Abstract
We report the presence of drug resistance mutations in 7.4% (2/27) of the treatment-naive, HIV-1-infected children in Pune, India, who had HIV-1 RNA levels >1,000 copies/ml. Nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations, namely A98G and K103N, were observed in two separate sequences. In addition, three study sequences displayed three separate HIV-1 protease minor resistance mutations—L10I, A71T, and T74S. These preliminary data from Pune, India, reporting the presence of HIVDR in treatment-naive, HIV-1-infected children, reinforces the need to conduct large-scale studies to assess the prevalence of primary HIVDR in the pediatric population, which in turn will aid in planning protocols and policies related to antiretroviral treatment for the pediatric population.
The main cause of pediatric HIV-1 infection is mother-to-child transmission (MTCT) of HIV-1, which may occur in utero, during delivery, or with breastfeeding.1 In 2011, about 3.4 million children were HIV infected worldwide, with more than 90% infected through mother-to-child transmission.2 The presence of the transmitted/primary drug resistance has important clinical and public health implications. It can reduce the efficacy of first-line antiretroviral treatment (ART) primarily affecting nonnucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside reverse transcriptase inhibitors (NRTIs).
While there are Indian studies describing the prevalence of (HIVDR) mutations in adults,3–7 the prevalence of HIV drug resistance in the pediatric HIV-1-infected Indian population is largely unknown. This study describes the prevalence of mutations associated with HIVDR by the genotypic method in treatment-naive, HIV-1-infected children in Pune, India.
The study population was composed of 50 HIV-1-infected children attending the ART center at a tertiary care center from May 2009 to November 2010. All these participants were ART naive. A detailed clinical history was recorded, and informed consent was obtained from the parent(s)/legal guardian of each participant before sample collection. The demographic and epidemiologic data were recorded for each patient after institutional ethical board (IEC) clearance.
Five milliliters of blood was collected from each study participant in a K3EDTA vial. While the CD4+ T lymphocytes count was carried out on the same day, multiple aliquots of plasma were stored at −70°C for HIV-1 RNA level estimation of and HIVDR genotyping.
The CD4+ T lymphocyte count of each participant was estimated using fluorescence activated cell sorter (FACS) count (Becton Dickinson, Biosciences Immunocytometry Systems, San Diego, CA). Absolute CD4+T lymphocyte count was calculated for the study participants whose age was equal to or more than 5 years, while CD4 percentage (%) was calculated for those whose age was less than 5 years.
Estimation of HIV-1 RNA levels was carried out by using Roche Amplicor HIV-1 Monitor Test version 1.5 (Roche Diagnostics, Branchburg, NJ) according to the manufacturer's instructions.
HIV-1 RNA extraction from plasma was done with the NucliSENS easyMAG (bioMérieux, Durham, NC) total Nucleic Acid Extraction Machine. The HIV-1 pol gene region encoding protease (PR) and the 5′ end of reverse transcriptase (RT) was amplified by reverse transcriptase polymerase chain reaction (PCR) with primer sets Pol 2021F (5′-AAGGCTGTTGGAAATGTGG-3′) and Pol4521R (5′-AGCTGTTTCTTGTCCTGTTTCTGC-3′). RT-PCR using the RobusT I RT-PCR kit (Finnzymes Oy, Finland), followed by nested PCR with the two inner primer sets mentioned below, were used to amplify entire protease (1–99 amino acids) and complete reverse transcriptase (1–560 amino acids), namely Pol2135F (5′-TTCAGAGCAAGACCAGAGCCAACAGC-3′) and Pol4470R (5′-CTGGCTACATGGACTGCTACC-3′).
The purified PCR amplicons were directly sequenced on an ABI PRISM 3100 genetic analyzer system (Applied Biosystems, Foster City, CA) using 10 sequencing primers, namely, Pol2135F (5′-TTCAGAGCAAGACCAGAGCCAACAGC-3′), Pol2493F (5′-CCTGTCAACATAATTGGAAG-3′), Pol3012F (5′-GGATCACCAGCAATATTC-3′), Pol3403F(5′-GGGCCAAAGTACTAACAG-3′), Pol3768F (5′-GCCAATAGTCTGTCCACCATG-3′), Pol2557R (5′-GGTACAGTTTCAATGGGAC-3′), Pol3117R (5′-CCCTATTTCTAAGTCAGATCC-3′), Pol3620R (5′-TAGTGTGGGCAGTCCTC-3′), Pol3999R (5′-CCTGAATCTTGCAAAGCTAG-3′), and Pol4381R (5′-CCTGGACTACAGTCTACTTGTCCAT3′).
The sequences for each sample were bidirectionally aligned using SeqScape v2.0 software (Applied Biosystems) with HXB-2 (K03455) as a reference HIV-1 sequence, assembled and edited. The nucleotide sequences were exported in fasta format and analyzed online using the Stanford University HIV Drug Resistance Database HIVdb program (Version 6.0.11) (available at http://hivdb.stanford.edu). The study sequences along with reference sequences alignment were done using Mega 5.0 software.8 The phylogenetic tree was generated using PhyML (www.hiv.lanl.gov/content/sequence/PHYML/interface.html). These included consensus sequences for the M group, A1, A2, B, C, D, F1, F2, G, H, K, Indian subtype C sequences (AF067155), non-Indian type from Botswana (AF110980, AF110967), Kenya (AF061640, AF061641, AF004885), Senegal (AF069670, AF061642, AF082395, AF082394), Zambia (AY772699), Brazil (U52953, AF005494), France (K03455, AJ249238), subtypeA1 from Kenya, Ethiopia (U46016), the United States (U21135, U63632, M17451, AY1331295, DQ853463), Uganda (U88824, U51190, AB253429, AF484509, M82320), and Thailand (AY17339511).
Out of 50 HIV-1-infected pediatric study participants, there were 28 whose HIV-1 RNA levels were >1,000 copies/ml. Plasma specimens of these 28 participants were processed for the sequencing of HIV-1 proteases and reverse transcriptase genes. The mean age of these 28 study participants was 9.026 years (range 2.3 years to 13 years). The male-to-female ratio among these study participants was 1:1.
The median CD4 T lymphocyte count in 26 patients over age 5 years was 232 cells/μl (range 8–1,086), while the CD4 T lymphocyte percentages in the remaining two patients under age 5 were 12% and 23%, respectively. The median HIV-1 RNA level was 156,822 copies/ml (range 1,262–1,816,524 copies/ml).
The PCR amplification of the protease and reverse transcriptase region of HIV-1 was successful for 27 out of 28 study samples. The HIVDR mutations were observed in 18.5% (5/27) of the study participants. The protease inhibitor-associated minor mutations observed were L10I, A71T, and T74S, whereas the NNRTI-associated resistance mutations included A98G and K103N. There were no NRTI-associated resistance mutations detected in the study sequences (Table 1).
Table 1.
Mutations at Positions Associated with Protease Inhibitor, Nucleoside Reverse Transcriptase Inhibitor, and Nonnucleoside Reverse Transcriptase Inhibitor Drug Resistance Observed in 27 Antiretroviral Treatment-Naive, HIV-1-Infected Children in Pune, India
| Subtype | Mutations associated with HIV-1 drug resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sr. No. | Sequence ID | Sample date (date/month/year) | Patient age (in years) | CD4+T lymphocyte count (cells/μl) | HIV-1 RNA level (copies/ml) | PR | RT | PI Major | PI Minor | NRTI | NNRTI |
| 1 | PAED AFMC-1 | 24/7/2009 | 11 | 31 | 197,685 | C | C | None | None | None | None |
| 2 | PAED AFMC-4 | 30/7/2009 | 12 | 155 | 46,180 | C | C | None | None | None | None |
| 3 | PAED AFMC-7 | 17/9/2009 | 7 | 1,086 | 9,321 | C | C | None | None | None | None |
| 4 | PAED AFMC-10 | 20/10/2009 | 5 | 12%a | 1,262 | C | C | None | None | None | None |
| 5 | PAED AFMC-11 | 3/11/2009 | 2.3 | 269 | 109,402 | C | C | None | None | None | A98G |
| 6 | PAED AFMC-12 | 12/11/2009 | 7 | 586 | 1,600 | C | C | None | None | None | None |
| 7 | PAED AFMC-14 | 20/11/2009 | 12 | 312 | 3,222 | C | C | None | None | None | None |
| 8 | PAED AFMC-15 | 20/11/2009 | 10 | 537 | 41,768 | C | C | None | None | None | None |
| 9 | PAED AFMC-16 | 20/11/2009 | 8 | 324 | 47,087 | C | C | None | None | None | None |
| 10 | PAED AFMC-18 | 20/11/2009 | 10 | 226 | 17,412 | C | C | None | None | None | None |
| 11 | PAED AFMC-19 | 22/11/2009 | 13 | 118 | 268,548 | A1 | C | None | None | None | None |
| 12 | PAED AFMC-21 | 22/11/2009 | 4 | 1,073 | 2,109 | C | C | None | T74S | None | None |
| 13 | PAED AFMC-24 | 9/2/2010 | 7 | 273 | 2,135 | C | C | None | None | None | None |
| 14 | PAED AFMC-26 | 19/2/2010 | 3 | 23%a | 4,194 | C | C | None | None | None | None |
| 15 | PAED AFMC-28 | 19/2/2010 | 7 | 1,017 | 1,675 | C | C | None | None | None | None |
| 16 | PAED AFMC-31 | 9/4/2010 | 9 | 247 | 418,643 | C | C | None | None | None | None |
| 17 | PAED AFMC-32 | 30/4/2010 | 12 | 363 | 77,025 | A1 | A1 | None | L10I | None | None |
| 18 | PAED AFMC-34 | 4/5/2010 | 12 | 38 | 138,883 | C | C | None | None | None | None |
| 19 | PAED AFMC-36 | 7/5/2010 | 11 | 31 | 353,481 | C | C | None | None | None | None |
| 20 | PAED AFMC-37 | 7/5/2010 | 11 | 71 | 450,081 | C | C | None | None | None | None |
| 21 | PAED AFMC-38 | 7/5/2010 | 8 | 8 | 1,816,524 | C | C | None | None | None | K103N |
| 22 | PAED AFMC-39 | 14/5/2010 | 11 | 30 | 24,222 | C | C | None | None | None | None |
| 23 | PAED AFMC-44 | 31/5/2010 | 7 | 90 | 105,822 | C | C | None | None | None | None |
| 24 | PAED AFMC-46 | 4/6/2010 | 12 | 238 | 24,187 | C | C | None | None | None | None |
| 25 | PAED AFMC-47 | 8/6/2010 | 11 | 128 | 83,651 | C | C | None | A71T | None | None |
| 26 | PAED AFMC-49 | 14/6/2010 | 12 | 589 | 128,588 | C | C | None | None | None | None |
| 27 | PAED AFMC-50 | 18/6/2010 | 12 | 216 | 14,885 | C | C | None | None | None | None |
Age of study participant <5 years.
PR, protease; RT, reverse transcriptase; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
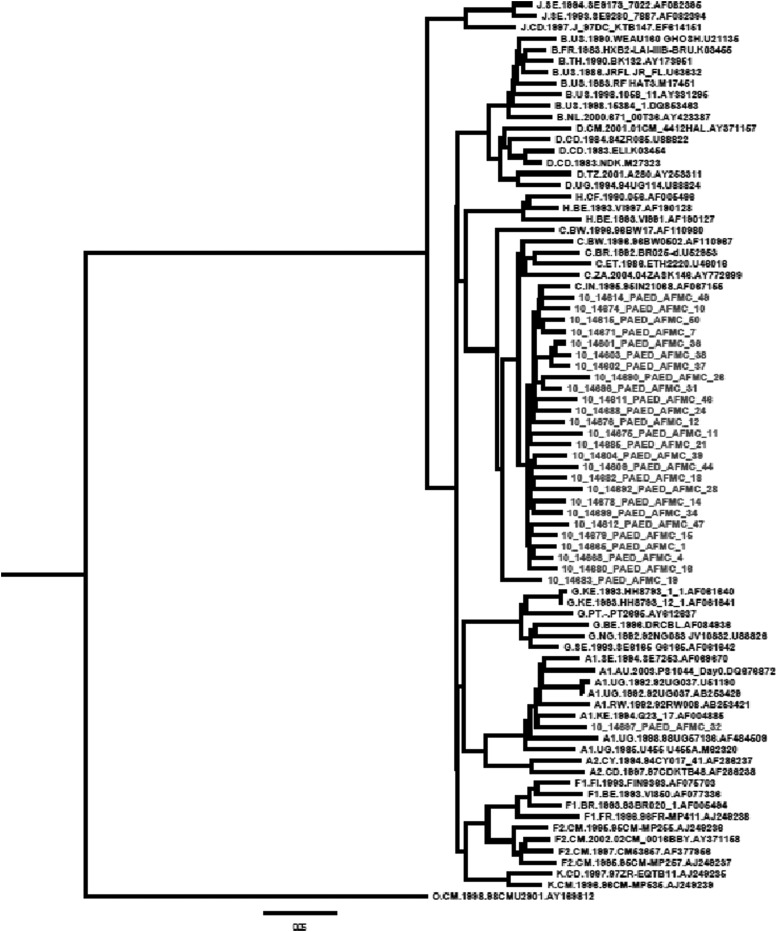
Phylogenetic analysis showed that 26 sequences clustered with HIV-1 subtype C sequences while one clustered with HIV-1 subtype A1, when rooted with the M group.
The 26 HIV-1 study subtype C pol gene sequences formed a separate subcluster along with the Indian subtype C reference sequence (AF067155) and segregated away from subtype C sequences from other countries, namely Botswana, Kenya, Senegal, Zambia, Brazil, France, Ethiopia, the United States, Uganda, and Thailand as shown in Fig. 1.
FIG. 1.
Phylogenetic tree constructed by using Mega 5.0 software, using the General Time Reversible (GTR) model and rooted with the M group consensus sequence. The sequences that were included were consensus sequences for the M group, A1, A2, B, C, D, AE, F1, F2, G, H, and K, and the study sequences.
Demonstration of HIVDR due to mutations in the virus in the pediatric population requires serial specimens from the neonate at birth and from mothers before starting treatment, at time of delivery, and thereafter. Thus, identification of transmission of resistant strains provides a timely and effective aid in clinical management.9
Reporting of primary HIVDR in up to 20% of HIV-infected adults in the United States and Europe10,11 has led to recommendations that all treatment-naive adults should undergo resistance testing at the time of entry for care.12 Similar recommendations to detect primary resistance have been made for all treatment-naive children before initiation of therapy.13
In infected infants in the United States Persaud et al.14 have demonstrated rates of primary resistance to at least one ARV drug that range between 19% and 24%, which are mostly associated with NNRTI resistance mutations, while Chakraborty et al.15 have reported 6.8% primary resistance among children in the United Kingdom. Pedroso et al.16 in a Brazilian study reported primary resistance in 27% of perinatally infected children, while a prospective cohort study conducted by Soto-Ramirez et al.17 in Brazil, Argentina, and Mexico (NISDI Pediatric Study) demonstrated primary HIVDR in 8.7% of ARV-naive children.
In our study, HIVDR mutations were observed in 18.5% of the HIV-1-infected treatment-naive pediatric population with HIV-1RNA levels >1,000 copies/ml. These included important HIVDR NNRTI-associated mutations (A98G, K103N) and protease minor mutations (L10I, A71T, T74S). In comparison, the prevalence of primary resistance in our Indian pediatric population appears to be similar to the figures reported from the United States and Brazil.14,16
To date the available study data on primary HIVDR mutation studies in India have been carried out mostly in adolescents and adults. Deshpande et al.3 reported a prevalence of 1.6% in Mumbai, while a study from South India conducted by Balakrishnan et al.4 reported mutation rates of 100%, 6%, and 14% at protease, NRTI, and NNRTI resistance positions, respectively. Sen et al.6 demonstrated that there were no reverse transcriptase inhibitor-associated or major PR resistance mutations seen in ARV-naive patients from Pune, while treatment-experienced participants with evidence of virologic failure revealed 81.81% with one or more HIVDR mutations in individuals. Subsequently, Lall et al.7 reported HIVDR mutations in 10% of treatment-naive HIV-1-infected individuals.
There are few Indian studies reporting HIVDR in the pediatric population, with most reporting HIVDR mutations in infants exposed to nevirapine (NVP). Kurle et al.18 demonstrated the presence of 10.5% and 46.15% NNRTI resistance mutations in infant samples collected after 48 h and 2 months postpartum, respectively. Subsequently, Moorthy et al.19 reported a higher prevalence of NVP resistance than those who received single dose NVP, by both standard population sequencing (92% of 12 vs. 38% of 29) and low-frequency clonal analysis (92% of 12 vs. 59% of 29). In this study, the Y181C variant was found to be predominant among infants diagnosed in the first 6 weeks of life, compared to Y188C/H during late breast-milk transmission.
Soundararajan et al. 20 studied HIVDR in 48 ARV-naive pediatric study participants and observed significant polymorphisms, but no major drug resistance mutations, in both the RT and protease genes of the study sequences. Although there is adequate availability of ARVs in India, including Pune where the present study was conducted, there is a definite need to generate greater awareness among the HIV-infected population about drug resistance, with special reference to the pediatric population.
Our study presents preliminary data highlighting the prevalence of primary HIVDR in treatment-naive HIV-1-infected children in Pune, India, thus highlighting the importance of resistance assays in this pediatric age group at the time of diagnosis, prior to initiation of ART. Further large-scale HIVDR studies in Indian HIV-1-infected children will contribute immensely to formulating specific ARV management guidelines.
Sequence Data
The PR and RT nucleotide sequences of the study isolates were submitted and are available under the following accession number in GenBank: JN639188, JN639189, JN639190, JN639191, JN639192, JN639193, JN639194, JN639195, JN639196, JN639197, JN639198, JN639199, JN639200, JN639201, JN639202, JN639203, JN639204, JN639205, JN639206, JN639207, JN639208, JN639209, JN639210, JN639211, JN639212, JN639213, and JN639214.
Acknowledgments
We acknowledge the National AIDS Research Organization (Indian Council of Medical Research), Pune, India for conducting the HIV-1 drug resistance genotyping. We are grateful to the HIV-1-infected patients who participated in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Becquet R, Ekouevi DK, Arrive E, Stringer JS, Meda N, Chaix ML, et al. : Universal antiretroviral therapy for pregnant and breast-feeding HIV-1-infected women towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis 2009;49(12):1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS/WHO: Paediatric HIV surveillance among infants and children less than 18 years of age World Health Organization 2013. Available at http://apps.who.int/iris/bitstream/10665/85513/1/9789241505833_eng.pdf Accessed on August22, 2013 [PubMed]
- 3.Deshpande A, Recordon P, Deshmukh R, Faure M, Jauvin V, Garrigue I, et al. : Molecular characterization of HIV type 1 isolates from unrelated patients of Mumbai (Bombay) India: A detection of rare resistance mutations. AIDS Res Hum Retroviruses 2004;20:1032–1035 [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan P, Kumaraswamy N, Kantor R, Solomon S, Vidya S, Mayer KH, et al. : HIV type 1 genotypic variation in an antiretroviral treatment-naive population in southern India. AIDS Res Hum Retroviruses 2005;21(4):301–305 [DOI] [PubMed] [Google Scholar]
- 5.Sen S, Tripathy SP, Chimanpure VM, Patil AA, Bagul RD, and Paranjape RS: Human immunodeficiency virus type 1 drug resistance mutations in peripheral blood mononuclear cell proviral DNA among antiretroviral treatment naïve and treatment experienced patients from Pune, India. AIDS Res Hum Retroviruses 2007;23(4):489–497 [DOI] [PubMed] [Google Scholar]
- 6.Sen S, Tripathy SP, Patil AA, Chimanpure VM, and Paranjape RS: High prevalence of human immunodeficiency virus type 1 drug resistance mutations in antiretroviral treatment experienced patients from Pune, India. AIDS Res Hum Retroviruses 2007;23(10):1303–1308 [DOI] [PubMed] [Google Scholar]
- 7.Lall M, Gupta RM, Sen S, Kapila K, Tripathi SP, and Paranjape RS: Profile of primary resistance in HIV-1 infected treatment naïve individuals from Western India. AIDS Res Hum Retroviruses 2008;24(7):987–990 [DOI] [PubMed] [Google Scholar]
- 8.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S: MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira FG, Pinto JA, Kakehasi FM, Cleto S, Tupinambás U, Aleixo AW, et al. : Prevalence of primary drug resistance-associated mutations among HIV Type 1 vertically infected children in Belo Horizonte, Brazil. AIDS Res Hum Retroviruses 2010;26(2):229–232 [DOI] [PubMed] [Google Scholar]
- 10.Cane P, Chrystie I, Dunn D, Evans B, Geretti AM, Green H, et al. : Time trends in primary resistance to HIV drugs in the United Kingdom: Multicentre observational study. BMJ 2005;331:7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wensing AM, van de Vijver DA, Angarano G, Asjö B, Balotta C, Boeri E, et al. : Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: Implications for clinical management. J Infect Dis 2005;192(6):958–966 [DOI] [PubMed] [Google Scholar]
- 12.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed on August16, 2013
- 13.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf Accessed on August16, 2013
- 14.Persaud D, Palumbo P, Ziemniak V, Chen J, Ray SC, Hughes M, et al. : Early archiving and predominance of non nucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis 2007;195(10):1402–1410 [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty R, Smith CJ, Dunn D, Green H, Duong T, Doerholt K, et al. : HIV-1 drug resistance in HIV-1-infected children in the United Kingdom from 1998 to 2004. Pediatr Infect Dis J 2008;27(5):457–459 [DOI] [PubMed] [Google Scholar]
- 16.Pedroso C, Queiroz ATL, Alcantara LC, Drexler JF, Diaz RS, Weyll N, et al. : High prevalence of primary antiretroviral resistance among HIV-1-infected adults and children in Bahia, a northeast state of Brazil. J Acquir Immune Defic Syndr 2007;45(2):251–253 [DOI] [PubMed] [Google Scholar]
- 17.Soto-Ramirez LE, Rodriguez-Diaz R, Harris DR, and Hazra R: HIV drug resistance-associated mutations in antiretroviral naïve HIV-1-infected Latin American children. Adv Virol 2010;2010:407476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurle S, Gangakhedkar RR, Sen S, Hayatnagarkar SS, Tripathy SP, and Paranjape RS: Emergence of NNRTI drug resistance mutations after single-dose nevirapine exposure in HIV Type 1 subtype C-infected infants in India. AIDS Res Hum Retroviruses 2007:23(5):682–685 [DOI] [PubMed] [Google Scholar]
- 19.Moorthy A, Gupta A, Bhosale R, Tripathy S, Sastry J, Kulkarni S, et al. : Nevirapine resistance and breast-milk HIV transmission: Effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One 2009;4(1):e4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soundararajan L, Karunaianandham R, Jauvin V, Schrive MH, Ramachandran R, Narayanan PR, et al. : Characterization of HIV-1 isolates from antiretroviral drug-naïve children in southern India. AIDS Res Hum Retroviruses 2007;23(9):1119–1126 [DOI] [PubMed] [Google Scholar]