Abstract
We have recently reported that mouse embryonic stem cells (mESCs) are deficient in expressing type I interferons (IFN) when exposed to viral infection and double-stranded RNA. In this study, we extended our investigation and demonstrated that single-stranded RNA and protein-encoding mRNA can induce strong IFN expression and cytotoxicity in fibroblasts and epithelial cells, but none of the effects associated with these antiviral responses were observed in mESCs. Our results provided additional data to support the conclusion that mESCs are intrinsically deficient in antiviral responses. While our findings represent a novel feature of mESCs that in itself is important for understanding innate immunity development, we exploited this property to develop a novel mRNA-mediated gene expression cell model. Direct introduction of synthetic mRNA to express desired genes has been shown as an effective alternative to DNA/viral vector-based gene expression. However, a major biological challenge is that a synthetic mRNA is detected as a viral RNA analog by the host cell, resulting in a series of adverse effects associated with antiviral responses. We demonstrate that the lack of antiviral responses in mESCs effectively avoids this problem. mESCs can tolerate repeated transfection and effectively express proteins from their synthetic mRNA with expected biological functions, as demonstrated by the expression of green fluorescent protein and the transcription factor Etv2. Therefore, mRNA-based gene expression could be developed into a novel ESC differentiation strategy that avoids safety concerns associated with viral/DNA-based vectors in regenerative medicine.
Introduction
The antiviral mechanisms have been extensively investigated and are presumably acquired by most types of somatic cells as a critical part of innate immunity [1,2], but few studies have investigated innate immunity in embryonic stem cells (ESCs). It is unclear if ESCs, which normally reside in the womb, have developed an active innate immunity. Recent studies suggest that human ESCs (hESCs) do not respond to a wide range of infectious agents, including bacterial endotoxins and viral RNA analogs [3,4]. Mouse ESCs (mESCs) similarly do not show inflammatory responses to cytokines, lipopolysaccharides [5], or even live bacteria [6]. These studies prompted us to investigate the antiviral responses in mESCs. We recently reported that mESCs do not express type I interferons (IFNα and IFNβ) in response to viral infections and double-stranded RNA (dsRNA), but they are susceptible to La Crosse virus-induced lytic cell death and inhibited cell proliferation by polyIC (a synthetic analog of viral dsRNA) [7]. In this study, we have further investigated the responses of mESCs to synthetic single-stranded RNA (ssRNA) and synthetic protein-encoding mRNA, which mimic viral RNA in inducing antiviral responses. Our results demonstrate that ssRNA and synthetic mRNA can induce strong IFN expression and cytotoxicity in fibroblasts and epithelial cells, but none of these effects were observed in mESCs, similar to their responses to viruses and dsRNA [7]. We conclude that mESCs are intrinsically deficient in antiviral responses. Together with the similar observations in hESCs [4], the lack of antiviral responses represents a unique property of ESCs that has not been previously characterized. While this finding in itself provides new insight into the development of the innate immunity during embryogenesis, the lack of antiviral responses makes ESCs an excellent model for developing mRNA-based gene expression.
The landmark achievement in generating induced pluripotent stem cells (iPSCs) has led to the new concept of cell reprogramming [8], but the fact that viral vectors are commonly used for effective expression of reprogramming factors prevents the therapeutic use of the reprogrammed cells [9,10]. Extensive effort to avoid this problem has led to the development of several alternatives, among which mRNA-mediated gene expression has shown great promise due to the nonintegrating nature [11]. This method directly introduces synthetic mRNA into the host cell for the expression of reprogramming factors, thus eliminating the need of viral or DNA vectors. The successful generation of RNA-induced iPSCs from fibroblasts [12–15] has led to the belief that this strategy is the beginning of the new era of cell reprogramming [11]. This strategy could in principle be expanded to reprogram any type of cell as long as the genes that control the cell fate are identified. A major biological challenge, however, is that a synthetic mRNA is detected as a viral RNA analog by host cells and induces strong antiviral responses resulting in IFN induction, protein synthesis inhibition, and reduced viability of host cells [16,17]. Synthetic mRNA must therefore be modified via a complex process to minimize their effects in eliciting antiviral responses (known as immunogenicity) [12,15]. The lack of antiviral responses in mESCs prompted us to investigate the feasibility of developing an mRNA-based gene expression strategy, with the expectation that mESCs would allow effective translation of synthetic mRNA without suffering the adverse effects associated with antiviral responses encountered in differentiated somatic cells.
ESCs have attracted enormous attention in recent years for their differentiation potential as a cell source for regenerative medicine [18,19]. Methods for the differentiation of ESCs to specific cell lineages have developed, most of which primarily depend on the spontaneous differentiation potential of ESCs and the influence of certain growth factors and/or cytokines. For example, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are commonly used to stimulate endothelial cell differentiation [20–22]. However, the efficiency of these methods is usually so low that isolating a pure cell population is difficult. The lack of effective differentiation methods to obtain specific types of cells in sufficient quality and quantity is a major challenge that limits clinical applications of ESC-derived cells. Cell lineage specification is mainly driven by the activation of cell-specific transcription factors during early embryogenesis. It is conceivable that the inefficient differentiation of existing methods is, at least in part, due to insufficient transcription activation during in vitro differentiation. A recent study reported that hESCs can be effectively differentiated into endothelial cells by viral vector-mediated expression of transcription factors that control vascular differentiation [23], demonstrating that intervention at the transcription level can provide a much stronger driving force for ESC differentiation into a specific cell fate.
In this study, we show that mESCs can tolerate repeated transfection and effectively express synthetic mRNA with expected biological functions without the need of any chemical modifications of synthetic mRNA. Therefore, by exploiting the underdeveloped antiviral mechanisms in mESCs, we demonstrated the feasibility that mRNA-mediated gene expression could be developed into novel strategies that achieve efficient expression of desired genes in ESCs.
Materials and Methods
In vitro synthesis and purification of short single-strand RNA (3p-ssRNA) and synthetic mRNA
For preparation of short random sequenced 3p-ssRNA and HO-ssRNA, the dsDNA templates for in vitro transcription were prepared by primer extension of the DNA oligo containing the T7 φ2.5 promoter sequence (5′-CGTAATACGACTTCACTATTAG) with a DNA template (5′-NnCTAATAGTGAGTCGTATTACG, N=random nucleotide (nt) sequence, A, G, C, T, n=18, 38, 58, or 78) using Taq DNA polymerase (Promega) [24,25]. The resulting dsDNA was ethanol-precipitated and used as templates for in vitro transcription with the Ampliscribe T7-Flash Transcription Kit (Epicentre) to produce 5′-3p ssRNA (5′-pppAGNn, n=18, 38, 58, or 78, the resulting RNA are termed 3p-20nt, 3p-40nt, 3p-60nt, and 3p-80nt) following the manufacturer's protocol. The RNA transcripts were ethanol-precipitated and purified by 8% denaturing polyacrylamide gel electrophoresis (PAGE). HO-ssRNA was prepared by removing the 5′ triphosphate of above 3p-ssRNA with calf intestinal alkaline phosphatase (Takara) followed by PAGE purification. The resulting RNAs are named as HO-20nt, HO-40nt, HO-60nt, and HO-80nt.
For preparation of protein-encoding mRNA, the DNA template for enhanced green fluorescence protein (EGFP) mRNA was generated by polymerase chain reaction (PCR) from a pEGFP-N1 plasmid (BD Biosciences) using two primers containing sequences of 5′-CGTAATACGACTCACTATTAGGAAGCTTCGAATTCTGCAGTCG and 5′-TTTACGCCTTGAGATACATTGATG. The DNA template for mouse Etv2 was amplified by PCR from cDNA library prepared from a mouse lung endothelial cells [26] using primers with the sequences of 5′-CCGTAATACGACTCACTATTAGGAACCGTCAGAACAAGCATCC and 5′-TTTTGTTTTTGTTTTTTGTTTTATTGGCC. PCR was performed with Q5 High-Fidelity DNA polymerase (New England Biolabs). The resulting dsDNA templates contain the T7 φ2.5 promoter for in vitro transcription [24,25], the 5′-UTR region with the Kozak sequence [27], and the open reading frame of EGFP or Etv2. In addition, EGFP has a 3′-UTR region.
Using the above EGFP DNA as the template, 3p-EGFP-mRNA (long 3p-ssRNA) was prepared by in vitro transcription under the same condition as the short ssRNA preparation described previously and was purified by an M100 Microcon membrane filter. To prepare functional EGFP and Etv2 mRNA, in vitro transcription from their DNA templates was carried out in the presence of the cap analog m7GpppA (chemically synthesized in our laboratory, unpublished) to generate 5′-capped EGFP and Etv2 mRNA transcripts. The purified RNA transcripts were polyadenylated by Escherichia coli Poly(A) polymerase (New England Biolabs), resulting in functional mRNA, m7GpppA-EGFP-polyA, and m7GpppA-Etv2-polyA.
Cell culture and treatment
ESCs (D3 cell line, ATCC) were cultured in the standard mESC medium that contains leukemia inhibitory factor as previously described [7]. Epithelial cells (SKOV3 cells, a human ovarian carcinoma cell line) and mouse fibroblasts (10T1/2 cell line, derived from mouse embryonic tissues that can differentiate into several cell lineages) [28,29] were cultured in the RPMI and DMEM medium, respectively, which contain 10% fetal calf serum and 100 units/mL penicillin and 100 μg/mL streptomycin. All cells were maintained at 37°C in a humidified incubator with 5% CO2.
Cells were plated at 40%–50% confluence and were usually cultured for 24 h before the experiments. Synthetic dsRNA (polyIC [polyinosinic-polycytidylic acid], Sigma), ssRNA, and mRNA in different formats were transfected into the cells with DharmaFECT reagent (Thermo Scientific) as previously described [7]. The control cells were transfected with DharmaFECT reagent only. The cells were collected and used for various analyses after incubation for the times indicated in individual experiments.
Real-time quantitative PCR
Total RNA was extracted using Tri-reagent (Sigma). cDNA was prepared by M-MLV reverse transcriptase (Promega). Real-time quantitative PCR (RT-qPCR) was performed using SYBR green ready mix on a MX3000PTM RT-PCR system (Stratagene), as previously reported [30]. The mRNA level from qRT-PCR was calculated using the comparative Ct method [31]. β-actin mRNA was used to normalize relative levels of mRNA for tested genes. The sequences of the primer sets are listed in Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd
Cell proliferation, viability, and cell cycle analysis
Cell proliferation and viability were determined by cell number and by cell morphology after toluidine blue (TB) staining as we previously described [32]. The absorbance at 630 nm of TB-stained cells was measured with a microtiter plate reader. The values, which correlate with the number of viable cells, were used as an indirect measurement of cell proliferation or viability. Cell cycle analysis by flow cytometry was performed after the cells were stained with 50 μg/mL propidium iodide. The cell cycle profiles were generated with the CFlow software [33].
Protein phosphorylation analysis by flow cytometry
Activation of dsRNA-activated protein kinase (PKR) was determined by the phosphorylation state of eukaryotic initiation factor 2α (eIF2α, a physiological substrate of PKR) [34]. Treated cells were incubated with the antibodies that specifically recognize phosphorylated eIF2α (Cell Signaling). The cells were then incubated with fluorescein isothiocyanate-conjugated secondary antibodies and examined by an Accuri C6 flow cytometer. The fluorescence intensity, which correlates with phosphorylated eIF2α level, was used as an indirect measurement of PKR activation as we previously described [7].
Microscopic analysis and quantitative measurement of GFP expression
Expression of GFP in live cells was visualized using a LSM confocal fluorescence microscope. To quantitatively measure the GFP expression level, the cells were grown in a black-walled, clear-bottom 96-well cell culture plate (Costar) and analyzed with a SpectraMax® M5 Scanning Multi-Detection Microplate Reader (Molecular Devices) according to the manufacture's instruction.
Results
Characterization of short synthetic ssRNA-induced antiviral responses in epithelial cells
Synthetic short ssRNA and long protein-encoding mRNA prepared by in vitro transcription have been used as viral RNA mimics to study antiviral responses. The length and the 5′-triphosphate (3p) group of RNA are considered to be the most important factors in eliciting antiviral responses, while the sequence seems to be less critical [35–37]. We synthesized a panel of short 3p-ssRNA that have 20, 40, 60, and 80 nt with a random sequence of A, U, C, and G, each of which was detected as a single band with expected size by PAGE analysis (Fig. 1A, gel inset).
FIG. 1.
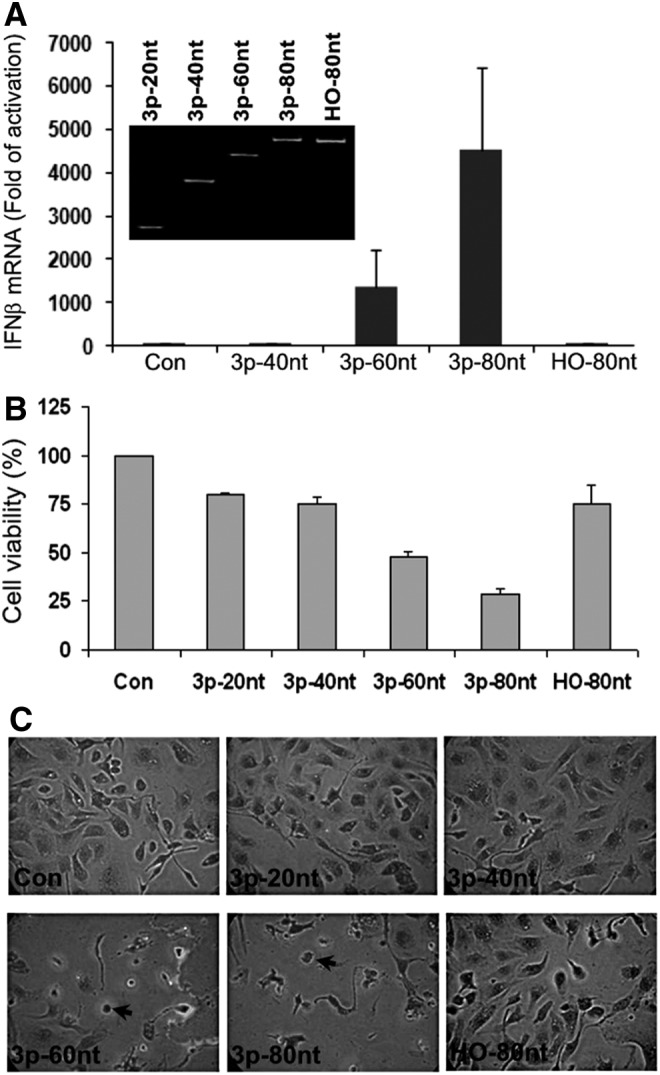
Single-stranded RNA (ssRNA) induced antiviral responses in SKOV3 cells. The cells were transfected with different format of ssRNA (40 nM) as indicated. (A) The induction of interferon (IFN)β mRNA were determined by real-time quantitative polymerase chain reaction (RT-qPCR) at 12 h post-transfection. Control (Con) represents cells transfected with DharmaFECT without ssRNA. The results are means±SD of three independent experiments. The gel inset shows the polyacrylamide gel electrophoresis analysis of purified ssRNA. (B) Cell viability was determined by toluidine blue (TB) cell staining of viable cells at 24 h post-transfection. The values are means±SD of a representative experiment performed in triplicates. (C) Cell morphology was examined under a phase-contrast microscope (200×) at 24 h after TB cell staining. The images were photographed from a representative experiment. Arrows denote apoptotic cells. All representative experiments were repeated at least twice with similar results.
We first determined potency of these short ssRNA in eliciting antiviral responses in a human ovarian epithelial carcinoma cell line (SKOV3 cells), which have been previously used as a model to study the immunogenicity of different types of RNA [38–40]. Using IFNβ expression as a benchmark for antiviral responses, our data showed that only 3p-ssRNA with longer than 60 nt induced IFNβ, whereas the removal of the 3p group completely abolished this effect, as demonstrated in the case of 3p-80nt-ssRNA (Fig. 1A, p-80nt vs. HO-80nt). 3p-60nt-ssRNA and 3p-80nt-ssRNA caused cell death (Fig. 1B, C), in parallel with their potency of IFNβ induction. The cytotoxicity was not seen when the cells were treated with HO-80nt-ssRNA, correlating with its inability to induce IFNβ (Fig. 1A). Similar results were observed with an ssRNA of 145 nt that encodes a segment of luciferase (data not shown). These results are in line with the characteristics of synthetic ssRNA previously described by other investigators [16,17,41].
Short 3p-ssRNA induced strong antiviral responses in fibroblasts but not in mESCs
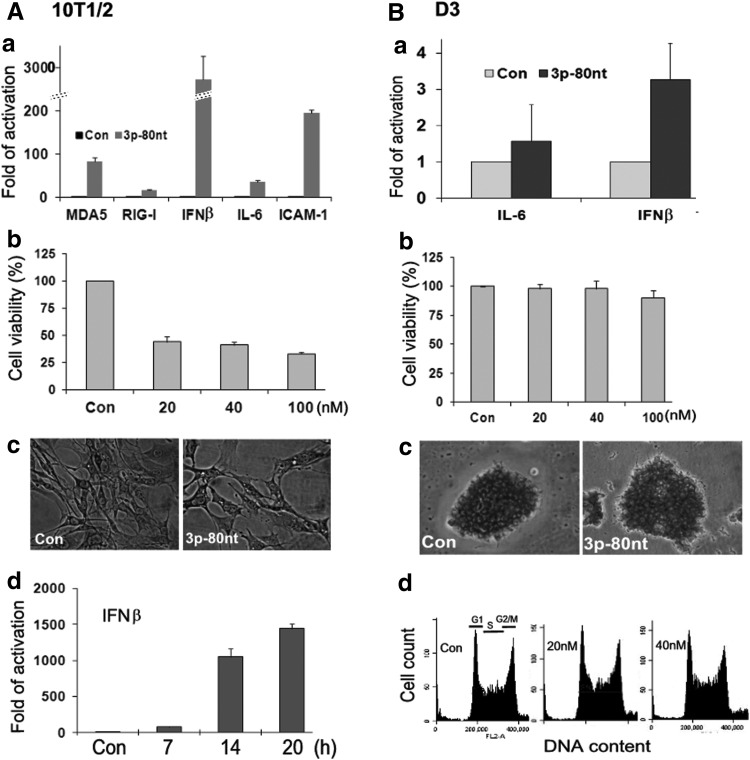
Using 3p-80nt-ssRNA characterized in SKOV3 cells, we investigated its effects on 10T1/2 cells, which are fibroblasts derived from early mouse embryonic tissues with mesenchymal stem cell properties that can differentiate into several cell lineages [28,29]. Similar to SKOV3 cells, 3p-80nt-ssRNA induced strong IFNβ expression in these cells, as well as the expression of several other genes commonly upregulated during antiviral responses, including IL-6 (an inflammatory cytokine), ICAM-1 (a cell adhesion molecule), and RIG-I and MDA5 (cytosolic RNA receptors) (Fig. 2A-a). 3p-80nt-ssRNA also reduced cell viability in a dose-dependent manner (Fig. 2A-b). The cytotoxicity caused by 3p-80nt-ssRNA was apparent at 12 h after transfection and significantly increased at 24 h as indicated by the reduced number of viable cells (Fig. 2A-c). A time course analysis indicated that IFNβ induction by 3p-80nt-ssRNA was detectable at 7 h and dramatically increased at 14 and 20 h post-transfection (Fig. 2A-d), coinciding with the onset of cytotoxicity and increased cell death. However, when mESCs (D3 cells) were treated with 3p-80nt-ssRNA under the same conditions, the induction of IFNβ was negligible at 12 h treatment (Fig. 2B-a) in comparison with 10T1/2 cells and was not detected at 6 or 24 h time points (data not shown). The upregulation of IL-6 (Fig. 2B-a) ICAM-1, MDA5, and RIG-I was not observed in D3 cells (data not shown). Undifferentiated ESCs are characterized by their rapid cell proliferation with about 60% of cells in S phase and their growth in compact colonies. Transfection with 3p-80nt-ssRNA did not have detectable effects on cell viability, colony morphology, or cell cycle profile (Fig. 2B-b, c, or d, respectively). Similar results were obtained when the cells were treated with 3p-80nt-ssRNA up to 100 μM (data not shown) The different responses in 10T1/2 cells and D3 cells were not due to the different transfection efficiency since both cell types effectively expressed transfected GFP mRNA, as will be discussed later.
FIG. 2.
Comparative analysis of 3p-ssRNA induced antiviral responses in 10T1/2 cells and D3 cells. The cells were transfected with 3p-80nt-ssRNA (40 nM). The mRNA levels of IFNβ and other gene as indicated were determined by RT-qPCR in cells transfected for 12 h (a). The results are means±SD of three independent experiments. The cell viability was determined by TB cell staining of viable cells at 24 h post-transfection of (b). The values are means±SD of a representative experiment performed in triplicates. A time course of IFNβ mRNA induction in 10T1/2 cells by 3p-80nt-ssRNA (40 nM) was determined by RT-qPCR. The values are means±SD of a representative experiment performed in triplicate (A, d). The cell cycle profiles and cell count of D3 cells were determined with CFlow software at 24 h post-transfection (B, d). The morphology of transfected 10T1/2 cells (A, c) and D3 cells (B, c) were examined under a phase-contrast microscope (400×) after TB cell staining. All representative experiments were repeated at least twice with similar results.
Protein-encoding mRNA (long ssRNA) induced antiviral responses in fibroblasts but not in mESCs
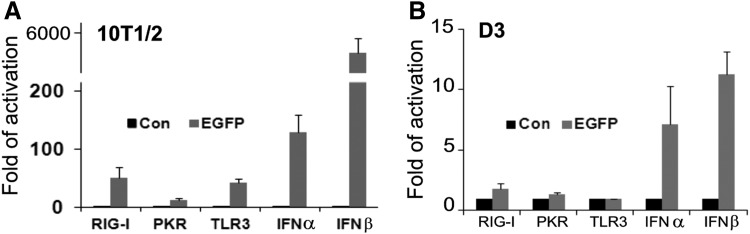
To test the effects of long ssRNA, we used the coding sequence of EGFP mRNA with a 3p group (3p-EGFP, 1033 nt, without cap and polyA tail). It not only strongly induced the expression of IFNα and IFNβ in 10T1/2 cells (∼150-fold and ∼5,500-fold, respectively) but also upregulated RNA receptors (RIG-I, TLR3 and PKR) that play key roles in antiviral responses (Fig. 3A) and caused cell death (data not shown, similar to the effects of 3p-80nt-ssRNA illustrated in Fig. 2A-b, c). On the other hand, 3p-EGFP mRNA only induced about 5- and 10-fold increase of IFNα and IFNβ, respectively, in D3 cells (Fig. 3B), which is negligible in comparison with its effects on 10T1/2 cells. The 3p-EGFP mRNA did not have a significant impact on cell viability or morphology of D3 cells (data not shown, as illustrated in Fig. 2B-b, d). It is noted that unlike 3p-80nt-ssRNA where the removal of the 3p group completely abolished its immunogenicity as shown in Fig. 1, the removal of the 3p group from EGFP mRNA significantly reduced but did not completely abolish IFNβ induction since HO-EGFP mRNA can still induce ∼40-fold increase of IFNβ in 10T1/2 cells. These observations were in line with the notion that the 3p group plays a critical role in type I IFN induction, but the length also contributes the immunogenicity of ssRNA [16,17,42].
FIG. 3.
Comparative analysis of enhanced green fluorescence protein (EGFP)-mRNA induced antiviral responses in 10T1/2 (A) and D3 cells (B). The cells were transfected with 3p-EGFP-mRNA (EGFP, 300ng/mL) for 12 h. The mRNA levels of genes involved in antiviral response were determined by RT-qPCR. The results are means±SD of three independent experiments.
dsRNA, but not ssRNA, activates PKR in mESCs
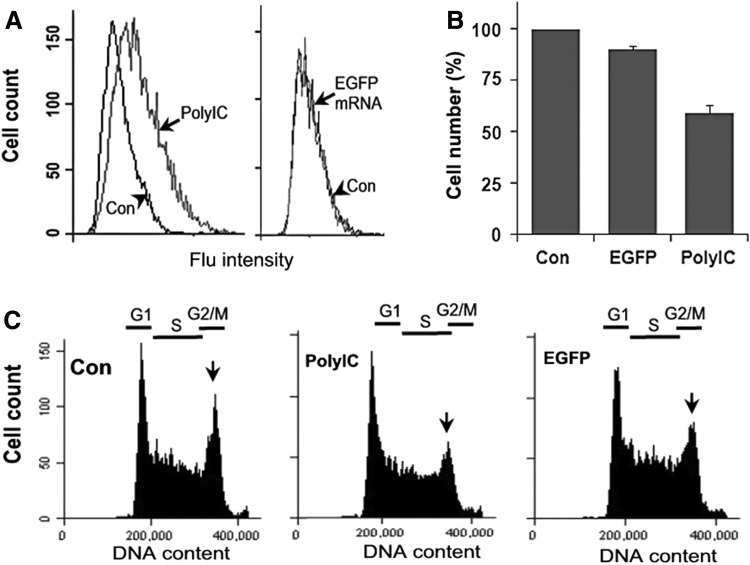
dsRNA directly activates PKR (dsRNA-activated protein kinase), which in turn phosphorylates eIF2α and results in a general inhibition of translation and cell proliferation, thereby limiting viral replication as a part of antiviral mechanisms [34,43]. It is noted that polyIC fails to induce type I IFN in D3 cells, but it can activate PKR and induce a strong inhibition of cell proliferation [7]. Since it has been reported that PKR can also be activated by ssRNA that have certain structural features [44], we analyzed the effect of 3p-EGFP mRNA on PKR activation in mESCs. As a positive control experiment, we show that transfection of D3 cells with polyIC significantly increased the level of phosphorylated eIF2α, indicating the activation of PKR (Fig. 4A, polyIC). However, transfecting the cells with 3p-EGFP mRNA did not show any effect compared with the control experiment (Fig. 4A, EGFP mRNA). We have previously demonstrated that PKR activation by polyIC significantly inhibited the synthesis of cyclins, thereby inhibiting D3 cell proliferation [7]. Although this effect of polyIC-induced PKR activation is reflected by the reduced cell number in cell proliferation analysis (Fig. 4B) and by the decreased cell population at G2/M phase in cell cycle analysis (Fig. 4C), the effects of 3p-EGFP mRNA on these cellular processes are rather limited, if there is any.
FIG. 4.
Effects of polyIC and 3p-EGFP-mRNA on PKR activation and cell proliferation. D3 cells were transfected with 300 ng/mL 3p-EGFP-mRNA (EGFP mRNA, coding sequence) or polyIC. (A) The activation of PRK was indicated by the increase of phosphorylated eIF2α (indicated by fluoresce intensity) with flow cytometry after immunostaining of the cells with antibodies against phosphorylated eIF2α. (B) Cell proliferation was determined by TB staining. The values are means±SD of a representative experiment performed in triplicate. (C) Cell cycle progression was analyzed by flow cytometry. Reduction of G2/M phase cells was indicated by arrows. All experiments were repeated at least twice with similar results.
mESCs can effectively express synthetic mRNA with expected biological functions
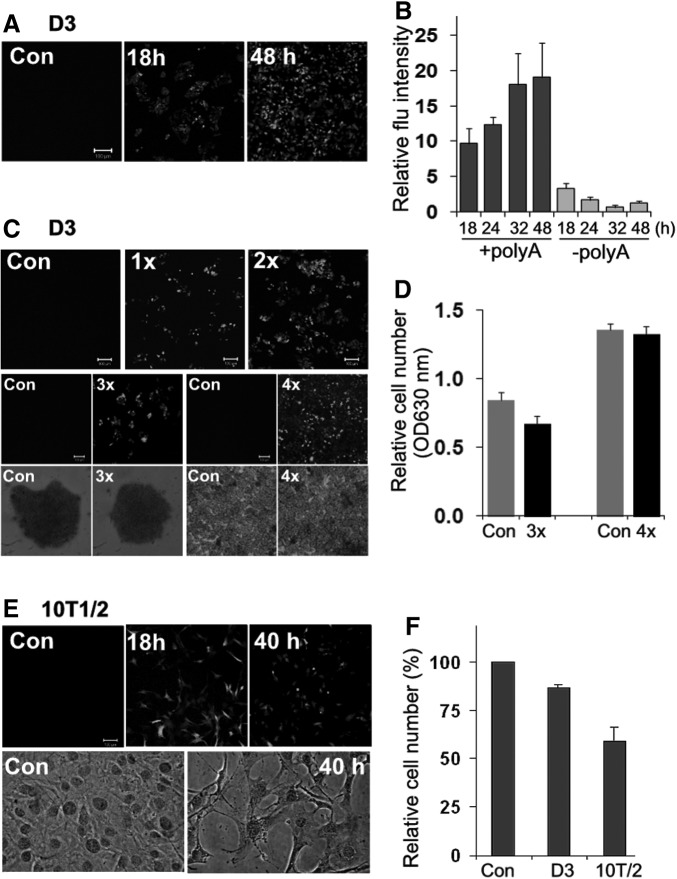
To determine if synthetic mRNA can be translated in the cells, we first made a functional EGFP by adding a cap and a polyA tail to EGFP-mRNA (m7GpppA-EGFP-polyA). After transfection, EGFP expression was detected in D3 cells as early as 12 h and the fluorescence intensity progressively increased in a time course from 18 to 48 h as determined by fluorescence microscopy and by spectrometric analysis (Fig. 5A, B, respectively), whereas very limited EGFP expression was detected with an EGFP mRNA without a poly A tail (m7GpppA-EGFP) (Fig. 5B, -polyA). In a separate experiment, D3 cells grown at low density were transfected with m7GpppA-EGFP-polyA and were then transfected a second time after 24 h. The expression of EGFP was analyzed under a fluorescence microscope at 24 h after each transfection. EGFP was effectively expressed after each transfection with a significant increase of EGFP florescence intensity after the second transfection (Fig. 5C, 1× vs. 2×). The cells after the second transfection quickly reached confluence. Therefore, they were split into new culture dishes where they received the third and the fourth transfection. Similar to the first two rounds of transfections, EGFP was effectively expressed under both low- and high-density growth conditions (Fig. 5C, 3× and 4×, respectively). Transfection of mESCs grown at low density slightly decreased the cell number, but such effect was not obvious in cells grown at high density (Fig. 5D, 3× and 4×, respectively). These results demonstrated that mESCs can tolerate repeated mRNA transfection and effectively translate the synthetic mRNA without affecting cell viability.
FIG. 5.
Expression of EGFP in 10T1/2 cells and D3 cells from synthetic mRNA. The cells were transfected with 300 ng/mL of m7GpppA-EGFP-polyA (+polyA). Con cells were transfected with DharmaFECT without mRNA. (A) The expression of EGFP in D3 cells was examined with a fluorescence microscope at 18 and 48 h after one time transfection. (B) The expression of EGFP in D3 cells from m7GpppA-EGFP-polyA (+polyA) or m7GpppA-EGFP (−polyA) was quantified with a fluorescence plate reader within a time course from 18–48 h. (C) D3 cells were transfected with m7GpppA-EGFP-polyA once or twice (1× and 2×, respectively). The expression of EGFP was examined with a fluorescence microscope at 24 h after each transfection. The cells were split into new culture dishes after the second transfection, where they received the third (3×) and fourth transfection (4×) following the same protocol used for the first two rounds of transfections. The cells were examined for EGFP expression with a fluorescence microscope (middle panels) and for cell morphology with a phase-contrast microscope (bottom panels). (D) The number of viable cells (determined by OD630 nm) was determined by TB staining at 24 h after the third (3×) and fourth (4×) transfections. (E) 10T1/2 cells were transfected m7GpppA-EGFP-polyA under the same conditions as described for D3 cells and examined for EGFP expression and for cell morphology. (F) The relative number of viable cells was determined by TB staining at the end of the experiment (40 h for 10T1/2 cells and 48 h for D3 cells for comparison). The values are means±SD of a representative experiment performed in triplicates. All experiments were repeated at least twice with similar results.
When 10T1/2 cells were transfected with m7GpppA-EGFP-polyA, EGFP was detected at 18 h, similar to its expression in D3 cells. However, the cells began to lose viability around 30 h after transfection, concurrent with a decrease in fluorescence signal. EGFP expression was significantly diminished with only about 60% of cells remaining viable compared with the control cells at 40 h (Fig. 5E, F). Therefore, even after being capped and polyadenylated, the functionalized mRNA can still cause a strong toxicity in 10T1/2 cells, although the synthetic mRNA can be translated at an early stage of transfection before the cells mount strong antiviral responses.
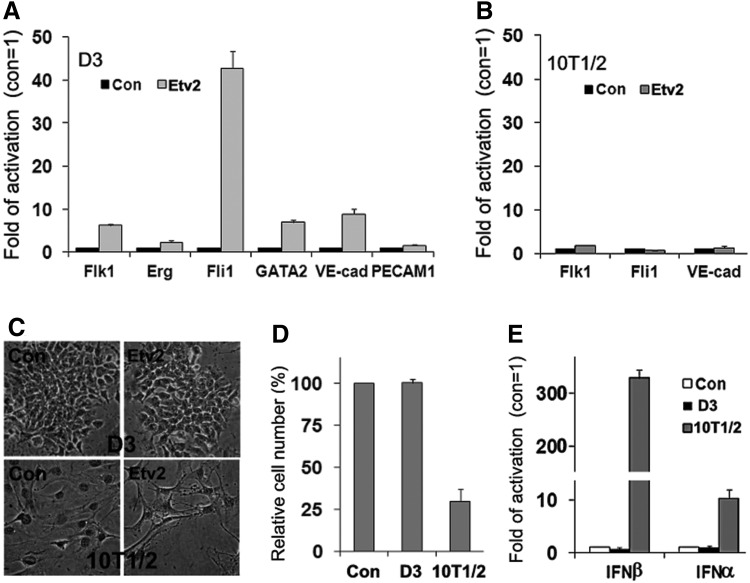
To further determine the functionality and compatibility of synthetic mRNA with mESCs, we transfected D3 cells with the Etv2 mRNA (m7GpppA-Etv2-polyA), a member of the E-twenty-six (ETS) transcription factor family that directly transactivates several genes required for vascular development [23,45]. As shown in Fig. 6, several genes essential for endothelial cell specification (known target genes of Etv2) were upregulated to different degrees in D3 cells transfected with Etv2 mRNA (Fig. 6A), demonstrating the expected transcription activity of Etv2. However, the three genes most upregulated in D3 cells, Flk1, Fli1, and VE-cadherin (VE-cad), were not significantly affected in 10T1/2 cells (Fig. 6B). Similar to EGFP mRNA, Etv2 mRNA transfection did not affect the viability of D3 cells but caused significant cytotoxicity in 10T1/2 cells (Fig. 6C, D). These results correlated with the strong induction of IFNα/β by Etv2 mRNA in 10T1/2 cells, but not in D3 cells (Fig. 6E). Therefore, the failure of Flk1, Fli1, and VE-cad expression in 10T1/2 cells can be logically explained by the fact the antiviral responses, especially PKR activation and cytotoxicity, are known factors that strongly inhibit protein synthesis in the host cells [34,43]. It is conceivable that the antiviral responses induced by Etv2 mRNA in 10T1/2 cells will inhibit its own translation, resulting in insufficient transcription activation required for the target genes (Flk1, Fli1, and VE-cad).
FIG. 6.
Expression of Etv2 from its synthetic mRNA in 10T1/2 cells and D3 cells. The cells were transfected with m7GpppA-Etv2-polyA (300 ng/mL) for 48 h. (A, B) The effects on the expression of Etv2 regulated genes were determined by RT-qPCR analysis. The results are means±SD of three independent experiments. (C) The morphology of transfected cells were examined under a phase-contrast microscope (400×) after TB cell staining. (D) The relative number of viable cells was determined by TB staining at the end of the experiment. (E) The mRNA levels of IFNβ and IFNα were determined by RT-qPCR in cells transfected for 12 h. The values are means±SD of a representative experiment performed in triplicates that was repeated at least twice with similar results.
Chemical modifications reduce, but not eliminate, the immunogenicity of synthetic mRNA
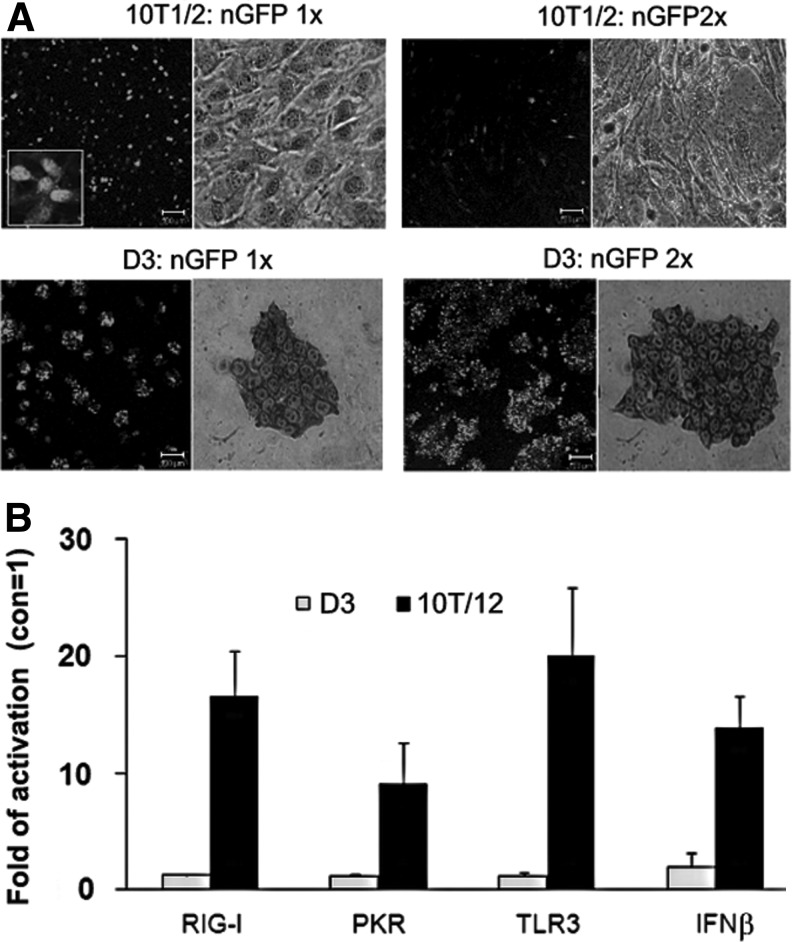
The 3p group is an intrinsic part of RNA synthesized by in vitro transcription [46]. When the mRNA is capped and polyadenylated, the functionalized synthetic mRNA still can induce strong antiviral responses as we have just shown in 10T1/2 cells and other investigators have reported in other types of fibroblasts [15]. Base modifications of the mRNA during in vitro transcription have been used to reduce the immunogenicity. However, cell reprogramming requires repeated transfection. Therefore, the modified mRNA must be used in combination with either siRNA knocking down the RNA receptors in the host cells [15] or inhibitors of IFN signaling pathway [12]. To test how mESCs respond to mRNA modifications, we used a fully functional GFP mRNA with base modifications (purchased from Stemgent). It also contains a nuclear localization signal sequence that directs the translated GFP to the nucleus (designated as nGFP-mRNA). The nGFP mRNA was effectively translated in both 10T1/2 cells and D3 cells and detected in the nucleus as expected after transfection for 24 h (Fig. 7A, 1×). The cells did not display detectable cytotoxicity within 48 h of transfection. However, subsequent transfection for the second time resulted in a dramatically increased cytotoxicity in 10T1/2 cells (30%–50% loss of viable cells) and significantly diminished GFP expression. On the contrary, the second transfection substantially increased GFP expression in D3 cells without causing cell death (Fig. 7A, 2×), similar to the results obtained from EGFP mRNA synthesized in our laboratory without base modifications (Fig. 5). It is noted that the first transfection with nGFP-mRNA did not induce IFNβ expression in 10T1/2 cells or in D3 cells (data not shown), but the second transfection induced the expression of IFNβ and RNA receptors in 10T1/2 cells (10- to 20-fold) while the effects in D3 cells were negligible (Fig. 7B). The results from 10T1/2 cells suggest that base modifications of synthetic mRNA can reduce, but cannot eliminate, the immunogenicity.
FIG. 7.
Base substitution of synthetic nGFP mRNA reduces but not eliminates its immunogenicity. The cells were transfected with a synthetic nGFP-mRNA (nGFP, 300 ng/mL) that has been modified by base modifications. (A) The transfection was performed twice (1× and 2×) with a 48 h interval. The expression of GFP was exampled with a LSM confocal flourescence microscope at 24 h after each transfection. The boxed area (10T1/2:nGFP, 1×) shows enlarged cells to illustrate the nuclear location of GFP. The morphology of transfected cells was examined under a phase-contrast microscope after TB staining (400×). (B) The mRNA levels of IFNβ, RIG-I, PKR, and TLR3 were determined by RT-qPCR in the cells that were transfected for a second time for 12 h. The results are means±SD of three independent experiments.
Discussion
We have recently reported that mESCs are deficient in expressing type I IFN when exposed to viral infection and dsRNA viral analogs [7]. In this study, we extended our investigation to their responses to different formats of synthetic ssRNA as viral ssRNA mimics. Short ssRNA induced antiviral responses in highly differentiated epithelial cells (SKOV3 cells) in a manner that depends on the length and the presence of the 5′-3p group. Even less differentiated fibroblasts with mesenchymal stem cell properties (10T1/2 cells) displayed strong antiviral responses to 3p-ssRNA and mRNA. These results are consistent with features of ssRNA characterized in other types of somatic cells [16]. None of the cellular effects associated with antiviral responses were observed in mESCs. Together with the results previously obtained from viral infection and dsRNA [7], the current study provided additional evidence to a general conclusion that mESCs have underdeveloped antiviral mechanisms.
RNA-induced type I IFN expression is mediated by toll-like receptors 3 and 7 localized on the cell surface or on the endosomal membrane and by RIG-I and MDA5 localized in the cytosol [1,2]. Apparently, none of these receptors are functional in mESCs with respect to IFN induction based on our results with live viral infection, dsRNA [7] and ssRNA (this study). Although the detailed molecular mechanisms remain to be elucidated, the low level expression of RNA receptors and their signaling components in ESCs could be at least partly responsible for the deficient antiviral responses [4,7]. RIG-I is the major receptor that mediates the effects of viral ssRNA and synthetic ssRNA, especially those with a 3p-group [16,17,41,42,47]. In addition to its well-defined function in inducing type I IFN, a recent study showed that RIG-I activation leads to apoptosis by a mechanism that is independent of IFN expression in SKOV3 cells [48]. Therefore, the RIG-I pathway may play a major role in mediating the IFNβ expression and cytotoxicity induced by ssRNA, as demonstrated in 10T1/2 cells where 3p-80nt-ssRNA induced IFNβ expression and cell death within a similar time course. Apparently, the antiviral responses elicited by ssRNA through the RIG-I pathway typically seen in differentiated cells are not induced in mESCs. The only pathway that is functional in mESCs seems to be the PKR pathway, which is activated by polyIC and responsible for polyIC-induced inhibition of translation and cell proliferation as we previously reported [7]. Although best characterized as dsRNA-activated kinase, recent studies have indicated that PKR can be also activated by ssRNA depending on certain structural features of ssRNA [44] and that there is a synergistic involvement of RIG-I and IFN [49]. The failure of ssRNA to activate PKR in mESCs can be explained by the inactive status of the RIG-I pathway and defected IFN expression mechanism in these cells. Therefore, the inactivation status of PKR in mESCs may play a key role for the effective translation of synthetic mRNA.
Expression of type I IFN in response to viral infection is the central part of innate immunity developed in most types of somatic cells [1,2]. The lack of this mechanism to viral infection and the lack of inflammatory responses to bacterial challenge [6] in ESCs indicated that “innate immunity” is, in fact, not developed in ESCs and has to be acquired during or after their differentiation to other cell types. While our findings opened up an important area in ESC research for understanding the development of innate immunity during embryogenesis, we are particularly excited about the possibility that the underdeveloped antiviral responses in ESCs may provide a “natural solution” to resolve the most challenging issue in the application of mRNA-mediated gene expression. As discussed earlier, the problems associated with antiviral responses induced by synthetic mRNA are not insurmountable, but the need of complex modifications has been a major barrier for the application of this method. We demonstrated that 5′ capping and 3′ polyadenylation of mRNA is not sufficient to repress the immunogenicity of long ssRNA since a single transfection of 10T1/2 cells with m7GpppA-EGFP-polyA (without base modifications) caused significant cell death. Base modifications of mRNA can indeed reduce the immunogenicity since the transfection of 10T1/2 cells with nGFP-mRNA (with base modifications) resulted in GFP expression without causing serious cytotoxicity. Therefore, the difference in immunogenicity between the two GFP-mRNA preparations is mainly due to base modifications. However, it is interesting to note that the effect of base modifications cannot eliminate the immunogenicity since the effect is limited to a single transfection and the subsequent transfection of 10T1/2 cells with nGFP-mRNA resulted in apparent antiviral responses. This can be explained by a well-documented phenomenon in antiviral responses; through a positive feedback loop, an initial weak stimulus (the first nGFP-mRNA transfection in our case) can upregulate the antiviral signaling pathways, thereby “priming” the cells to mount stronger antiviral responses to the subsequent stimulus (second nGFP-mRNA transfection) [50–52]. Therefore, immunogenicity intrinsically associated with mRNA (long ssRNA, sequence independent) can sensitize the host cells that have active antiviral mechanisms to repeated mRNA transfection. Without other interventions, such as knockdown of RNA receptors in the host cells [15] or using inhibitors of IFN signaling pathway [12], base modification of mRNA alone is not sufficient for successful cell reprogramming that needs multiple mRNA transfections. On the other hand, mESCs can be repeatedly transfected even with unmodified 3p-mRNA (ssRNA with the most potent immunogenicity) and can effectively translate functional proteins from the synthetic mRNA either with or without nucleotide modifications.
The ultimate goal of ESC research is to generate clinically usable ESC-derived cells for regenerative medicine. It is increasingly clear that obtaining ESC-derived cells with clinical quality by existing methods is a difficult task. Based on the same principle and methodology for iPSC reprogramming, a recent study demonstrated that forced expression of three transfection factors (Etv2, Fli1, and Erg1) that control vascular differentiation effectively converted hESCs to endothelial cells with significantly increased yield and maturity [23,45]. This study demonstrates that intervention at the transcriptional level can provide a strong internal driving force for cell-specific differentiation. However, the expression of the aforementioned transcription factors in that study was also mediated by viral vectors [23]. Using synthetic mRNA as an alternative gene expression approach could avoid the safety concerns associated with viral vectors. While the present study has proven the principle of this approach and demonstrated the feasibility of increasing the transcription activity of Etv2 from its synthetic mRNA, it should be pointed out that the experiments described in this study were carried out in undifferentiated mESCs. Generating specific cell types must be performed under differentiation conditions and meet cell-specific requirements. In the case of generating mature and functional endothelial cells from ESCs, simultaneous expression of Etv2, Fli1, and Erg1 and repression of TGFβ signaling is required [23]. The effort toward that goal is under way in our laboratories.
Conclusions
Our current and previous studies demonstrate that mESCs have underdeveloped antiviral mechanisms. This finding represents a unique and previously uncharacterized property of mESCs that is not only important for understanding innate immunity development but can also be exploited for developing mRNA-based gene expression methods that may transform the existing differentiation paradigms.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants R21HL082731 (Y.-L.G.) and R15CA152822 (F.H.) and by Mississippi INBRE, funded by the National Institute of General Medical Sciences (8 P20 GM103476-11). We thank Baobin Kang for microscopy analysis and Mississippi INBRE for the use of the imaging facility. We are grateful to Rachel Payne for critical reading of the article.
Author Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Kawai T. and Akira S. (2011). Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650 [DOI] [PubMed] [Google Scholar]
- 2.Stetson DB. and Medzhitov R. (2006). Type I interferons in host defense. Immunity 25:373–381 [DOI] [PubMed] [Google Scholar]
- 3.Foldes G, Liu A, Badiger R, Paul-Clark M, Moreno L, Lendvai Z, Wright JS, Ali NN, Harding SE. and Mitchell JA. (2010). Innate immunity in human embryonic stem cells: comparison with adult human endothelial cells. PLoS ONE 5:e10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LL, Yang L. and Carmichael GG. (2010). Molecular basis for an attenuated cytoplasmic dsRNA response in human embryonic stem cells. Cell Cycle 9:3552–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zampetaki A, Xiao Q, Zeng L, Hu Y. and Xu Q. (2006). TLR4 expression in mouse embryonic stem cells and in stem cell-derived vascular cells is regulated by epigenetic modifications. Biochem Biophys Res Commun 347:89–99 [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Rossi R, Hale C, Goulding D. and Dougan G. (2009). Interaction of enteric bacterial pathogens with murine embryonic stem cells. Infect Immun 77:585–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Wang J, Paul AM, Acharya D, Bai F, Huang F. and Guo YL. (2013). mouse embryonic stem cells are deficient in type i interferon expression in response to viral infections and double-stranded RNA. J Biol Chem 288:15926–15936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K. and Yamanaka S. (2006). induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 9.Buganim Y, Faddah DA. and Jaenisch R. (2013). Mechanisms and models of somatic cell reprogramming. Nat Rev Genet 14:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller LU, Daley GQ. and Williams DA. (2009). Upping the ante: recent advances in direct reprogramming. Mol Ther 17:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daubman S. (2011). Landmark approach to generating human stem cells. Circ Res 108:161–163 [DOI] [PubMed] [Google Scholar]
- 12.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakubov E, Rechavi G, Rozenblatt S. and Givol D. (2010). Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun 394:189–193 [DOI] [PubMed] [Google Scholar]
- 14.Plews JR, Li J, Jones M, Moore HD, Mason C, Andrews PW. and Na J. (2010). Activation of pluripotency genes in human fibroblast cells by a novel mRNA based approach. PLoS ONE 5:e14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angel M. and Yanik MF. (2010). Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS ONE 5:e11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, et al. (2006). 5'-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997 [DOI] [PubMed] [Google Scholar]
- 17.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljeström P, Weber F. and Reis e Sousa C. (2006). RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science 314:997–1001 [DOI] [PubMed] [Google Scholar]
- 18.Wobus AM. and Boheler KR. (2005). Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev 85:635–678 [DOI] [PubMed] [Google Scholar]
- 19.Lerou PH. and Daley GQ. (2005). Therapeutic potential of embryonic stem cells. Blood Rev 19:321–331 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. and Nishikawa S. (2000). Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408:92–96 [DOI] [PubMed] [Google Scholar]
- 21.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J. and Langer R. (2002). Endothelial cells derived from human embryonic stem cells. PNAS 99:4391–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCloskey KE, Stice SL. and Nerem RM. (2006). In vitro derivation and expansion of endothelial cells from embryonic stem cells. Methods Mol Biol 330:287–301 [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler J, Schachterle W, Pulijaal V, Mathew S, et al. (2012). Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGF-beta suppression. Cell 151:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman TM, Wang G. and Huang F. (2004). Superior 5' homogeneity of RNA from ATP-initiated transcription under the T7 {phi}2.5 promoter. Nucleic Acids Res 32:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang F. (2003). Efficient incorporation of CoA, NAD and FAD into RNA by in vitro transcription. Nucleic Acids Res 31:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang B, Oo TN. and Rizzo V. (2006). Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J 20:1501–1503 [DOI] [PubMed] [Google Scholar]
- 27.Kozak M. (1987). At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196:947–950 [DOI] [PubMed] [Google Scholar]
- 28.Darland DC. and D'Amore PA. (2001). TGF-β is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4:11–20 [DOI] [PubMed] [Google Scholar]
- 29.Haas AR. and Tuan RS. (2000). Murine C3H10T1/2 multipotential cells as an In vitro model of mesenchymal chondrogenesis. Dev Biol Protocol 137:383–389 [DOI] [PubMed] [Google Scholar]
- 30.Guo YL, Ye J. and Huang F. (2007). p38alpha MAP kinase-deficient mouse embryonic stem cells can differentiate to endothelial cells, smooth muscle cells, and neurons. Dev Dyn 236:3383–3392 [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YL, Chakraborty S, Rajan S, Wang R. and Huang F. (2010). Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-Renewal. Stem Cell Dev 19:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R. and Guo YL. (2012). Transient inhibition of cell proliferation does not compromise self-renewal of mouse embryonic stem cells. Exp Cell Res 318:2094–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia MA, Meurs EF. and Esteban M. (2007). The dsRNA protein kinase PKR: virus and cell control. Biochimie 89:799–811 [DOI] [PubMed] [Google Scholar]
- 35.Gondai T, Yamaguchi K, Miyano-Kurosaki N, Habu Y. and Takaku H. (2008). Short-hairpin RNAs synthesized by T7 phage polymerase do not induce interferon. Nucleic Acids Res 36:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlee M, Hartmann E, Coch C, Wimmenauer V, Janke M, Barchet W. and Hartmann G. (2009). Approaching the RNA ligand for RIG-I? Immunol Rev 227:66–74 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Ludwig J, Schuberth C, Goldeck M, Schlee M, Li H, Juranek S, Sheng G, Micura R, et al. (2010). Structural and functional insights into 5'-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat Struct Mol Biol 17:781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubler K, Gehrke N, Riemann S, Bohnert V, Zillinger T, Hartmann E, Polcher M, Rudlowski C, Kuhn W, Hartmann G. and Barchet W. (2010). Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 70:5293–5304 [DOI] [PubMed] [Google Scholar]
- 39.Kubler K, Tho PC, Gehrke N, Riemann S, Dassler J, Coch C, Landsberg J, Wimmenauer V, Polcher M, et al. (2011). Immunogenic cell death of human ovarian cancer cells induced by cytosolic poly(I:C) leads to myeloid cell maturation and activates NK cells. Eur J Immunol 41:3028–3039 [DOI] [PubMed] [Google Scholar]
- 40.Van DN, Roberts CF, Marion JD, Lépine S, Harikumar KB, Schreiter J, Dumur CI, Fang X, Spiegel S. and Bell JK. (2012). Innate immune agonist, dsRNA, induces apoptosis in ovarian cancer cells and enhances the potency of cytotoxic chemotherapeutics. FASEB J 26:3188–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim DH, Longo M, Han Y, Lundberg P, Cantin E. and Rossi JJ. (2004). Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat Biotech 22:321–325 [DOI] [PubMed] [Google Scholar]
- 42.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, et al. (2009). Recognition of 5'-triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuel CE. (2001). Antiviral actions of interferons. Clin Microbiol Rev 14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nallagatla SR, Toroney R. and Bevilacqua PC. (2011). Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struc Biol 21:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Val S. and Black BL. (2009). Transcriptional control of endothelial cell development. Dev Cell 16:180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang F, He J, Zhang Y. and Guo Y. (2008). Synthesis of biotin-AMP conjugate for 5[prime] biotin labeling of RNA through one-step in vitro transcription. Nat Protoc 3:1848–1861 [DOI] [PubMed] [Google Scholar]
- 47.Yoneyama M. and Fujita T. (2007). Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem 282:15315–15318 [DOI] [PubMed] [Google Scholar]
- 48.Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S. and Hartmann G. (2009). Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon independent apoptosis in human melanoma cells. J Clin Invest 119:2399–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE. and Bevilacqua PC. (2007). 5'-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 318:1455–1458 [DOI] [PubMed] [Google Scholar]
- 50.Pan ZK, Fisher C, Li JD, Jiang Y, Huang S. and Chen LY. (2011). Bacterial LPS up-regulated TLR3 expression is critical for antiviral response in human monocytes: evidence for negative regulation by CYLD. Int Immunol 23:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto M. and Seya T. (2008). TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev 60:805–812 [DOI] [PubMed] [Google Scholar]
- 52.Huang CC, Duffy KE, San Mateo LR, Amegadzie BY, Sarisky RT. and Mbow ML. (2006). A pathway analysis of poly(I:C)-induced global gene expression change in human peripheral blood mononuclear cells. Physiol Genomics 26:125–133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.