forces are an attractive means to direct differentiation, because they can be applied in a controlled and homogeneous manner to large populations of cells, and are, therefore, amenable for integration into many culture systems for the scalable production of differentiated stem cells.10 In addition, the reproducibility of well-defined physical perturbations enables facile translation between laboratories and scale-up bioprocessing of differentiated stem cells.11
The role of physical forces in tissue homeostasis, remodeling, and pathological conditions has become increasingly appreciated due to the importance of biophysical cues in regulating cell phenotype and tissue morphogenesis.12 As a result, physical forces have been employed in vitro to recapitulate physiological mechanical forces experienced by tissues such as the heart and the vasculature.4,6 Within the vasculature, fluid shear stress is the dominant physical force experienced by endothelial cells (ECs) that line blood vessels, and it has been well established that the modulation of various shear stress parameters (i.e., magnitude, duration, pulsatility, etc.) can dynamically manipulate EC function and phenotype.13,14 Fluid shear mechanotransduction has also been implicated as a critical regulator of cell fate specification and morphogenesis during embryonic development, as blood islands derived from the Flk-1+ hemangioblast composed of more mature hematopoietic (Runx1, Tal1) and endothelial (VE-cadherin, PECAM) progenitors arise coincident with the onset of vascular flow.15 While studies on EC differentiation in response to fluid shear stress have primarily focused on the acute phenotypic effects of fluid shear on endothelial specification of stem cells, the downstream effects of physical stimulation on stem cell organization, morphogenesis, and differentiation remain less well defined.
ESCs are commonly differentiated as 3D aggregates of cells in suspension culture, referred to as embryoid bodies (EBs) that can spontaneously give rise to derivatives of the three germ layers—ecto-, endo-, and mesoderm, and undergo morphogenesis analogous to embryonic development in vivo.16,17 EB-based methods have frequently been employed to differentiate ECs from ESCs,3,18,19 and the establishment of 3D cell–cell and cell–matrix adhesions supports morphogenic events within EBs that resemble aspects of primitive vasculogenesis.20–22 Efforts to control and modulate the differentiation of ESCs toward endothelial and many other cell types have focused primarily on manipulating physical and chemical cues during and after EB formation. For example, the composition of intercellular adhesions,23–26 soluble growth factors, cytokines, and materials27,28 and culture environments, such as hypoxia,23,29 have been used to modulate EB differentiation. However, a few studies to date have examined how “pre-conditioning” of ESCs before EB formation may influence the trajectory of differentiated stem cell phenotypes and multicellular morphogenesis.
Therefore, the objective of this study was to examine the effects of fluid shear stress pre-conditioning on mouse ESC endothelial differentiation and morphogenesis within EBs. After exposing ESCs to 0 or 5 dyn/cm2 for 48 h in monolayer culture, the ESC populations were differentiated as EBs in rotary orbital suspension culture for approximately 10 days to examine subsequent differentiation and morphogenesis. Immediately after pre-conditioning, ESCs were examined for expression of pluripotency markers and lineage-specific genes. During the course of EB differentiation, EB morphology and size, as well as endothelial gene and protein expression were analyzed. In addition, ESCs stably transfected with pVE-cadherin-GFP were pre-conditioned and similarly differentiated, in order to monitor the spatiotemporal presence of VE-cadherin+ cells within EBs. Overall, fluid shear stress pre-conditioning of ESCs represents a novel application of physical forces to manipulate EB differentiation and could prove useful for the study of endothelial morphogenesis within the context of pluripotent 3D multicellular aggregates for tissue engineering and developmental biology studies.
Materials and Methods
ESC and EB culture
D3 murine ESCs (nontransfected or pVE-cadherin-GFP,30 the latter a kind gift from Dr. Joseph Wu) were cultured on 0.1% gelatin-coated tissue-culture polystyrene cultureware (Corning) in Dulbecco's modified Eagle's medium (DMEM; Mediatech) containing 15% fetal bovine serum (FBS; Hyclone), 1×nonessential amino acids, 2 mM l-glutamine, 100 mg/mL streptomycin, 0.25 mg/mL amphotericin, 100 U/mL penicillin, 103 U/mL leukemia inhibitory factor (LIF; Millipore), and 0.1 mM β-mercaptoethanol. ESCs were re-fed every other day and passaged with 0.05% trypsin-EDTA (Mediatech) every 2–3 days before reaching 70%–80% confluence. EBs were formed from a single-cell suspension of 2×106 cells in 10 mL of differentiation media (DMEM with FBS and supplements without LIF) and maintained in a 100-mm Petri dish on a rotary orbital shaker (Lab-Line Lab Rotator; Barnstead) at 40 rpm.31 Rotary orbital shakers were calibrated daily to ensure consistent speed throughout the period of EB suspension culture. EBs were re-fed every 2 days after gravity-induced sedimentation in a 15-mL conical tube by exchanging 90% of the spent media with fresh media.
Fluid shear pre-conditioning
Before EB differentiation studies, ESCs were seeded onto 28.5 cm2 glass slides pre-coated with mouse collagen type IV (50 μg/mL; BD Biosciences) at a density of 20,000 cells/cm2 and cultured for 48 h under static conditions to enables the ESCs to form a confluent monolayer. The confluent monolayer of ESCs was then subjected to either 0 dyn/cm2 (static) in a 150-mm Petri dish or 5 dyn/cm2 (shear) of continuous fluid flow for 48 h using a parallel plate flow chamber connected to a peristaltic pump.6 During pre-conditioning, ESCs were cultured in Minimal Essential Media Alpha (Mediatech) supplemented with 10% FBS, 100 U/mL penicillin streptomycin, and 0.1 mM β-mercaptoethanol, similar to media commonly used for ESC endothelial differentiation.6,32,33
Quantitative reverse-transcriptase–polymerase chain reaction
Total RNA was extracted from samples using an RNeasy Mini Kit (Qiagen). The quantity and quality of RNA was determined using a Nanodrop® Spectrophotometer ND1000 reading at 280 nm and the ratio between the absorbance at 260 and 280 nm, respectively. Samples with ratios between 1.7 and 2.0 were used to synthesize complementary DNA (cDNA) from 1 μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad). Quantitative polymerase chain reaction was performed using SYBR green technology on an MyIQ cycler (Bio-Rad); amplification was performed using a two-step cycling program that was run at the appropriate annealing temperatures for each primer set (40 cycles, 1 min). Primer sequences (Invitrogen) were designed using Beacon Designer software, validated using appropriate cell controls for target genes, vascular endothelial growth factor receptor 2 (Flk-1), vascular endothelial growth factor receptor 1 (Flt-1), vascular endothelial cadherin (VE-cadherin), platelet-endothelial cell adhesion molecule (PECAM), runt-related transcription factor 1 (Runx1), and T-cell acute lymphocytic leukemia protein 1 (Tal1), alpha fetaprotein (AFP), paired box gene 6 (Pax6), cardiac-specific homeobox protein (Nkx2.5), myocyte-specific enhance factor 2C (Mef2c), and global transcription factor 4 (GATA4). Primer sequences and specific annealing temperatures are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea). The absolute quantities of the specific gene products were calculated using a standard curve and normalized to expression levels of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Histology
EB samples were prepared for histology after being fixed in 10% formalin for 30 min at room temperature, washed thrice with phosphate-buffered saline (PBS), and resuspended in 200–400 μL of Histogel (Richard Allan Scientific). Histogel-embedded EB samples were dehydrated through a graded series of alcohol solutions (70%–100%) and xylene rinses before being infiltrated and embedded in paraffin. Histological samples were sectioned at 5 μm using a microtome (Microm HM 355S), affixed to glass slides, and de-paraffinized before histological staining. Samples were stained with hematoxylin and eosin (H&E) using a Leica AutoStainer XL. After staining, samples were rinsed with xylene, then cover slipped using mounting media, and imaged shortly thereafter using a Nikon Eclipse 80i upright microscope that was equipped with a SpotFLEX camera (Diagnostic Instruments, Inc.).
Immunostaining of cell monolayers and embryoid bodies
ESC monolayers were washed with Dulbecco's PBS before fixation in 4% paraformaldeyde for 10 min at room temperature. After fixation, adherent cells were blocked and permeabilized with PBS containing 2% donkey serum (blocking buffer) and 0.05% Triton X-100 at room temperature for 45 min. Monolayers were then rinsed twice with PBS and incubated in goat anti-Oct-4 (1:200; Santa Cruz Biotechnology) primary antibody overnight at 4°C. Samples were rinsed in blocking buffer before incubation in a donkey anti-goat AlexaFluor™ 488 (Invitrogen) secondary antibody (1:200) and Hoescht (1:100) diluted in blocking buffer for 1 h at room temperature. Monolayers were rinsed in PBS, cover slipped using Fluoro-Gel™ mounting media, and imaged shortly thereafter using a Zeiss LSM 510 NLO multiphoton confocal microscope.
For whole mount immunofluorescence, EBs were washed in PBS and fixed in 4% paraformaldehyde for 1 h at 4°C. After fixation, EBs were washed in blocking buffer containing 2% donkey serum and 0.1% Tween-20. EBs were permeabilized with 1.5% Triton X-100 for 30 min and subsequently re-fixed in 4% paraformaldehyde for 15 min. EBs were incubated in blocking buffer for 1–3 h at 4°C and incubated in the following primary antibodies from Santa Cruz Biotechnology: goat anti-VE-cadherin (CD144; 1:200), rabbit anti-PECAM (CD31; 1:200), or rabbit anti-vWF (1:200) overnight at 4°C. After the overnight incubation, EBs were washed with blocking buffer and incubated with a donkey anti-goat AlexaFluor®488 or donkey anti-rabbit AlexaFluor®546 (Invitrogen) secondary antibodies (1:200) and Hoescht 33258 (10 μg/mL) for 4 h at 4 C. Stained EBs were washed with blocking buffer and imaged the next day using a Zeiss LSM 510 NLO multiphoton confocal microscope.
Morphometric image analysis
Phase images of EBs were acquired on days 2, 4, 7, and 10 during the course of EB differentiation with a Nikon TE 2000 inverted microscope (Nikon, Inc.) that was equipped with a SpotFLEX camera (Diagnostic Instruments, Inc.). A minimum of three fields of EBs per sample were analyzed using ImageJ image analysis software such that the cross-sectional area of shear and static pre-conditioned EB samples was measured for a minimum of 100 EBs from independent samples (n=6). The percentage of EBs with dark, centrally located foci was determined by counting the number of such EBs relative to the total number in the same field. Only EBs which contained a dark or optically opaque, centrally located foci that constituted ≥10% of the EB cross-sectional area were included for a statistical analysis of the population.
Flow cytometry analysis
The starting ESC population and day 0 samples, after exposure to static and shear conditions, were analyzed for expression of Oct-4. Briefly, cells were trypsinized, washed thrice with PBS, and fixed with 4% paraformaldehyde for 15 min at 4°C. Cells were permeabilized in 0.05% Triton X-100 in blocking buffer (1 mg/mL BSA and 0.1% Tween20 in PBS) for 30 min and washed in blocking buffer for 15 min. Cells were incubated at room temperature with either the primary antibody against OCT-4 (1 μg/million cells; Santa Cruz Biotechnology) or IgG isotype control (20 μL/million cells; Santa Cruz Biotechnology) for 1 h. Cells were washed with blocking buffer and incubated with the secondary antibody (AlexaFluor488 donkey anti-goat, 1 μg/million cells; Invitrogen) for 30 min at room temperature. Cells were resuspended in 300 μL blocking buffer and analyzed with an Accuri C6 flow cytometer and FlowJo software (Tree Star, Inc.).
Flow cytometric analyses of EBs were performed by dissociating the EBs with incubation in 0.05% trypsin-EDTA (Mediatech) for either 15 min (day 2 and 4 EBs) or 30 min (day 7 EBs) at 37°C. Trypsin was inactivated by the addition of ESC media to the dissociated cell suspension before centrifugation for 5 min at 1000 rpm to harvest the cells. The dissociated cells were rinsed with cold PBS containing 0.1% BSA, passed through a 35-μm strainer, and analyzed for pVE-cadherin GFP expression using an Accuri C6 flow cytometer. Undifferentiated naïve D3s and pVE-cadherin GFP D3s30 were used as negative controls. pVE-Cadherin GFP D3s contain a construct composed of a VE-cadherin promoter that drives the expression of enhanced green fluorescence protein.30 The percentage of VE-cadherin GFP-positive cells was determined by applying a gate, which assumed <1% of undifferentiated pVE-cadherin GFP D3's expressed GFP. A minimum of 25,000 events was recorded from six independent samples for each experimental group.
Statistical analysis
All experiments were performed in triplicate, and the results are presented as mean±standard error. Statistical analysis was performed using SYSTAT 12 software (Systat Software, Inc.). Comparisons were examined using a two-way analysis of variance (ANOVA) followed by a post hoc Tukey–Kramer test to determine significant (p<0.05) differences between pre-conditioning regimens across and within different time points. Comparisons between static and shear pre-conditioning regimens measured at individual time points were assessed using Student's t-test, with statistical significance determined by p<0.05.
Results
Fluid shear conditioning of ESCs
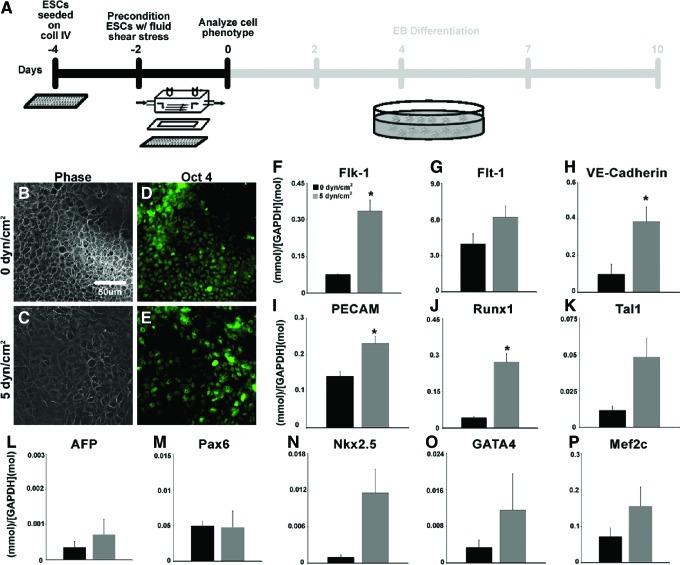
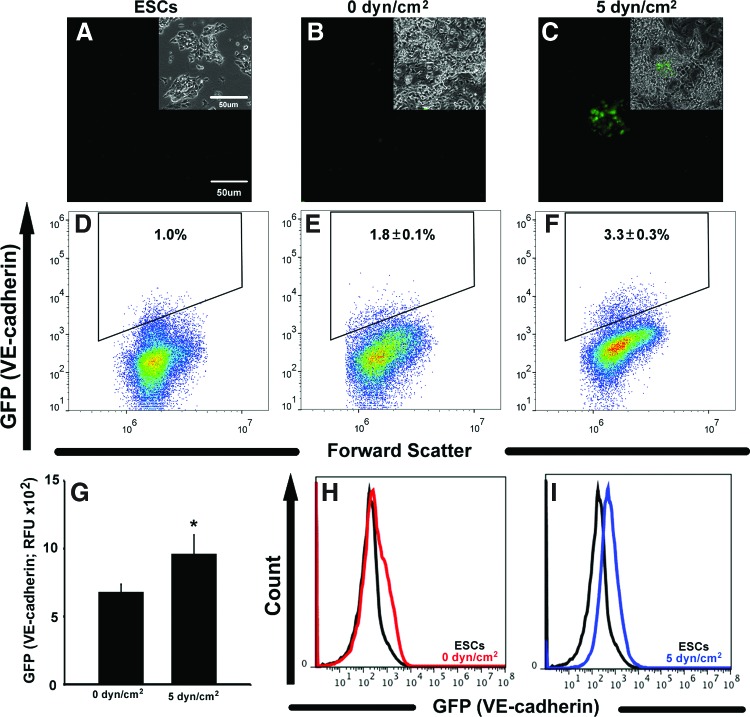
Pre-conditioning of ESCs, after 2 days of adhesion to collagen IV-coated slides, was performed for 2 days (Fig. 1A) at either 0 dyn/cm2 (Fig. 1B) or 5 dyn/cm2 (Fig. 1C), after which ESC populations did not exhibit any noticeable morphological differences. Both ESC populations largely retained their undifferentiated stem cell phenotype, with the majority of static and shear pre-conditioned ESCs retaining expression of Oct-4 (Fig. 1D, E and Supplementary Fig. S1). Immediately after fluid shear pre-conditioning, the expression of Flk-1 (VEGFR2), an endothelial and mesoderm gene, was increased ∼3-fold (p=0.005) in shear pre-conditioned ESCs compared with ESCs cultured at 0 dyn/cm2 (Fig. 1F); however, Flt-1 (VEGFR1) expression (Fig. 1G) was comparable in static and shear pre-conditioned ESCs. ESCs pre-conditioned at 5 dyn/cm2 also expressed higher levels of VE-cadherin (p=0.041) (Fig. 1H), PECAM (p=0.02) (Fig. 1I), and the hematopoietic transcription factor Runx1 (p=0.002) (Fig. 1J), compared with ESCs not subjected to fluid shear stress. Tal1 (Scl), a hematopoietic gene (Fig. 1K), did not differ between ESCs pre-conditioned with fluid shear and static ESCs; similarly no significant differences were found in the expression of cardiac genes GATA4, Nkx2.5, or Mef2c between static and shear pre-conditioned ESCs (Fig. 1N–P). Static and shear pre-conditioned ESCs also expressed similar low levels of AFP (Fig. 1L) and Pax6 (Fig. 1M) expression, suggesting that fluid shear stress pre-conditioning did not significantly affect endoderm or ectoderm differentiation. Although the majority of ESCs subjected to static and shear conditions acutely retained pluripotent characteristics, exposure to fluid shear stress increased the expression of several endothelial and hematopoietic genes, which was consistent with previous reports.3,6,34 However, although fluid shear stress may be sufficient to promote vascular gene expression, the prolonged effects of physical pre-conditioning on ESCs before differentiation remained ill defined.
FIG. 1.
Fluid shear pre-conditioning of embryonic stem cells (ESCs). ESCs were pre-conditioned via fluid shear stress for 48 h after adhesion on collagen IV (A). No distinct differences in morphology were noted between ESCs cultured at 0 dyn/cm2 (static; B) and 5 dyn/cm2 (C), cells from both pre-conditioning regimens expressed Oct-4 (D, E). ESCs pre-conditioned at 5 dyn/cm2 exhibited increased expression of hematopoietic and endothelial genes (F–K) compared with those at 0 dyn/cm2. Expression of genes related to endoderm (L), ectoderm (M), and cardiac mesoderm (N–P) differentiation was not altered by pre-conditioning. Scale bar=50 μm; n=4; *p<0.05. Color images available online at www.liebertpub.com/tea
Embryoid body differentiation
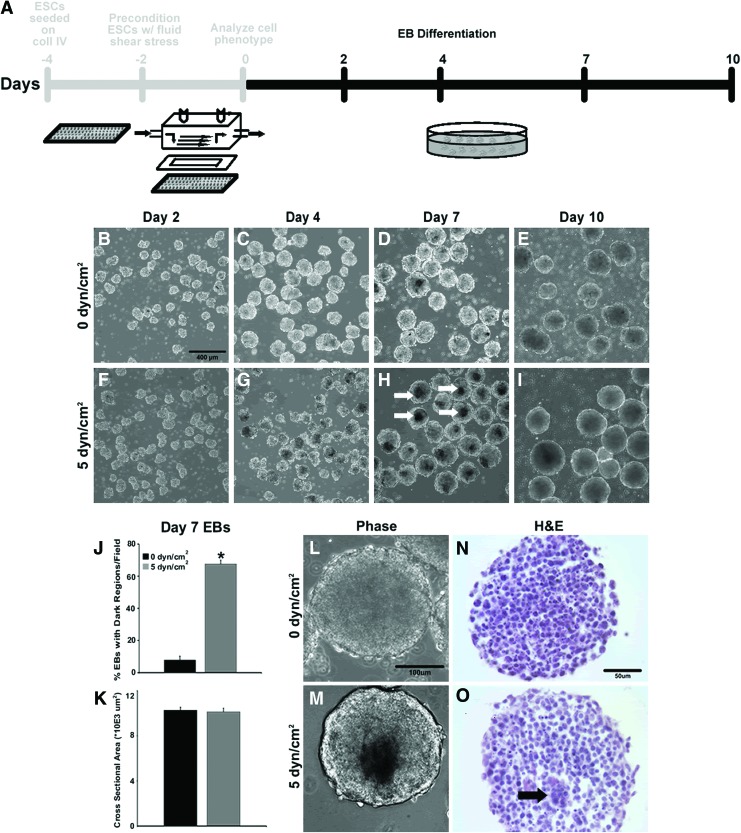
Following the two different pre-conditioning regimens (+/− shear), EBs were formed from single-cell suspensions of the respective ESC populations using rotary orbital suspension culture to examine subsequent effects of shear pre-conditioning on ESC differentiation (Fig. 2A). On the second day of EB culture, EBs formed from ESCs pre-conditioned under static (Fig. 2B) or shear (Fig. 2F) conditions appeared similar in size and gross morphology, and yielded similar quantities of EBs (Supplementary Fig. S2). After 4 days of EB differentiation, morphological differences became evident, as dark foci appeared in EBs formed from ESCs pre-conditioned with shear (Fig. 2G), whereas EBs formed from static pre-conditioned ESCs (Fig. 2C) were devoid of any such dark regions. Differences in EB morphology were more apparent on day 7 (Fig. 2D, H) with the presence of centrally located dark regions within EBs formed from shear pre-conditioned ESCs (Fig. 2H); however, after 10 days of EB differentiation, substantial differences in EB morphology between static and shear pre-conditioned ESCs were no longer observed (Fig. 2E, I). At day 7, an increased proportion of EBs formed from shear pre-conditioned ESCs exhibited dark regions compared with those from statically pre-conditioned ESCs (68.9%±12.4% vs. 6.1%±6.4%; p<0.001) (Fig. 2J). However, at the same time point, the cross-sectional area of EBs formed from ESCs pre-conditioned under static and shear did not significantly differ (Fig. 2K; 10,270±2550 and 10,120±2210 μm2, respectively), suggesting that differences in morphogenic events between EB groups were independent of EB size. Statically pre-conditioned ESCs exhibited uniform EB (Fig. 2L) and cell (Fig. 2N) morphologies, with little spatially distinct cellular organization; however, the optically opaque dark foci (Fig. 2M) centrally located within EBs from shear pre-conditioned ESCs were similar in size to the centrally located cluster of cells observed by histological analysis (Fig. 2O). The striking difference in morphology between EBs composed of static and shear pre-conditioned ESCs observed several days to a week after pre-conditioning suggested that acute physical perturbation of ESCs via fluid shear induces subsequent effects on 3D ESC morphogenesis.
FIG. 2.
Time course of embryoid body (EB) differentiation and morphogenesis. After pre-conditioning, ESCs were differentiated as EBs for 10 days (A). EBs formed from ESCs pre-conditioned at either 0 dyn/cm2 (static) (B–E) or 5 dyn/cm2 (shear) (F–I) exhibited altered morphogenesis, including the appearance of dark foci (indicated by white arrows) in shear pre-conditioned ESCs, which were absent in statically pre-conditioned ESCs (J), despite the similar cross-sectional areas of EBs from both pre-conditioning regimens (K). The morphogenic changes in EBs pre-conditioned in static (L) and shear (M) conditions were paralleled by changes in cellular organization, as exhibited by hematoxylin and eosin (H&E) (N, O), with tightly packed clusters of cells (arrow) centrally located within day 7 shear pre-conditioned EBs. Scale bars=400 μm (B), 100 μm (L), 50 μm (N). n=6; *p<0.05. Color images available online at www.liebertpub.com/tea
Gene expression
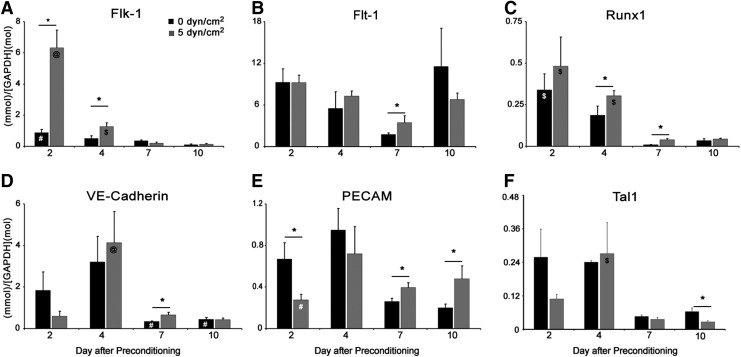
To examine the subsequent effects of fluid shear stress pre-conditioning on mesoderm differentiation within EBs, the temporal gene expression patterns of endothelial and hematopoietic differentiation markers were assessed. Flk-1, which was higher in shear pre-conditioned ESCs immediately after shear pre-conditioning (i.e., day 0, Fig. 1F), remained elevated in EBs formed from shear pre-conditioned ESCs at day 2 (p<0.002) and day 4 (p=0.009). However, by days 7 and 10, Flk-1 gene levels were similar, regardless of pre-conditioning regimen. Flk-1 gene expression levels were highest in the shear pre-conditioned EBs at day 2 (p<0.001 at all time point comparisons). At day 4, Flk-1 gene expression levels were significantly higher in shear pre-conditioned EBs compared with expression levels at day 7 (p=0.035) and day 10 (p=0.021) (Fig. 3A). In contrast, although Flt-1 gene levels were initially similar (day 0, 2, and 4) (Fig. 1G, B) in both populations, by day 7, shear pre-conditioned EBs expressed (p=0.047) elevated Flt-1 expression compared with static pre-conditioned EBs (Fig. 3B). Runx1 levels, which were higher in shear pre-conditioned ESCs before EB formation (i.e., day 0, Fig. 1J), were also greater in shear pre-conditioned EBs compared with static pre-conditioned EBs at day 4 (p=0.03) and day 7 (p<0.002), and similar at day 10. Runx1 expression levels in both static pre-conditioned EBs and shear pre-conditioned EBs decreased from day 2 to 7 (static p<0.001, shear p<0.001) and day 10 (static p<0.002, shear p<0.001) (Fig. 3C). VE-cadherin expression levels were greater in shear pre-conditioned ESCs (day 0, Fig. 1H) and day 7 EBs formed from shear pre-conditioned ESCs (p=0.012) when compared with EBs formed from statically pre-conditioned ESCs. However, VE-cadherin expression levels peaked at day 4 in shear pre-conditioned EBs (p<0.001 at all time point comparisons). In static pre-conditioned EBs, VE-cadherin expression was higher on day 4 compared with on day 7 (p<0.002) and day 10 (p<0.002) (Fig. 3D). While PECAM expression levels were initially higher in shear pre-conditioned ESCs (i.e., day 0, Fig 1I), on day 2, PECAM expression levels were less (p=0.014) in shear pre-conditioned EBs. However, PECAM expression was higher on day 7 (p=0.01) and day 10 (p=0.02) in shear pre-conditioned EBs compared with static pre-conditioned EBs. Between days 2 and 4, PECAM expression levels (p=0.004) increased in shear pre-conditioned EBs (Fig. 3E). No differences in Tal1 gene expression were detected between static and shear pre-conditioned ESCs (i.e., day 0, Fig. 1K) or pre-conditioned EB groups until day 10, when static pre-conditioned EBs expressed more Tal1 (p<0.002) than shear pre-conditioned EBs (Fig. 3F). Tal1 expression levels in shear pre-conditioned EBs were higher at day 4 compared with at day 7 (p=0.049) and day 10 (p=0.036). The differences in EB expression levels of endothelial and hematopoietic genes 10 days after static and shear pre-conditioning of ESCs demonstrate the prolonged effect that shear pre-conditioning has on EB endothelial and hematopoietic differentiation. After 7 days of EB differentiation, the time point at which distinct differences in EB morphology between shear and static pre-conditioned EBs were observed, three of the four endothelial genes examined, Flt-1, VE-cadherin, and PECAM, were higher in shear pre-conditioned EBs. Overall, the correlation between morphological differences and gene expression indicated that fluid shear stress pre-conditioning of ESCs exerted subsequent effects on EB morphogenesis and differentiation that may promote endothelial lineage commitment.
FIG. 3.
Time course of endothelial and hematopoietic gene expression in shear pre-conditioned EBs. EBs formed from fluid shear stress pre-conditioned ESCs exhibited increased expression of vascular endothelial growth factor receptor 2 (Flk-1) (A) at early stages of differentiation compared with statically pre-conditioned EBs. After 7 days of differentiation, EBs shear pre-conditioned EBs exhibited increased expression of vascular endothelial growth factor receptor 1 (Flt-1) (B), runt-related transcription factor 1 (Runx1) (C), vascular endothelial cadherin (VE-cadherin) (D), and platelet-endothelial cell adhesion molecule (PECAM) (E) compared with EBs formed from static pre-conditioned ESCs. While Flk-1 (A), Runx1 (C), and T-cell acute lymphocytic leukemia protein 1 (Tal1) (F) expression decreased over time, static and shear pre-conditioned EBs exhibited significant changes in expression at different stages, indicating possible changes in differentiation kinetics. n=3; *p<0.05 between groups indicated with solid line. For all other significance noted, p<0.05 between similar conditions over various time points was as follows: # as compared with day 4, $ as compared with day 7 and day 10, and @ as compared with all other time points.
Embryoid body protein expression
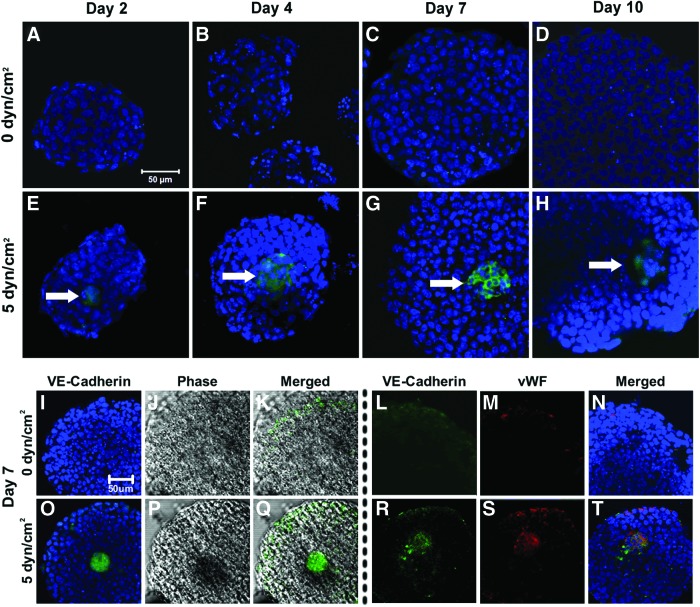
The persistent effects of fluid shear stress pre-conditioning on ESC endothelial and hematopoietic differentiation within EBs was assessed via immunostaining of VE-cadherin, von Willebrand factor (vWF), and PECAM. EBs formed from ESCs cultured under static conditions (0 dyn/cm2) did not appear to contain VE-cadherin-positive cells at any of the time points examined (days 2, 4, 7, 10, Fig. 4A–D). However, EBs composed of shear pre-conditioned ESCs exhibited VE-cadherin expression at all time points examined, with the VE-cadherin-positive cells centrally clustered within the EBs throughout differentiation (Fig. 4E–H). In addition, the lack of VE-cadherin+ cells was consistent with the decreased observation of dark regions within statically pre-conditioned EBs (Fig. 4I–K), whereas shear pre-conditioned ESCs exhibited co-localization of dark regions with similar-sized VE-cadherin-positive cell clusters after 7 days of differentiation (Fig. 4O–Q). In addition, VE-cadherin+ cell clusters within day 7 EBs formed from shear pre-conditioned ESCs exhibited co-localized staining for vWF expression, a more mature endothelial marker (Fig. 4R–T), while day 7 EBs formed from statically cultured ESCs were negative for vWF expression (Fig. 4L–N). Interestingly, while vWF was detected at day 7, staining was not detected at days 2 or 4 (data not shown), suggesting that the VE-cadherin-positive ESCs were undergoing differentiation toward an EC-like phenotype within the EB during this time period. PECAM staining was not detected in EBs formed from shear or static pre-conditioned ESCs (data not shown). Together, the increased expression of endothelial genes VE-cadherin, PECAM, and Flt-1 on day 7 of EB differentiation, along with the expression of endothelial proteins VE-cadherin and vWF, revealed that fluid shear stress pre-conditioning promoted EB endothelial differentiation and morphogenesis. Moreover, fluid shear stress pre-conditioned ESCs exhibited distinct spatial differentiation, with centrally clustered VE-cadherin-positive cells subsequently expressing vWF at later stages of differentiation, indicating continued endothelial differentiation and morphogenesis.
FIG. 4.
Expression of VE-cadherin and von Willebrand factor (vWF) in shear pre-conditioned EBs. EBs formed from static pre-conditioned ESCs did not exhibit VE-cadherin expression through the initial 10 days of differentiation (A–D). However, EBs formed from shear pre-conditioned ESCs contained clusters of VE-cadherin-positive cells (arrows) as early as 2 days of differentiation, which persisted through day 10 (E–H). In addition to the lack of VE-cadherin (I, L) and optically opaque regions (J, K), static pre-conditioned ESCs also did not express vWF (M, N). Conversely, EBs formed from shear pre-conditioned ESCs exhibited co-localization of VE-cadherin-positive cell clusters (O, R) with the dark foci (P, Q) and vWF (S, T), suggesting the appearance of endothelial-like cells at the center of shear pre-conditioned EBs. Scale bars=50 μm. Color images available online at www.liebertpub.com/tea
Fluid shear pre-conditioning of pVE-cadherin GFP ESCs
In order to visualize and quantify VE-cadherin expression with increased temporal resolution, pVE-cadherin GFP ESCs (VE-GFP ESCs) were assessed after the static and shear pre-conditioning regimens and during subsequent EB differentiation. Undifferentiated VE-GFP ESCs displayed no detectable levels of GFP expression via fluorescence microscopy or flow cytometry (Fig. 5A, D). After the two pre-conditioning regimens, GFP+ ESCs were not observed in monolayers of ESCs pre-conditioned statically (Fig. 5B); however, GFP+ ESCs were visible by fluorescence microscopy in shear pre-conditioned populations (Fig. 5C). In addition, 3.3%±0.3% of cells within shear pre-conditioned ESCs expressed GFP (VE-cadherin), compared with 1.8%±0.1% within statically pre-conditioned EBs (p=0.01) and above the baseline 1.0% of ESCs before pre-conditioning (Fig 5D–F). The intensity of GFP expression was also significantly increased (p=0.015) in shear pre-conditioned VE-GFP ESCs (1146±119 RFU) compared with statically pre-conditioned VE-GFP ESCs (964±82 RFU) (Fig. 5G). Furthermore, there was a more pronounced shift in the population GFP expression between undifferentiated and shear pre-conditioned VE-GFP ESCs compared with the shift in GFP mean peak fluorescence intensity between undifferentiated and statically pre-conditioned VE-GFP ESCs (Fig. 5H–I).
FIG. 5.
Fluid shear stress pre-conditioned VE-GFP ESCs. GFP (VE-cadherin) expression was not observed in undifferentiated (A) and statically pre-conditioned VE-GFP ESCs (B); however, shear pre-conditioned VE-GFP ESCs (C) exhibited GFP after pre-conditioning. Single cell analyses of GFP expression (D) revealed a significant increase in the percentage of GFP-positive cells between static (E) and shear (F) pre-conditioned VE-GFP ESCs. Significant differences were also observed in GFP mean fluorescence intensity (G) between pre-conditioning regimens, and the shift in GFP fluorescence distribution between undifferentiated and differentiated populations (H, I) was more pronounced after shear pre-conditioning (I). Scale bar=50 μm; n=3; *p<0.05. Color images available online at www.liebertpub.com/tea
EBs formed from statically pre-conditioned VE-GFP ESCs were devoid of a dark foci and GFP+ cell clusters after 7 days (Fig. 6A, B), which was consistent with the morphology previously observed in statically pre-conditioned nontransfected ESCs (Fig. 2F). The diffuse darker areas observed centrally in statically pre-conditioned EBs were a result of contrast due to size of the EB and were distinctly different from the dark condensed foci observed at various locations in shear pre-conditioned EBs (Supplementary Fig. S3). Throughout EB differentiation, a few GFP+ cells were observed in statically pre-conditioned VE-GFP EBs (Fig. 6C–E). However, EBs formed from shear pre-conditioned VE-GFP ESCs exhibited dark regions at the center of the EB at day 7 (Fig. 6F), which co-localized with GFP+ expression (Fig. 6G), similar to those previously observed with nontransfected shear pre-conditioned ESCs (Fig. 2G). Furthermore, GFP+ cell clusters were observed in EBs formed from shear pre-conditioned VE-GFP ESCs (Fig. 6H–J) at all time points examined. The dark regions and GFP+ cell clusters observed in EBs formed from shear pre-conditioned VE-GFP ESCs confirmed the spatiotemporal effects of fluid shear stress pre-conditioning previously observed with the nontransfected ESCs.
FIG. 6.
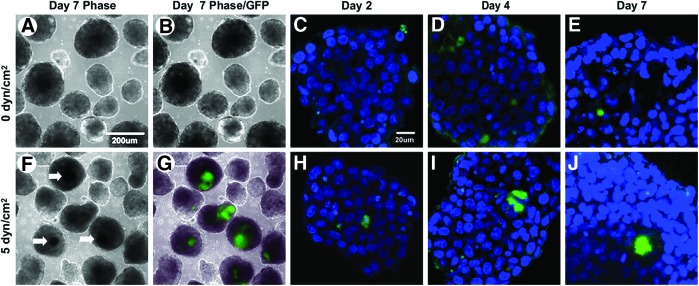
Centrally localized clusters of GFP positive cells in shear pre-conditioned VE-GFP EBs. EBs formed from static pre-conditioned VE-GFP ESCs did not develop dark foci (A) and exhibited minimal GFP expression (B–E). In contrast, EBs composed of shear pre-conditioned VE-GFP ESCs developed dark foci after 7 days of differentiation, (F) which co-localized with GFP expressing cells (G). GFP-positive cell clusters were observed at day 2 (H), 4 (I), and 7 (J) in shear pre-conditioned VE-GFP EBs. Scale bars (A)=200 μm, (C)=20 μm. Color images available online at www.liebertpub.com/tea
Discussion
The results of this study demonstrate that fluid shear stress pre-conditioning of ESCs results in endothelial differentiation and morphogenesis within EBs. Immediately after exposure to fluid shear stress, ESCs expressed elevated levels of endothelial genes while still retaining pluripotent phenotypic traits. In addition, EBs composed of shear pre-conditioned ESCs exhibited significantly increased expression of endothelial genes on day 7 compared with EBs formed from static pre-conditioned ESCs. Centrally organized clusters of VE-cadherin+ cells were observed as early as 2 days after the initial formation of EBs from shear pre-conditioned ESCs, and the centrally located clusters of cells subsequently expressed vWF co-localized with VE-cadherin. These findings reveal that fluid shear stress increases the expression of endothelial genes and proteins in ESCs, and it promotes spatially organized aggregation of cells and endothelial differentiation within EBs. Moreover, this study demonstrates that fluid shear stress stimulation of ESCs during early differentiation has persistent effects on ESC endothelial differentiation and morphogenesis.
Previous studies examining the acute effects of fluid shear stress on ESCs have reported similar results on endothelial differentiation5,6,34 as those reported here. The application of fluid shear stress to adherent monolayers of ESCs and ESC-derived vascular progenitors increased the expression of endothelial genes and proteins, such as Flk-1, Flt-1, PECAM, and VE-cadherin, and promoted the formation of tubular structures and acetylated LDL uptake.3,4,6–8 In previous studies, the effects of varying the manner (pulsatile or continuous) and magnitude (∼1–10 dyn/cm2) of fluid shear stress on phenotype were examined.4,34 For the studies reported here, only 5 dyn/cm2 was investigated, because this magnitude is close to the wall shear stress experienced by cells in the dorsal aorta of E10.5 mouse embryos,3 and preliminary studies did not indicate significant phenotypic or morphological results in EBs with ESCs preconditioned with greater (15 dyn/cm2) or lesser (1.5 dyn/cm2) magnitudes of fluid shear stress (data not shown). The observed increase in the expression of Runx1 in shear-preconditioned ESCs parallels the results of a recent study in which mouse ESC-derived CD41+Flk-1+ cells demonstrated increased expression of hematopoietic markers Runx1, Myb, and Klf2 after 30 h of shear at 5 dyn/cm2.3 In addition, ESC-derived ECs, vascular progenitor cells, and hematopoietic progenitor cells have been reported to exhibit changes in morphology and increased expression of endothelial, vascular, and hematopoietic genes in response to fluid shear stress exposure.3,5,35,36 In contrast to previous studies that focused primarily on examining the phenotypic effects of fluid shear stress on stem cells immediately after the application of shear stress, this study establishes an increased understanding of the continued lineage commitment of fluid shear pre-conditioned cells in the context of multicellular ESC differentiation.
Many studies have used biochemical cues such as VEGF to successfully promote ESC endothelial differentiation.22,32 Early studies have reported that treating EBs with basic fibroblast growth factor (bFGF) promotes EC differentiation and vasculogenesis.37 Furthermore, studies that utilized a combinatorial approach, using bFGF and VEGF, demonstrated the potency of these biochemical cues to promote EB endothelial morphogenesis and vasculogenesis.38 Studies performed by Zhang et al. revealed that exposing amniotic fluid-derived stem cells to shear stress using an orbital shaker in the presence of angiogenic factors significantly increased their ability to perform EC functions such as forming capillary-like structures.39 While biochemical and biomechanical cues are important for both ESC endothelial differentiation and morphogenesis, this work aimed at systematically studying and understanding the effect of solely utilizing fluid shear stress as a biomechanical cue to promote ESC endothelial differentiation and understand its subsequent effects on EB endothelial morphogenesis.
During development, endothelial and hematopoietic cells originate from a common mesoderm precursor, the hemangioblast,40 defined by its expression of Flk-1, SCL/Tal1, CD34, and CD133.41,42 Aggregates of hemangioblast cells in the extraembryonic yolk sac (primitive hematopoiesis) and aorta-gonad-mesonphros (definitive hematopoiesis) mature and form blood islands during early embryogenesis, which consist of an inner core of blood cells and an outer layer of ECs.43,44 The organization of hematopoietic and ECs into blood islands initiates the onset of vascular development and expansion, by which blood island cells migrate, divide, and create connections to form the yolk sac vasculature.44,45 Therefore, the morphogenesis of hematopoietic-primed ESCs in the 3D EB microenvironment may recapitulate aspects of yolk sac hematopoiesis. Moreover, the co-expression of VE-cadherin and vWF by differentiating ESCs within EBs46 suggests that the central clusters of cells undergo organization and morphogenesis analogous to hemangioblast maturation. The potential maturation of the fluid shear stress pre-conditioned ESCs at the center of EBs reflects the permissive morphogenic nature of EB microenvironments that support endothelial differentiation.
Cadherins play an important role in selective cell adhesion, tissue organization, segregation, and morphogenesis.47,48 The organization of VE-cadherin+ cells within EBs formed from shear pre-conditioned ESCs suggests a role for cadherin-mediated selective cell adhesion in the organization and differentiation of putative ECs. VE-cadherin, a cadherin specifically expressed at the intercellular junctions of ECs,49 is essential for vascular morphogenesis,50,51 a process imperative during embryogenesis for the development of the cardiovascular system. Lack of VE-cadherin leads to impairment of tissue morphogenesis, vasculogenesis, and angiogenesis within the embryo.50 The presence of VE-cadherin+ cells at the core of EBs in this work is analogous to previous in vitro studies in which VE-cadherin+ cell clusters were observed in day 6 EBs cultured with an angiogenic growth factor mixture,22 suggesting that fluid shear stress pre-conditioning may influence the vascular lineage commitment and morphogenesis of ESCs through similar mechanisms as angiogenic factors.5
The findings of this study reveal that fluid shear stress has prolonged effects on ESC endothelial differentiation and morphogenesis within EBs. The results illustrate that pre-conditioning ESCs via fluid shear stress before EB formation enhanced endothelial differentiation within EBs and yielded ∼70% of EBs containing cells undergoing endothelial differentiation. In addition, fluid shear stress induced ESCs to express an endothelial specific cadherin (VE-cadherin), which appeared to promote specific cell adhesion and organization, and vascular morphogenesis within EBs. Furthermore, the morphogenic events and gene expression patterns observed in EBs formed from shear pre-conditioned ESCs after physical stimulation provide evidence that physical modulation of ESCs during early differentiation affects the differentiation trajectory of ESCs. Taken together, these results suggest that fluid shear stress pre-conditioning may be used to initiate primitive vascularization within cellular constructs derived from pluripotent cell sources, which has important implications for broad applications that are aimed at engineering modular in vitro models of vasculogenesis and regenerative tissue therapeutics.
Supplementary Material
Acknowledgments
D3 pVE-cadherin GFP ESCs were generously provided by Joseph Wu (Stanford University). The authors would like to thank Melissa Kinney for critically reviewing this article and Christian Mandrycky and Elizabeth Peijnenburg for their technical assistance. This work was supported by funding from the National Science Foundation through the Emergent Behaviors of Integrated Cellular Systems Science and Technology Center (CBET 0939511) and the Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues Engineering Research Center (EEC 9731643). B.A.N. was supported by an Alliance for Graduate Education, the Professoriate Fellowship (AGEP), as well as the Georgia Institute of Technology President's Fellowship and previously supported by a National Science Foundation Graduate Research Fellowship (NSF GRFP).
Disclosure Statement
No competing financial interests exist.
References
- 1.Discher D.E., Mooney D.J., and Zandstra P.W.Growth factors, matrices, and forces combine and control stem cells. Science 324,1673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry C.E., and Keller G.Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132,661, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Adamo L., Naveiras O., Wenzel P.L., McKinney-Freeman S., Mack P.J., Gracia-Sancho J., et al. . Biomechanical forces promote embryonic haematopoiesis. Nature 459,1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H., Nakayama Y., Qin K., Yamamoto K., Ando J., Yamashita J., et al. . Differentiation from embryonic stem cells to vascular wall cells under in vitro pulsatile flow loading. J Artif Organs 8,110, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Zeng L., Xiao Q., Margariti A., Zhang Z., Zampetaki A., Patel S., et al. . HDAC3 is crucial in shear- and VE GF-induced stem cell differentiation toward endothelial cells. J Cell Biol 174,1059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahsan T., and Nerem R.M.Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng Part A 16,3547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto K., Sokabe T., Watabe T., Miyazono K., Yamashita J.K., Obi S., et al. . Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 288,H1915, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wang H., Riha G.M., Yan S., Li M., Chai H., Yang H., et al. . Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol 25,1817, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Masumura T., Yamamoto K., Shimizu N., Obi S., and Ando J.Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol 29,2125, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Kinney M.A., Sargent C.Y., and McDevitt T.C.The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng Part B Rev 17,249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Placzek M.R., Chung I.-M., Macedo H.M., Ismail S., Mortera Blanco T., Lim M., et al. . Stem cell bioprocessing: fundamentals and principles. J R Soc Interface 6,209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith L.G., and Swartz M.A.Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7,211, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Levesque M.J., Nerem R.M., and Sprague E.A.Vascular endottielial cell proliferation in culture and the influence of flow. Biomaterials 11,702, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Levesque M.J., and Nerem R.M.The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng 107,341, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Ji R.P., Phoon C.K.L., Aristizábal O., McGrath K.E., Palis J., and Turnbull D.H.Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res 92,133, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Doetschman T.C., Eistetter H., Katz M., Schmidt W., and Kemler R.The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87,27, 1985 [PubMed] [Google Scholar]
- 17.Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., et al. . Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6,88, 2000 [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn G., Ochiya T., Terada M., and Yoshida T.Mouse flt-1 promoter directs endothelial-specific expression in the embyroid body model of embryogenesis. Biochem Biophys Res Commun 276,1089, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira L.S., Gerecht S., Shieh H.F., Watson N., Rupnick M.A., Dallabrida S.M., et al. . Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res 101,286, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Wang R., Clark R., and Bautch V.L.Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development 114,303, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Risau W., Sariola H., Zerwes H.G., Sasse J., Ekblom P., Kemler R., et al. . Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102,471, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Vittet D., Prandini M.H., Berthier R., Schweitzer A., Martin-Sisteron H., Uzan G., et al. . Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 88,3424, 1996 [PubMed] [Google Scholar]
- 23.Niebruegge S., Bauwens C.L., Peerani R., Thavandiran N., Masse S., Sevaptisidis E., et al. . Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng 102,493, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Bauwens C.L., Peerani R., Niebruegge S., Woodhouse K.A., Kumacheva E., Husain M., et al. . Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 26,2300, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Mohr J.C., de Pablo J.J., and Palecek S.P.3-D microwell culture of human embryonic stem cells. Biomaterials 27,6032, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kinney M.A., Saeed R., and McDevitt T.C.Systematic analysis of embryonic stem cell differentiation in hydrodynamic environments with controlled embryoid body size. Integr Biol 4,641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenedo R.L., Bratt-Leal A.M., Marklein R.A., Seaman S.A., Bowen N.J., McDonald J.F., et al. . Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials 30,2507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratt-Leal A.M., Carpenedo R.L., Ungrin M.D., Zandstra P.W., and McDevitt T.C.Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32,48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang S.M., Gerecht-Nir S., Chen J., Itskovitz-Eldor J., and Zandstra P.W.Controlled, scalable embryonic stem cell differentiation culture. Stem Cells 22,275, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Wu J.C., Sheikh A.Y., Kraft D., Cao F., Xie X., et al. . Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116,I46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenedo R.L., Sargent C.Y., and McDevitt T.C.Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 25,2224, 2007 [DOI] [PubMed] [Google Scholar]
- 32.McCloskey K.E., Lyons I., Rao R.R., Stice S.L., and Nerem R.M.Purified and proliferating endothelial cells derived and expanded in vitro from embryonic stem cells. Endothelium 10,329, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa S.I., Nishikawa S., Hirashima M., Matsuyoshi N., and Kodama H.Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125,1747, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Wolfe R.P., and Ahsan T.Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng 110,1231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCloskey K.E., Smith D.A., Jo H., and Nerem R.M.Embryonic stem cell-derived endothelial cells may lack complete functional maturation in vitro. J Vasc Res 43,411, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Metallo C.M., Vodyanik M.A., de Pablo J.J., Slukvin II, and Palecek S.P.The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol Bioeng 100,830, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Krah K., Mironov V., Risau W., and Flamme I.Induction of vasculogenesis in quail blastodisc-derived embryoid bodies. Dev Biol 164,123, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Kazemi S., Wenzel D., Kolossov E., Lenka N., Raible A., Sasse P., et al. . Differential role of bFGF and VEGF for vasculogenesis. Cell Physiol Biochem 12,55, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Zhang P., Baxter J., Vinod K., Tulenko T.N., and Di Muzio P.J.Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev 18,1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi K., Kennedy M., Kazarov A., Papadimitriou J.C., and Keller G.A common precursor for hematopoietic and endothelial cells. Development 125,725, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Kennedy M., D'Souza S.L., Lynch-Kattman M., Schwantz S., and Keller G.Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109,2679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fehling H.J., Lacaud G., Kubo A., Kennedy M., Robertson S., Keller G., et al. . Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130,4217, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Sabin F.R.Studies on the origin of blood-vessels and of red blood-corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol 9,215, 1920 [Google Scholar]
- 44.Medvinsky A., and Dzierzak E.Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86,897, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Palis J., and Yoder M.C.Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol 29,927, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Vischer U.M.von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost 4,1186, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Halbleib J.M., and Nelson W.J.Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20,3199, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Takeichi M.The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102,639, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Lampugnani M.G., Resnati M., Raiteri M., Pigott R., Pisacane A., Houen G., et al. . A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 118,1511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gory-Faure S., Prandini M.H., Pointu H., Roullot V., Pignot-Paintrand I., Vernet M., et al. . Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126,2093, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Breier G., Breviario F., Caveda L., Berthier R., Schnurch H., Gotsch U., et al. . Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 87,630, 1996 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.