Abstract
The phenotype of articular chondrocytes is dependent on the cytoskeleton, specifically the actin microfilament architecture. Articular chondrocytes in monolayer culture undergo dedifferentiation and assume a fibroblastic phenotype. This process can be reversed by altering the actin cytoskeleton by treatment with cytochalasin. Whereas dedifferentiation has been studied on chondrocytes isolated from the whole cartilage, the effects of cytoskeletal alteration on specific zones of cells such as superficial zone chondrocytes are not known. Chondrocytes from the superficial zone secrete superficial zone protein (SZP), a lubricating proteoglycan that reduces the coefficient of friction of articular cartilage. A better understanding of this phenomenon may be useful in elucidating chondrocyte dedifferentiation in monolayer and accumulation of the cartilage lubricant SZP, with an eye toward tissue engineering functional articular cartilage. In this investigation, the effects of cytoskeletal modulation on the ability of superficial zone chondrocytes to secrete SZP were examined. Primary superficial zone chondrocytes were cultured in monolayer and treated with a combination of cytoskeleton modifying reagents and transforming growth factor β (TGFβ) 1, a critical regulator of SZP production. Whereas cytochalasin D maintains the articular chondrocyte phenotype, the hallmark of the superficial zone chondrocyte, SZP, was inhibited in the presence of TGFβ1. A decrease in TGFβ1-induced SZP accumulation was also observed when the microtubule cytoskeleton was modified using paclitaxel. These effects of actin and microtubule alteration were confirmed through the application of jasplakinolide and colchicine, respectively. As Rho GTPases regulate actin organization and microtubule polymerization, we hypothesized that the cytoskeleton is critical for TGFβ-induced SZP accumulation. TGFβ-mediated SZP accumulation was inhibited by small molecule inhibitors ML141 (Cdc42), NSC23766 (Rac1), and Y27632 (Rho effector Rho Kinase). On the other hand, lysophosphatidic acid, an upstream activator of Rho, increased SZP synthesis in response to TGFβ1. These results suggest that SZP production is dependent on the functional cytoskeleton, and Rho GTPases contribute to SZP accumulation by modulating the actions of TGFβ.
Introduction
The articular chondrocyte phenotype is dependent on cell shape. When cultured on tissue culture plastic in monolayer conditions, articular chondrocytes flatten and assume a fibroblastic morphology. This change in cell shape is called dedifferentiation, with attendant decreases in the synthesis of collagen II and proteoglycans, hallmarks of the articular chondrocyte phenotype.1,2 When chondrocytes are reverted to a three-dimensional (3D) configuration in agarose, the characteristic collagen II and proteoglycan phenotype is restored.2 In an important experiment, Brown and Benya demonstrated the restoration of the chondrocyte phenotype, or redifferentiation of dedifferentiated chondrocytes, by treatment with cytochalasin D and dependent modulation of the actin microfilament cytoskeleton.3 Thus, cell shape and the morphology of the actin cytoskeleton are critical regulators of the chondrocyte phenotype,4 and therefore in chondrogenesis.5
Articular cartilage in the ends of long bones in diarthrodial joints permits smooth and nearly friction-free joint articulation. Weight bearing of loads up to 18 MPa is accomplished through an extracellular matrix architecture that combines the compressive properties of hydrated glycosaminoglycans (GAG) with the tensile strength of crosslinked type II collagen fibrils.6 In addition, articular cartilage possesses multiple modes of lubrication that reduces friction forces and wear, permitting decades of joint movement and mobility.7 In cases of osteoarthritis and other cartilage pathologies, there exists an acute need for replacement tissue that repairs and restores joint functionality. Tissue engineering of articular cartilage has proven to be a very difficult problem due to the remarkable and imposing nature of the native tissue's bulk and surface mechanical properties. One impediment in articular cartilage tissue engineering is a plentiful cell source. Growing substantial numbers of chondrocytes through methods such as monolayer culture is challenging due to dedifferentiation.8 If a bountiful supply of articular chondrocytes is identified, methods such as the self-assembly process may be able to exploit this resource to produce an engineered tissue capable of replicating the mechanical behavior of native articular cartilage.9 One can address this bottleneck to tissue engineering by exploring the role of the cytoskeleton to optimize the articular cartilage phenotype.
Articular cartilage is an anisotropic structure with a zonal design and consists of surface or superficial, middle, and deep zones. The superficial zone protein (SZP) is a mucinous proteoglycan found and produced in the surface zone of articular cartilage and reduces the coefficient of friction and wear in articular cartilage through a sacrificial, boundary lubrication mechanism.10–13 SZP and alternatively spliced isoforms such as lubricin and PRG4, are all products of the prg4 gene.14 The importance of functional SZP/lubricin is evident in patients with camptodactyly-arthropathy-coxa vara-pericarditis syndrome. They possess a mutation in the prg4 gene that encodes for SZP and suffer from precocious joint failure and synovial hyperplasia.15 Growth factors and morphogens such as bone morphogenetic proteins and transforming growth factor β (TGFβ) have been shown to be powerful anabolic agents of SZP synthesis.16,17 Whereas the effects of actin microfilament modulation have been examined in articular chondrocytes, these cells have been usually derived from a mixed population containing all three zones: superficial, middle, and deep. However, each zone of articular cartilage possesses a unique phenotype, such as differences in the cell shape and proteoglycan synthesis as well as SZP synthesis.18–20 Thus, work remains in elucidating the effects of zonal phenotype on the cytoskeletal regulation of articular chondrocytes.
The cytoskeleton is a pleiotropic system that interacts with many different aspects of cellular function. For instance, the Rho family of GTPases modulates actin polymerization to control the shape and movement of the cell. The most common and well studied members include Rho, Rac, and Cdc42.21 Like all small GTPases, their activity is controlled by their nucleotide binding partner. Rho GTPases assume an active conformation when bound to GTP, and are inactive when partnered with GDP. Regulatory molecules such as GTPase-Activating Proteins (GAPs) promote GTP hydrolysis to GDP to inactivate these proteins. The switch can be flipped and Rho family GTPases activated by guanine nucleotide exchange factors (GEFs), which catalyze a GDP to GTP conversion.22 Rho family GTPases are able to regulate many different processes by partnering with effector molecules such as Rho kinase (ROCK).23 Through ROCK, RhoA is able to enhance actin-myosin contractility through myosin light chain (MLC) phosphorylation and inhibition of MLC phosphatase.24 In addition, the Rho family of GTPases has also been demonstrated to mediate other processes such as mechanotransduction, transcription factor activation, and stem cell fate.25–28
The principal objective of this study was to determine the role of the cytoskeleton in SZP synthesis in superficial zone chondrocytes. The secondary goal was to identify a potential mechanism by which cytoskeletal alteration could affect SZP production. Therefore, it was hypothesized that the cytoskeleton will modulate SZP synthesis through Rho family GTPases. The hypothesis was evaluated by assessing the effects of cytoskeletal and Rho family GTPase modulators on basal and TGFβ1-stimulated SZP synthesis in a monolayer culture system. Given that superficial zone chondrocytes could prove a useful source of SZP for the boundary lubrication of engineered cartilage,29 the global aim of this experiment was to elucidate new mechanisms of SZP regulation to assist in the development of the tissue engineering of articular cartilage.
Materials and Methods
Isolation of superficial zone articular chondrocytes
Bovine stifle joints from 3-month-old calves were obtained from an abattoir and dissected under aseptic conditions to expose the femoral condyles.16 Superficial zone articular cartilage (∼100 μm thick) was harvested from load-bearing regions of the lateral and medial condyles (L1–L3, M1–M2) of bovine stifle joints as previously described using a dermatome.30 The cartilage was enzymatically digested for 2.5 h in 0.2% collagenase P (Roche, Indianapolis, IN) in a culture medium (DMEM/F-12, 50 μg/mL ascorbate 2-phosphate, 0.1% bovine serum albumin [BSA], and 1% penicillin/streptomycin) supplemented with 3% heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY). Isolated superficial zone articular chondrocytes were washed, plated at a density of 100,000 cells/well (∼25,000 cells/cm2) in 12-well dishes (Corning, Corning, NY), and seeded overnight in culture medium containing 10% FBS.
Monolayer culture of primary articular chondrocytes
After overnight attachment, chondrocytes were cultured in serum-free, defined conditions consisting of culture media supplemented with 1% ITS+Premix (BD Biosciences, Bedford, MA) and 0.5% Fungizone in a humidified, 5% CO2 incubator at 37°C.16 All reagents were obtained from Life Technologies (Grand Island, NY) or Sigma-Aldrich (St. Louis, MO), unless otherwise noted. After the media exchange, chondrocytes were pretreated with an inhibitor for 1 h, before the addition of 3 ng/mL TGFβ1 (R&D Systems, Minneapolis, MN). All control cell cultures received an equal volume of the equivalent carrier solution at the time of treatment. Chondrocytes were cultured for 96 h before the media were collected and stored at −80°C for later analysis of SZP accumulation.
Cytoskeleton and Rho GTPase inhibiting drugs
Cytochalasin D, jasplakinolide, and paclitaxel were solubilized in dimethyl sulfoxide (DMSO) at stock concentrations of 10, 1, and 10 mM, respectively. Colchicine was prepared as a stock solution in sterile water at a 10 mM concentration. NSC23766 and Y27632 (Tocris, Minneapolis, MN) were solubilized in a 25 mM HEPES solution at 50 and 10 mM, respectively. Lysophosphatidic acid (LPA) (Enzo Life Sciences, Farmingdale, NY) was prepared as a 10 mM solution in phosphate-buffered saline (PBS). ML141 (Tocris) was solubilized in DMSO at a concentration of 50 mM. Recombinant, human TGFβ1 was reconstituted in a filter sterilized solution of 0.1% BSA in 5 mM hydrochloric acid at 10 μg/mL. Stock concentrations of all reagents were prepared and stored at −20°C, until they were diluted in culture media to their final concentrations. Actin microfilaments were modified through cytochalasin D (1, 6, and 10 μM) and jasplakinolide (15 and 50 nM).31–33 Microtubules were inhibited through the use of colchicine and paclitaxel (1 and 10 μM).34,35 NSC23766 (50 and 150 μM), ML141 (5 and 20 μM), Y27632 (10 and 50 μM), and LPA (5 and 20 μM) were employed to modify the activity of Rho family GTPases.27,36,37 The inhibitors and dosages employed have been demonstrated in the literature to have virtually no cytotoxic effects.32,34,35,38,39 During the course of this investigation, no adverse changes in cellular morphology or appearance were observed.
Enzyme-linked immunosorbent assay for SZP
SZP media accumulation was quantified by a sandwich enzyme-linked immunosorbent assay (ELISA) using antibody S6.79.40 The capture reagent, peanut lectin (EY Laboratories, San Mateo, CA), was prepared as a 1 μg/mL solution in a 50 mM sodium carbonate buffer (pH 9.5), and used to coat black 96-well plates (50 μL/well) overnight at 4°C. The next day, plates were blocked with 100 μL/well of 1% BSA in identical buffer at room temperature for 2 h. After this and each subsequent step, plates were washed with PBS containing 0.05% Tween 20 (PBS-T). SZP standards and samples were serially diluted in PBS and incubated for 1 h at room temperature.30 Plates were then incubated overnight at 4°C with the primary SZP monoclonal antibody, S6.79 (2.3 mg/mL), which was diluted 1:5000 in PBS-T. Finally, the secondary, detection antibody, goat anti-mouse IgG-horseradish peroxidase (HRP) conjugate (Bio-Rad, Hercules, CA), was added to each plate at a 1:3000 dilution in PBS-T and incubated for 1 h at room temperature. After washes with PBS-T and PBS, the SuperSignal ELISA Femto substrate (Thermo Scientific, Rockford, IL) was added to each well and the chemiluminescent signal was recorded using a chemiluminescent imaging system (Alpha Innotech, Santa Clara, CA). Sample SZP levels were quantified using a serially diluted, bovine SZP standard. SZP from the synovial fluid and TGFβ-treated articular cartilage explant conditioned media were pooled, dialyzed, and purified through affinity chromatography using peanut lectin-linked agarose beads (EY Laboratories). The SZP standard concentration was determined using a bicinchoninic acid (BCA) assay (Thermo Scientific) based on a BSA protein standard.41
Immunoblot analysis of SZP
To confirm the ELISA data, SZP media accumulation was measured by immunoblot. Equal volumes of media samples were denatured and electrophoretically separated under reducing conditions in 4–12% Bis–Tris polyacrylamide gels using MOPS buffer (Life Technologies). Proteins were then transferred to polyvinylidene fluoride (PVDF) membranes using a semidry transfer cell (Bio-Rad). After the membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20, it was incubated overnight in the primary SZP antibody S6.79 (1:5000 in blocking buffer). Following a washing step, the membrane was incubated in a 1:3000 solution of the secondary antibody, goat anti-mouse IgG HRP. The SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) was added after a subsequent wash and the immunoblot was imaged using film.16
Statistical analysis
Each treatment group consisted of n=12 samples, produced from two separate experiments that comprised groups of genetically different animals. Results were evaluated in a statistical software package (JMP; SAS, Cary, NC) using a one-way analysis of variance (ANOVA) with a Tukey's post hoc test. Statistical significance was established at p-values less than 0.05. Results are presented as mean±standard deviation. In a figure, treatment groups not connected by the same letter were found to be significantly different.
Results
Actin microfilament modulation
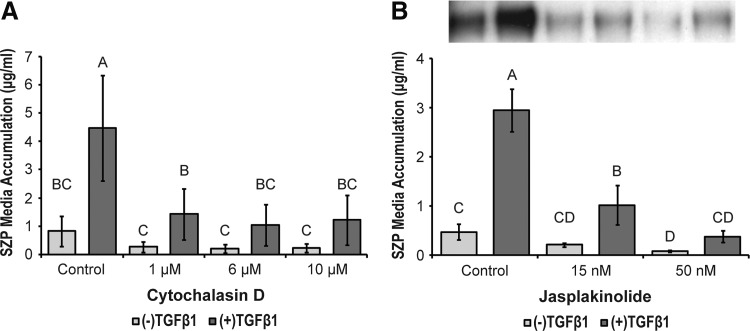
Cytochalasin D induced superficial zone articular chondrocytes to assume a round cell shape in control and TGFβ1-treated samples at all treatment levels (Fig. 1C, D). Untreated, control chondrocytes flattened and acquired a spread, fibroblastic morphology (Fig. 1A, B). TGFβ1-induced SZP media accumulation was significantly reduced in the presence of cytochalasin D (Fig. 2A). Whereas there was a trend toward cytochalasin treatment decreasing basal SZP media accumulation (i.e., cells that received TGFβ1 vehicle controls), the differences were not statistically significant. In the absence of cytochalasin, TGFβ1 (3 ng/mL) upregulated SZP accumulation fivefold greater than control. At the lowest cytochalasin D dosage, 1 μM, TGFβ1 was still able to induce a fivefold increase in SZP synthesis. However, at the 6- and 10-μM cytochalasin D dosages, TGFβ1-induced SZP levels in the media were not significantly different than control values.
FIG. 1.
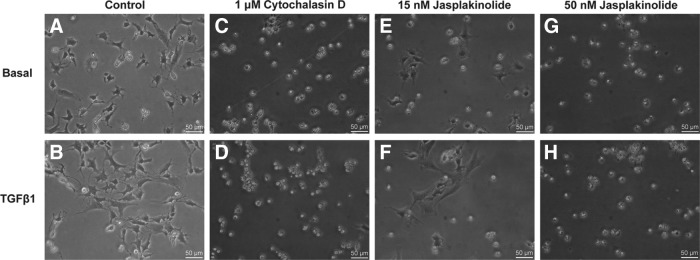
The effects of actin microfilament modulation on the cellular morphology of superficial zone articular chondrocytes. Primary, bovine, superficial zone articular chondrocytes were cultured for 4 days in serum-free, defined component media supplemented without (Basal) and with 3 ng/mL transforming growth factor β (TGFβ)1. By day 4 of culture, the majority of chondrocytes not treated with a cytoskeletal inhibitor (A, B) had attained a fibroblastic morphology. Compared with basal control cells (A), TGFβ1 treatment promoted increased cell spreading (B). When treated with 1 μM cytochalasin D, an actin polymerization inhibitor, chondrocytes remained rounded in the presence and absence of TGFβ1 (C, D). Cells treated with 6 and 10 μM cytochalasin D also retained a similar morphology (data not shown). Fifteen nanomolar jasplakinolide, an actin depolymerization inhibitor, elicited a rounded morphology in a large fraction of the cell population (E, F). However, a portion of the chondrocytes were unaffected by the drug and flattened to assume a fibroblastic shape (E), and cell spreading among these cells was enhanced by TGFβ1 (F). At the 50 nM jasplakinolide dosage, all the cells attained a rounded shape in cultures containing and lacking TGFβ1 (G, H).
FIG. 2.
The effects of actin microfilament inhibition on basal and TGFβ1-stimulated superficial zone protein (SZP) media accumulation. Primary, superficial zone articular chondrocytes were treated with (A) 1, 6, and 10 μM cytochalasin D and (B) 15 and 50 nM jasplakinolide, in the presence and absence of 3 ng/mL TGFβ1. SZP media accumulation was quantified by enzyme-linked immunosorbent assay (ELISA) and immunoblot. A representative immunoblot of SZP accumulated in the media of cell cultures receiving jasplakinolide treatment is inset of (B). The bands of the immunoblot correspond to the treatment labels of the bar graph below. Values are presented as mean±standard deviation (SD) for n=12 samples from two separate experiments.
Similar results were observed when superficial zone chondrocytes were treated with jasplakinolide. Jasplakinolide induced complete cell rounding at the high dosage (50 nM), while only a majority of cells rounded at the low concentration (15 nM) (Fig. 1E–H). The low and high concentrations of jasplakinolide (15 and 50 nM) elicited a statistically significant, dose-dependent decrease in SZP accumulation in TGFβ1-treated cultures (Fig. 2B). Fifty nanomolar jasplakinolide reduced TGFβ1-stimulated SZP accumulation to control levels. Basal SZP synthesis was moderated by jasplakinolide, but this effect was only significant at the 50 nM dosage.
Microtubule modulation
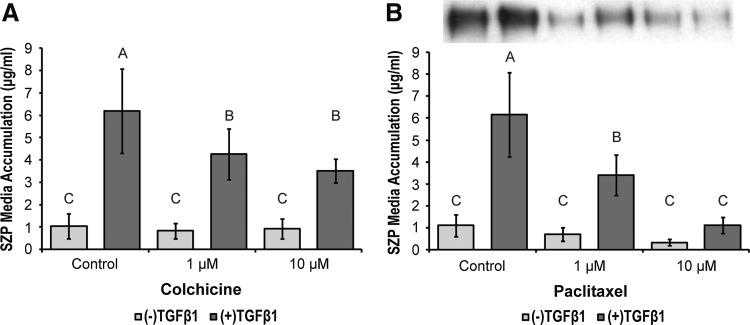
Inhibitors of microtubule dynamics, paclitaxel and colchicine, did not induce any gross changes in cell morphology (data not shown). Nevertheless, SZP media accumulation was affected. Paclitaxel inhibited TGFβ1-stimulated SZP in a dose-dependent manner (Fig. 3B). Basal SZP accumulation in the presence of paclitaxel trended downward, but was not significantly different. Whereas TGFβ1 was able to induce a significant increase in SZP synthesis at the 1 μM concentration, 10 μM paclitaxel reduced TGFβ1-stimulated SZP production to control levels. Colchicine had no discernible effect on basal SZP media concentrations, but it did significantly reduce SZP accumulation in the presence of TGFβ at both the low and high dosages (Fig. 3A).
FIG. 3.
The effects of microtubule modulation on basal and TGFβ1-stimulated SZP media accumulation. Primary, superficial zone articular chondrocytes were treated with (A) 1 and 10 μM colchicine, a microtubule polymerization inhibitor, and (B) 1 and 10 μM paclitaxel, a microtubule depolymerization inhibitor, in the presence and absence of 3 ng/mL TGFβ1. SZP media accumulation was quantified by ELISA and immunoblot. A representative immunoblot of SZP collected from the media of cell cultures treated with paclitaxel is inset of (B). The bands of the immunoblot correspond to the treatment labels of the bar graph below. Values are presented as mean±SD for n=12 samples from two separate experiments.
Rho family GTPase modulation
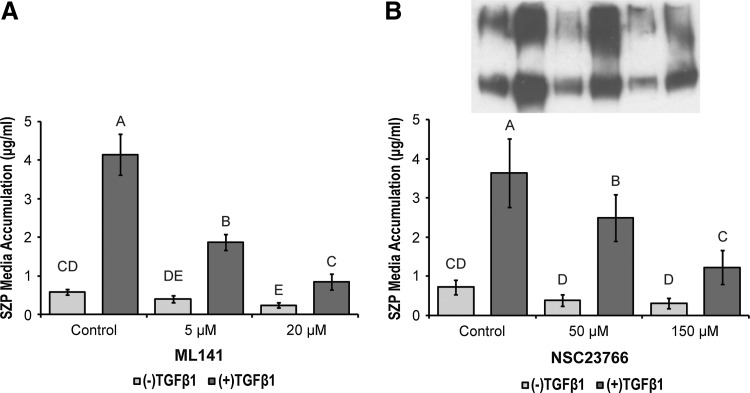
To determine whether Rho family GTPases may be playing a role in the cytoskeletal modulation of SZP synthesis, superficial zone chondrocytes were treated with reagents that inhibit or increase the activity of these small GTPases. Rac1 activity was inhibited by NSC23766, a small molecular compound that inhibits Rac1 activation by GEFs.42 NSC23766, reduced TGFβ1-stimulated SZP accumulation in a dose-dependent manner (Fig. 4B). Interestingly, TGFβ was able to significantly upregulate SZP synthesis >3.9-fold in the presence of NSC23766 at both low and high (50 and 150 μM) concentrations. ML141, a reversible and noncompetitive inhibitor of Cdc42,43 also decreased SZP accumulation stimulated by TGFβ in a dose-dependent manner (Fig. 4A). Whereas TGFβ treatment significantly increased SZP synthesis in the 5 μM ML141 group, 20 μM ML141 reduced TGFβ-induced SZP synthesis to near basal control levels. At the high dose, basal SZP accumulation was significantly reduced compared with basal control.
FIG. 4.
The effects of Cdc42 and Rac1 inhibition on basal and TGFβ1-stimulated SZP media accumulation. Primary, superficial zone articular chondrocytes were treated with (A) 5 and 20 μM ML141, a reversible, noncompetitive inhibitor of Cdc42 and (B) 50 and 150 μM NSC23766, a Rac1-guanine nucleotide exchange factor (GEF) inhibitor, in the presence and absence of 3 ng/mL TGFβ1. SZP media accumulation was quantified by ELISA and immunoblot. A representative immunoblot of SZP accumulated in the media of cell cultures receiving NSC23766 treatment is inset of (B). The bands of the immunoblot correspond to the treatment labels of the bar graph below. Values are presented as mean±SD for n=12 samples from two separate experiments.
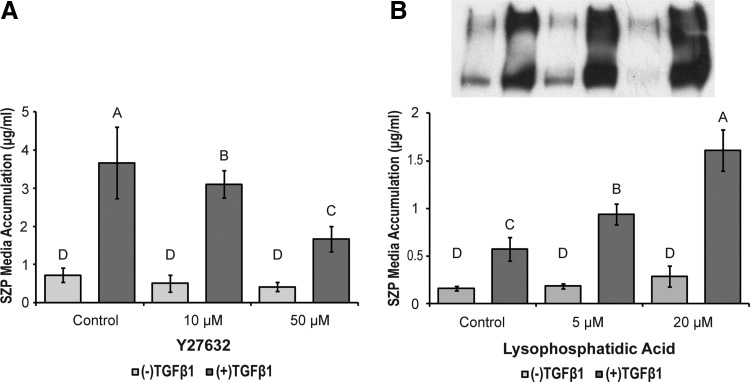
One of the effector proteins of Rho is ROCK. Instead of directly targeting Rho, the pyridine derivative Y27632 was employed to competitively inhibit this immediate, downstream effector.44 TGFβ-induced SZP synthesis was reduced by Y27632 in a dose-dependent manner, while basal SZP accumulation was not significantly affected by the drug (Fig. 5A). The ability of TGFβ to significantly increase SZP production (>3.9-fold) was also retained in Y27632-treated chondrocytes. Conversely, Rho activity was increased by LPA.45 LPA increased TGFβ-stimulated SZP media accumulation in a dose-dependent manner (Fig. 5B). Basal SZP production in the presence of LPA trended upward, but the effect was not significant.
FIG. 5.
The effects of Rho Kinase (ROCK) inhibition and RhoA activation on basal and TGFβ1-stimulated SZP media accumulation. Primary, superficial zone articular chondrocytes were treated with (A) 10 and 50 μM Y27632, a competitive inhibitor of ROCK and (B) 5 and 20 μM lysophosphatidic acid (LPA), an upstream activator of RhoA, in the presence and absence of 3 ng/mL TGFβ1. SZP media accumulation was quantified by ELISA and immunoblot. A representative immunoblot of SZP collected from the media of cell cultures treated with LPA is inset of (B). The bands of the immunoblot correspond to the treatment labels of the bar graph below. Values are presented as mean±SD for n=12 samples from two separate experiments.
Immunoblots were performed to corroborate the SZP media accumulation values quantified by ELISA. The media samples from the Rho-family GTPase treatments were subjected to additional freeze–thaw cycles during the course of analysis, compared with the media collected from the cytoskeletal inhibitor experiments. The additional freeze–thaw cycles altered the electrophoretic migration of a portion of the sample and created the pair of bands observed. The combined intensity of the band pairs corresponds with the respective ELISA values.
Discussion
This study sought to determine the role of the cytoskeleton in the production of SZP in superficial zone chondrocytes. Cell shape and the actin cytoskeleton are key determinants of the articular chondrocyte phenotype as demonstrated by Benya and others.2,3 In response to the pharmacological modulation of actin and microtubule polymerization, there was a significant decrease in the TGFβ-stimulated synthesis of SZP. Rho family GTPases were hypothesized to serve as a possible mediator between the cytoskeleton and the SZP signaling pathway, as measured by SZP media accumulation. When Rho GTPase activity was similarly inhibited using small molecular drugs, TGFβ-induced SZP media accumulation was inhibited in a dose-dependent manner. LPA, an upstream activator of RhoA activity, synergistically enhanced TGFβ-mediated SZP synthesis. These results suggest that modulation of Rho family GTPases may serve as a method of tuning SZP synthesis in articular chondrocytes, and offer a means of improving boundary lubrication in tissue engineered cartilage.
Cytoskeletal modification has long been identified as a method of chondrogenesis.46,47 This strategy is also being applied in cartilage tissue engineering, as a means of cellular expansion and as an exogenous stimulus of engineered constructs.48,49 Since engineered articular cartilage must possess lubrication in addition to bulk mechanical properties, it was an open question of how actin microfilament modification would affect the production of the boundary lubricant SZP from superficial zone chondrocytes. The data show that inhibition of actin dynamics by cytochalasin D and jasplakinolide significantly decreased TGFβ1-mediated SZP synthesis in superficial zone articular chondrocytes (Fig. 2A, B). These results are in alignment with a report by Klein et al.50 They demonstrated that in 3D alginate culture, the percentage of SZP producing superficial zone chondrocytes decreased by 80%. Induction of cell rounding through either actin microfilament modulation or 3D culture is not conducive toward SZP synthesis. In contrast, SZP secretion has been shown to increase over time in monolayer culture. Schmidt et al.51 reported that in monolayer cultures seeded at either 50,000 or 200,000 cells/cm2, SZP media accumulation increased over time with no significant differences between either seeding density at the end of the culture period. Whereas a rounded cell shape is conducive to a predominantly middle/deep zone phenotype (as defined by collagen II and proteoglycan synthesis), the native, flattened shape of surface zone chondrocytes52 supports the superficial zone phenotype (e.g., SZP secretion). The actin cytoskeleton and cell shape may not only be a determinant of the articular chondrocyte phenotype, but zone-specific traits as well.
An examination of the effects of cytoskeletal alteration on SZP synthesis would be incomplete without including microtubules (intermediate filaments were not studied due to the lack of specific inhibitors for modulating polymerization/depolymerization). Although microtubule dynamics do not play a role in the regulation of the chondrocyte phenotype,53 microtubules do play a key role in the elongation of fibroblasts and may affect SZP.54 Similar to actin, inhibition of microtubules by paclitaxel and colchicine reduced SZP media accumulation in the presence of TGFβ (Fig. 3A, B). Basal SZP secretion appeared unaffected by colchicine and was not significantly decreased by paclitaxel treatment. To reduce the possibility of these results arising from nonspecific effects of the reagents used, each cytoskeletal component was treated with two separate compounds that differed in their mechanism of action. One half of the pair of cytoskeletal modulators inhibited polymerization (cytochalasin and colchicine), while the other half affected depolymerization (jasplakinolide and paclitaxel).55,56
Cytoskeletal modulation could potentially reduce SZP media accumulation by disrupting cellular secretory mechanisms. However, Lohmander et al.34 demonstrated that cytochalasin B-induced decreases in GAG were primarily due to a reduction in synthesis, as secretion was not significantly affected. Whereas colchicine was shown to decrease both the synthesis and secretion of GAG, intracellular retention was increased by only 17%. And in the presence of colchicine, β-d-xyloside continued to elicit a greater than 100% increase in GAG synthesis and secretion compared with untreated controls. Lohmander et al. concluded that microtubules serve a “facilitatory rather than obligatory role” in the intracellular transport and secretion of GAG, and by extension proteoglycans.34,57 Based on these studies, modulation of the actin microfilaments and microtubules is likely to affect SZP accumulation largely through modulation of synthesis.
The cellular cytoskeleton is a pleiotropic structure as evidenced by the chondrocytes' dependence on actin architecture. To determine if there could be intermediaries linking cytoskeletal dynamics and SZP signaling, it was hypothesized that the Rho family of GTPases could modulate expression of TGFβ-induced SZP. The Rho family of GTPases consists of molecular switches that control actin microfilament assembly.21 The regulatory reach of Rho family GTPases also extends to microtubule assembly through effectors such as mDia and stathmin.58 In the presence of reagents that inhibit the activity of Rho family GTPases, basal SZP media accumulation decreased, but not to a significant degree with the exception of 20 μM ML141. TGFβ-stimulated SZP accumulation was reduced in a dose-dependent manner by the group of Rho family inhibitors (Figs. 3 and 4). However, TGFβ was still able to upregulate SZP compared with basal expression at every respective inhibitor dosage, and also relative to the untreated control. The only exceptions were the 20 μM ML141 and 150 μM NSC23766 groups, where the trend of greater TGFβ-induced SZP production relative to the basal control did not reach significance. These results suggest that Rho family GTPases may serve as a mediator in the signaling pathway linking SZP synthesis and cytoskeletal organization.
Interestingly, SZP accumulation was synergistically enhanced by a combination of TGFβ1 and LPA in a dose-dependent manner. These results suggest that it may be possible to enhance the boundary lubrication of tissue engineered constructs containing superficial zone chondrocytes by applying this phospholipid and morphogen combination. However, more work is needed to elucidate the mechanism of these effects due to the pleiotropic effects of LPA, such as stimulating cellular proliferation, migration, and survival.59–61 Lastly, morphogens such as TGFβ superfamily member BMP7 have been shown to interact with the cytoskeleton by increasing the expression of cytoskeletal proteins in articular chondrocytes in a context-dependent manner.31 Specifically, BMP7 upregulated cytoskeletal proteins talin and paxillin in monolayer, but not suspension cultures. It is thus important to determine whether the synergistic effects of LPA and TGFβ are similarly context dependent, and examine their effects in 3D and explant cultures.
Additional experiments in the future will need to be conducted to validate these results. The regulation of Rho family GTPases should be confirmed by studies employing siRNA knockdowns as well as dominant negative and dominant positive mutants of each GTPase. However, the stated aims of this investigation were satisfied as we sought to open a line of inquiry important for the advancement of articular cartilage tissue engineering. These data support the hypothesis that Rho family GTPases can serve as a possible mediator between the cytoskeleton and SZP signaling. Whereas cytoskeletal inhibition and cell rounding preserve a fundamentally middle/deep zone phenotype in terms of the expression of collagen II and proteoglycans, it inhibits the superficial zone phenotype as measured by SZP. Rho family GTPases modulate the activity of TGFβ in stimulating SZP, and thus, may perhaps be used to increase the production of SZP and enhance the boundary lubrication of engineered cartilage. In devising strategies and approaches, tissue engineers of articular cartilage need to consider the role cell shape and cytoskeletal dynamics play in the regulation of the articular chondrocyte phenotype, expression of SZP, and articular cartilage lubrication as a whole.
Acknowledgments
The authors thank Dr. Tom M. Schmid of Rush University for generously providing the antibody S6.79. This work is supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS R01 AR061496).
Disclosure Statement
No competing financial interests exist.
References
- 1.Benya P.D., Padilla S.R., and Nimni M.E.Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15,1313, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Brown P.D., and Benya P.D.Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin B-induced reexpression. J Cell Biol 106,171, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benya P.D., Brown P.D., and Padilla S.R.Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol 106,161, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanetti N.C., and Solursh M.Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol 99,115, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mow V.C., Ratcliffe A., and Poole A.R.Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13,67, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Neu C.P., Komvopoulos K., and Reddi A.H.The interface of functional biotribology and regenerative medicine in synovial joints. Tissue Eng Part B Rev 14,235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey D.J., Hu J.C., and Athanasiou K.A.Unlike bone, cartilage regeneration remains elusive. Science 338,917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natoli R.M., Skaalure S., Bijlani S., Chen K.X., Hu J., and Athanasiou K.A.Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 62,1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swann D.A., Hendren R.B., Radin E.L., Sotman S.L., and Duda E.A.The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum 24,22, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt T.A., and Sah R.L.Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage 15,35, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Jay G.D., Harris D.A., and Cha C.J.Boundary lubrication by lubricin is mediated by O-linked beta(1–3)Gal-GalNAc oligosaccharides. Glycoconj J 18,807, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ludwig T.E., McAllister J.R., Lun V., Wiley J.P., and Schmidt T.A.Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum 64,3963, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ikegawa S., Sano M., Koshizuka Y., and Nakamura Y.Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet 90,291, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Marcelino J., Carpten J.D., Suwairi W.M., Gutierrez O.M., Schwartz S., Robbins C., et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23,319, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Niikura T., and Reddi A.H.Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum 56,2312, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt T.A., Gastelum N.S., Han E.H., Nugent-Derfus G.E., Schumacher B.L., and Sah R.L.Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthritis Cartilage 16,90, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Aydelotte M.B., and Kuettner K.E.Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res 18,205, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Aydelotte M.B., Greenhill R.R., and Kuettner K.E.Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res 18,223, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Schumacher B.L., Block J.A., Schmid T.M., Aydelotte M.B., and Kuettner K.E.A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 311,144, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Burridge K., and Wennerberg K.Rho and Rac take center stage. Cell 116,167, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Jaffe A.B., and Hall A.Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21,247, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Bishop A.L., and Hall A.Rho GTPases and their effector proteins. Biochem J 348(Pt 2),241, 2000 [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens E.V., and Der C.J.Overview of rho GTPase history. In: van Golen K., ed. The Rho GTPases in Cancer. New York: Springer, 2010, pp. 3–27 [Google Scholar]
- 25.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S.Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6,483, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Haudenschild D.R., D'Lima D.D., and Lotz M.K.Dynamic compression of chondrocytes induces a Rho kinase-dependent reorganization of the actin cytoskeleton. Biorheology 45,219, 2008 [PubMed] [Google Scholar]
- 27.Arnsdorf E.J., Tummala P., Kwon R.Y., and Jacobs C.R.Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 122,546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haudenschild D.R., Chen J., Pang N., Lotz M.K., and D'Lima D.D.Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum 62,191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNary S.M., Athanasiou K.A., and Reddi A.H.Engineering lubrication in articular cartilage. Tissue Eng Part B Rev 18,88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neu C.P., Khalafi A., Komvopoulos K., Schmid T.M., and Reddi A.H.Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum 56,3706, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Vinall R.L., Lo S.H., and Reddi A.H.Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res 272,32, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Nofal G.A., and Knudson C.B.Latrunculin and cytochalasin decrease chondrocyte matrix retention. J Histochem Cytochem 50,1313, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Woods A., Wang G., Dupuis H., Shao Z., and Beier F.Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem 282,23500, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Lohmander S., Madsen K., and Hinek A.Secretion of proteoglycans by chondrocytes. Influence of colchicine, cytochalasin, B., and beta-D-xyloside. Arch Biochem Biophys 192,148, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Hui A., Kulkarni G.V., Hunter W.L., McCulloch C.A., and Cruz T.F.Paclitaxel selectively induces mitotic arrest and apoptosis in proliferating bovine synoviocytes. Arthritis Rheum 40,1073, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Woods A., Pala D., Kennedy L., McLean S., Rockel J.S., Wang G., et al. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthritis Cartilage 17,406, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Chen H.Y., Yang Y.M., Stevens B.M., and Noble M.Inhibition of redox/Fyn/c-Cbl pathway function by Cdc42 controls tumour initiation capacity and tamoxifen sensitivity in basal-like breast cancer cells. EMBO Mol Med 5,723, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman P., and Watt F.M.Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Exp Cell Res 178,199, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Woods A., Wang G., and Beier F.RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem 280,11626, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Su J.L., Schumacher B.L., Lindley K.M., Soloveychik V., Burkhart W., Triantafillou J.A., et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma 20,149, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Neu C.P., Reddi A.H., Komvopoulos K., Schmid T.M., and Di Cesare P.E.Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum 62,2680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y., Dickerson J.B., Guo F., Zheng J., and Zheng Y.Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101,7618, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surviladze Z., Waller A., Strouse J.J., Bologa C., Ursu O., Salas V., et al. A potent and selective inhibitor of Cdc42 GTPase. In: Probe Reports from the NIH Molecular Libraries Program, National Institutes of Health, Bethesda, MD, 2010 [Google Scholar]
- 44.Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389,990, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Moolenaar W.H., van Meeteren L.A., and Giepmans B.N.The ins and outs of lysophosphatidic acid signaling. Bioessays 26,870, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Solursh M., Linsenmayer T.F., and Jensen K.L.Chondrogenesis from single limb mesenchyme cells. Dev Biol 94,259, 1982 [DOI] [PubMed] [Google Scholar]
- 47.Bobick B.E., Chen F.H., Le A.M., and Tuan R.S.Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today 87,351, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Hsieh-Bonassera N.D., Wu I., Lin J.K., Schumacher B.L., Chen A.C., Masuda K., et al. Expansion and redifferentiation of chondrocytes from osteoarthritic cartilage: cells for human cartilage tissue engineering. Tissue Eng Part A 15,3513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoben G.M., and Athanasiou K.A.Use of staurosporine, an actin-modifying agent, to enhance fibrochondrocyte matrix gene expression and synthesis. Cell Tissue Res 334,469, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Klein T.J., Schumacher B.L., Blewis M.E., Schmidt T.A., Voegtline M.S., Thonar E.J., et al. Tailoring secretion of proteoglycan 4 (PRG4) in tissue-engineered cartilage. Tissue Eng 12,1429, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Schmidt T.A., Schumacher B.L., Klein T.J., Voegtline M.S., and Sah R.L.Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum 50,2849, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Langelier E., Suetterlin R., Hoemann C.D., Aebi U., and Buschmann M.D.The chondrocyte cytoskeleton in mature articular cartilage: structure and distribution of actin, tubulin, and vimentin filaments. J Histochem Cytochem 48,1307, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Vinall R.L., and Reddi A.H.The effect of BMP on the expression of cytoskeletal proteins and its potential relevance. J Bone Joint Surg Am 83-A(Suppl 1),S63, 2001 [PubMed] [Google Scholar]
- 54.Tomasek J.J., and Hay E.D.Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J Cell Biol 99,536, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng G.E., Wilson S.R., and Weiner O.D.A pharmacological cocktail for arresting actin dynamics in living cells. Mol Biol Cell 22,3986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan M.A., and Wilson L.Microtubules as a target for anticancer drugs. Nat Rev Cancer 4,253, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Lohmander S., Moskalewski S., Madsen K., Thyberg J., and Friberg U.Influence of colchicine on the synthesis and secretion of proteoglycans and collagen by fetal guinea pig chondrocytes. Exp Cell Res 99,333, 1976 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz M.Rho signalling at a glance. J Cell Sci 117,5457, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Itoh R., Miura S., Takimoto A., Kondo S., Sano H., and Hiraki Y.Stimulatory actions of lysophosphatidic acid on mouse ATDC5 chondroprogenitor cells. J Bone Miner Metab 28,659, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Choi J.W., Herr D.R., Noguchi K., Yung Y.C., Lee C.W., Mutoh T., et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50,157, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Kim M.K., Lee H.Y., Park K.S., Shin E.H., Jo S.H., Yun J., et al. Lysophosphatidic acid stimulates cell proliferation in rat chondrocytes. Biochem Pharmacol 70,1764, 2005 [DOI] [PubMed] [Google Scholar]