Abstract
Growth factor delivery systems incorporating chondroprogenitor cells are an attractive potential treatment option for damaged cartilage. The rapid isolation, processing, and implantation of therapeutically relevant numbers of autologous chondroprogenitor cells, all performed “in-theatre” during a single surgical procedure, would significantly accelerate the clinical translation of such tissue engineered implants by avoiding the time, financial and regulatory challenges associated with in vitro cell expansion, and differentiation. The first objective of this study was to explore if rapid adherence to a specific substrate could be used as a simple means to quickly identify a subpopulation of chondroprogenitor cells from freshly digested infrapatellar fat pad (IFP) tissue. Adhesion of cells to tissue culture plastic within 30 min was examined as a mechanism of isolating subpopulations of cells from the freshly digested IFP. CD90, a cell surface marker associated with cell adhesion, was found to be more highly expressed in rapidly adhering cells (termed “RA” cells) compared to those that did not adhere (termed “NA” cells) in this timeframe. The NA subpopulation contained a lower number of colony forming cells, but overall had a greater chondrogenic potential but a diminished osteogenic potential compared to the RA subpopulation and unmanipulated freshly isolated (FI) control cells. When cultured in agarose hydrogels, NA cells proliferated faster than RA cells, accumulating significantly higher amounts of total sGAG and collagen. Finally, we sought to determine if cartilage tissue could be engineered by seeding such FI cells into a transforming growth factor-β3 delivery hydrogel. In such a system, both RA and NA cell populations demonstrated an ability to proliferate and produced a matrix rich in sGAG (∼2% w/w) that stained positively for type II collagen; however, the tissues were comparable to that generated using FI cells. Therefore, while the results of these in vitro studies do not provide strong evidence to support the use of selective substrate adhesion as a means to isolate chondroprogenitor cells, the findings demonstrate the potential of combining a growth factor delivery hydrogel and FI IFP cells as a single stage therapy for cartilage defect repair.
Introduction
Traditional cartilage tissue engineering strategies involve the isolation and expansion of cells (either primary chondrocytes, chondroprogenitors, or stem cells) followed by their subsequent culture over several week or months in vitro in a three-dimensional scaffold or hydrogel before the implantation of this construct into a defect site.1 This approach has several limitations that are impeding its widespread clinical adoption, including the high cost and time associated with expanding cells and engineering such a tissue, as well as the requirement for two surgical procedures—one to isolate the cells and the second to implant the engineered tissue. A theoretically more appealing approach would be to combine freshly isolated (FI) stromal cells with a chondroinductive scaffold as a putative one-step surgical procedure for cartilage repair.2,3 FI cells from the infrapatellar fat pad (IFP) of the knee have been shown to be highly chondrogenic with a strong potential for cartilage tissue formation.3 To translate such findings into a single-stage therapy for cartilage repair will most likely require a number of key scientific and technical hurdles to be overcome. These include the development of a relatively simple, fast, and affordable method to isolate a sufficiently large number of chondroprogenitors from the IFP, a scaffold or hydrogel to support these cells in vivo, and potentially a means to control the delivery of stimulatory factors (e.g., one or more chondrogenic growth factors) to the defect site to promote chondrogenesis of the implanted cells.
A number of approaches have been described that can potentially be used to isolate a more homogenous cell population from FI stromal cells. Magnetic bead cell separation is one approach to isolating different cell populations;4–7 however, this method can become expensive and is technically challenging. Furthermore, it is still unclear what specific cell surface markers, if any, can be used to define chondroprogenitors within a heterogeneous cell population. A simpler method of cell separation is via centrifugation and plastic adhesion. Centrifugation allows cells of different densities to be separated (e.g., adipocytes and the stromal vascular fraction in digested adipose tissue). Differential cell adhesion to tissue culture plastic has also been used to isolate mesenchymal progenitor cells from umbilical core blood8 and bone marrow,9,10 and fibroblast precursors from several tissues.11 Cell adhesion to a scaffold has also been used to separate adipose stromal cells from other cell types found in digested adipose tissue.2 Cell attachment to different scaffolds was reported to be rapid (within as little as 10 min), with the majority of the putative adipose derived stem cells adhering to the scaffold within 60 min of seeding.2
The objective of this study was to first exploit the adhesive behaviour of multipotent stromal cells to tissue culture plastic as a mechanism to rapidly isolate progenitor cells from digested IFP tissue, and to then combine this cell source with a transforming growth factor-β3 (TGF-β3) delivery hydrogel as a putative single stage therapy for cartilage regeneration. Incorporation of growth factor releasing microspheres into a construct seeded with culture expanded mesenchymal stem/progenitor cells (MSCs) has previously been shown to facilitate cartilage tissue formation.12–16 Two subpopulations of FI IFP cells, those that adhered to cell culture plastic within 30 min of collagenase digestion (termed rapidly adhering or “RA” cells) and those that did not adhere in that time (termed nonadherent or “NA” cells) were first characterized and their differentiation capacity was compared using traditional assays. Unmanipulated FI control cells that were not selected based on adherence to tissue culture plastic were also analyzed. The second phase of the study examined the capacity of the FI cell subpopulations to generate cartilage tissue after encapsulation into a growth factor delivery-hydrogel. Demonstrating that such a construct can generate hyaline-like cartilage will provide compelling support for the continued preclinical evaluation of novel single-stage or “in-theatre” cell-based therapies as an alternative to microfracture, autologous cell implantation, and its variants for articular cartilage regeneration.
Materials and Methods
All chemicals used were purchased from Sigma-Aldrich unless otherwise stated.
Cell isolation and culture
Cells were isolated from porcine IFP tissue removed from 4 month old pigs as previously described.15 These cells have been previously identified as multipotent stem/progenitor cells,17 capable of differentiating down chondrogenic, osteogenic, and adipogenic pathways. IFPs were harvested under sterile conditions and digested in 750 U/mL collagenase at 37°C for 4 h under constant rotation. Once digested, the solution was sieved using a 100 μm cell strainer and centrifuged at 650 g. The floating fraction, primarily consisting of adipocytes and digested extracellular matrix was aspirated and discarded. The cell pellet was resuspended and sieved using a 40 μm cell strainer. The number of live cells were counted using trypan blue.
The FI IFP stromal cells were placed in T-175 culture flasks (Nunc) with 5 mL of culture media at a seeding density of 3 million cells per mL, and then placed in an incubator at 37°C, 21% O2, and 5% CO2. After 30 min, nonadherent cells were collected from the flask, centrifuged and recounted. Those cells which did adhere to the plastic were trypinized, collected, and recounted. Both the cells which rapidly adhered in 30 min (termed “RA” cells) and those which did not adhere after 30 min (termed “NA” cells) were used in subsequent experiments. FI cells that were not placed onto culture plastic were used as a control (termed “FI” cells).
Flow cytometry
RA, NA, and FI cells were centrifuged and washed with flow cytometry staining buffer (eBioscience). The cells were then incubated with fluorescently labeled primary antibodies (all from eBioscience) following the manufacturers recommendations. 50 μL of the antibodies in buffer solution was added to 50 μL of cell suspension and incubated for 30 min in the dark at 4°C. The cells were washed and centrifuged twice to remove unbound antibody. Cells were stained with propidium iodide to eliminate dead cells from our analysis. Compensation controls using compensation beads (eBioscience) were performed in parallel. All the samples were analyzed using a LSRFortessa flow cytometer (BD Biosciences). CD14, CD44, CD45, and CD90 were examined using this technique. Data were correlated using FlowJo software (Tree Star). This experiment was repeated using cells taken from a second pig to confirm our initial findings.
Colony forming unit fibroblasts
FI RA and NA cells (as well as control FI cells) were separately plated at a seeding density of 10 cells per cm2 onto 58 cm2 petri dishes and cultured in a standard expansion media consisting of Dulbecco's modified Eagle's medium (DMEM)+GlutaMAX supplemented with 10% (v/v) fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin (all Gibco). The media were changed every 2 to 3 days. After 10 days the media were removed, the dishes washed with phosphate-buffered saline (PBS) and the cells fixed in 2% (w/v) paraformaldehyde (PFA). The colonies were then stained using 1% (w/v) crystal violet and washed with water. Images of the colonies were recorded using a digital camera. This experiment was repeated in triplicate using cells derived from different pigs.
Osteogenic and chondrogenic potential of isolated cell populations
To determine their osteogenic potential, cells were seeded onto six-well plates at a density of 5×104 cells per well and maintained in expansion media until they reached 80% confluence. The cells were then cultured in a chemically defined osteogenic media consisting of expansion media additionally supplemented with 100 nM dexamethasone, 10 mM β-glycerol phosphate, and 0.05 mM ascorbic acid for 21 days. Cells cultured in regular expansion media were used as a negative control. After 21 days, cells were fixed using ethanol and stained with alizarin red solution for 2 min. The wells were then washed with distilled water until the negative control wells appeared clear. Images of the well were taken using a digital camera. The area stained red in each well was quantified using ImageJ.
To determine the chondrogenic potential of FI, RA, and NA cells, pellets were formed via centrifuge, each containing 2.5×105 FI cells per pellet. The pellets were cultured in a chemically defined chondrogenic media consisting of DMEM+GlutaMAX supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 1.5 mg/mL bovine serum albumin, 1× insulin-transferrin-selenium, 100 nM dexamethasone, 50 μg/mL ascorbic acid, 4.7 μg/mL linoleic acid and 10 ng/mL transforming growth factor β3 (TGF-β3; Prospec). After 21 days, the pellets were either fixed with 4% (w/v) PFA and wax embedded for histological analysis or were weighed and frozen at −80°C for subsequent biochemical analysis. The biochemical analysis was repeated in triplicate using cells derived from three different pigs.
Fabrication of agarose hydrogels
Agarose hydrogels were fabricated using 2% (w/v) low gelling temperature agarose solution in PBS. This solution was autoclaved and allowed to gel. The agarose was then reheated to 80°C to allow it to melt, and then cooled to 37°C. The molten agarose solution was mixed with FI RA, NA, or FI cells and 60 μL of the solution was pipetted into cylindrical silicone moulds, 5 mm in diameter. A cell seeding density of 5×106 cells per mL was used. The agarose was allowed to set at room temperature for 20 min. Hydrogels were removed from the agarose moulds and cultured in 2.5 mL chemically defined prochondrogenic media for 28 days in a low oxygen incubator (37°C, 5% O2, 5% CO2). A low oxygen incubator was used since it provided an environment more similar to the in vivo conditions expected within a chondral defect. Such hypoxic conditions have previously been shown to enhance chondrogenesis.18–21
Fabrication of growth factor releasing hydrogels
Gelatin microspheres were fabricated in a similar manner to that described previously.15 Briefly, an 11% (w/v) gelatin solution in deionized water was added dropwise to preheated olive oil (45°C) under constant stirring. After 10 min, the solution was cooled on ice, while still undergoing stirring. After 30 min, prechilled acetone was added and this was stirred for a further 60 min. The microsphere solution was poured through a 50 μm sieve allowing the microspheres to be isolated from the solution. The microspheres were washed with acetone and crosslinked in 0.1% (v/v) glutaraldhyde solution with Tween 80 for 1 h. The microspheres were washed several times with 50 mM glycine solution, and then left overnight in glycine before being washed with deionized water. Microspheres were then sieved to control the sphere size (50–70 μm). The microspheres were freeze-dried at −1°C overnight and sterilized using dehydrothermal treatment at 110°C under vacuum of 50 mbar for 24 h. Microspheres were then loaded with a volume of TGF-β3 which was less than the volume required to fully swell the microspheres. Microspheres were stored at 4°C overnight. 200 ng of TGF-β3 was loaded into each hydrogel.
Growth factor releasing hydrogels were fabricated by mixing the TGF-β3 loaded microspheres with 2% (w/v) agarose and RA, NA, or FI cells before gelation. These hydrogels were cultured at low oxygen for 28 days in chemically defined media described previously (without TGF-β3 since this was already present in the microspheres) with the addition of 5% (v/v) FCS.
Histology and immunohistochemistry
Hydrogels were fixed with 4% (w/v) PFA, dehydrated using ethanol and xylene, and embedded in paraffin wax. 5 μm sections were cut using a microtome and attached to glass slides. Alcian blue and 0.1% (w/v) nuclear fast red solution were used to stain for sulphated glycosaminoglycans (sGAGs) and nuclei, respectively. Picrosirius red solution was used to stain for collagen. For collagen I and II staining, sections were treated with hydrogen peroxide solution, washed with PBS, and treated with chondroitinase ABC in a humidified chamber at 37°C. Sections were then washed again and blocked using goat serum. Sections were incubated with a collagen type I or II mouse monoclonal antibody (Abcam), washed with PBS, and incubated with a biotin conjugated anti-mouse secondary antibody. After washing, the sections were treated using Vectastain ABC reagent (Vectstain ABC kit; Vector Laboratories) followed by treatment with DAB peroxide substrate (Vector Laboratories), which was stained brown in the presence of collagen I or II. Cartilage and ligament slides were used to verify the specificity of the collagen staining.
Biochemical assays
Hydrogels were digested for 18 h at 60°C in papain (125 μg/mL) in 0.1 M sodium phosphate buffer, 5 mM Na2EDTA, 10 mM L-cystine, pH 6.5. DNA was measured using the Hoechst 33258 DNA quantitation kit (DNA-QF) following the manufacturers guidelines. Total sGAG content was measured using a DMMB assay (Biocolor Ltd) following the manufacturer's instructions. Collagen content was measured using a hydroxyproline assay as previously described22 where a hydroxyproline to collagen ratio of 1:7.69 was used.23
Statistical analysis
Data were analyzed using MINITAB software (Minitab Ltd). ANOVA with a 90% Tukey test was used to compare RA, NA, and IF data. Significance was accepted at a level of p<0.05.
Results
IFP derived cells that rapidly adhere to tissue culture plastic express a specific cell surface profile
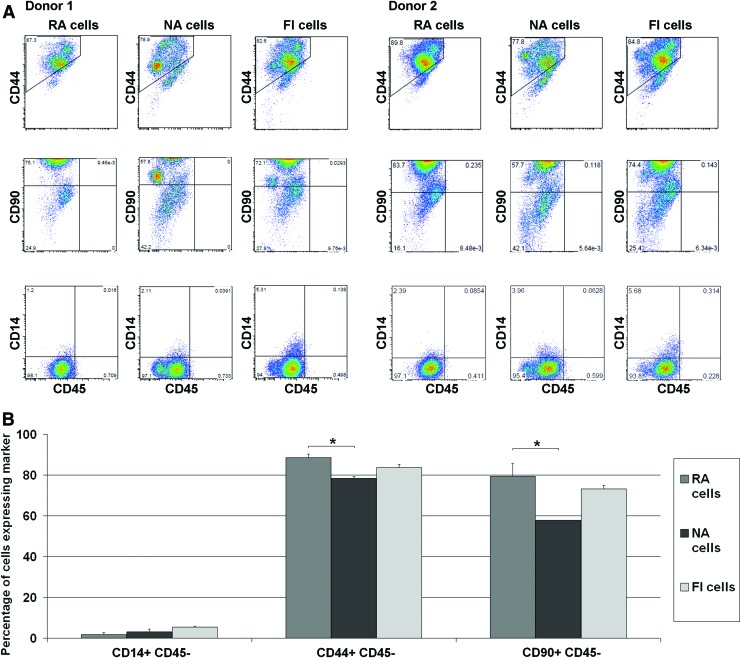
73% (±4%) of FI IFP derived stromal cells adhered to tissue culture plastic within 30 min, and this population were termed RA cells. The cells that did not adhere in this timeframe were termed NA cells. RA, NA, and control unmanipulated FI cells were analyzed using flow cytometry (Fig. 1). All three groups had a large population of CD44 positive cells (88.5% of RA cells, 78.3% of NA cells, and 83.7% of FI cells) and small subpopulation of CD14 positive cells. There was a statistically significant difference (p=0.013) between the number of CD44 positive cells in the RA and NA groups. The most noticeable difference found between all three groups was that there were significantly (p=0.021) fewer CD90 positive cells in the NA population (57.8%) compared to the RA population (79.5%), with an intermediate number of CD90 positive cells found in the FI control group (73.3%).
FIG. 1.
(A) Distribution of CD14, 44, 45, and 90 positive cells in rapidly adhering (RA), nonadherent (NA) and freshly isolated (FI) subpopulations from two different donors; (B) percentage mean of each marker (*represents a significant difference, p<0.05). Color images available online at www.liebertpub.com/tea
RA IFP cells display an enhanced osteogenic capacity but a diminished chondrogenic capacity
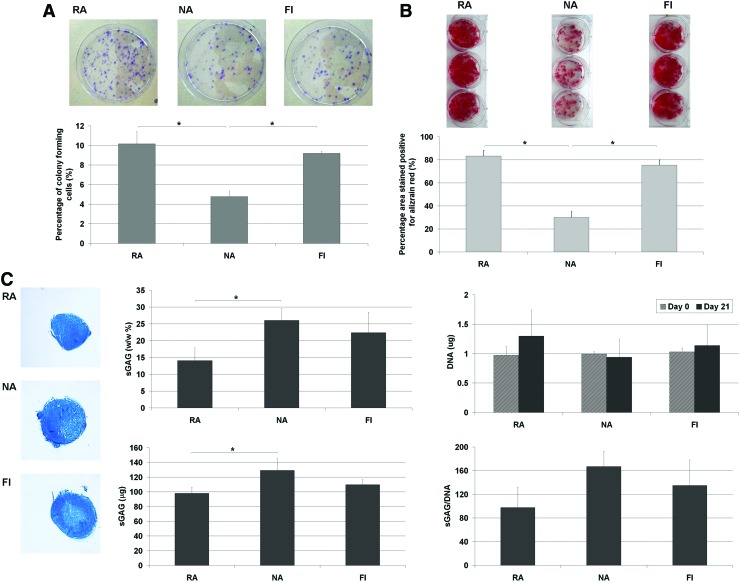
A colony forming unit fibroblasts (CFU-f ) assay was used to determine the percentage of colony forming cells within the RA, NA, and FI subpopulations. There were significantly more colonies present in the RA and FI groups than the NA group, with almost twice as many colonies formed (Fig. 2A). FI RA and FI cells cultured in osteogenic media also stained strongly for alizarin red indicating calcium deposition, with a significantly smaller area staining positive in the NA group, suggesting that overall this subpopulation has a lower osteogenic capacity (Fig. 2B). Pellets generated using FI (i.e., not culture expanded) RA, NA, and FI cells that were maintained in chondrogenic media all stained positive for alcian blue indicating sGAG deposition (Fig. 2C). When sGAG content was quantified for these groups, the NA subpopulation produced significantly more sGAG than the RA subpopulation, although this difference was no longer significant when sGAG accumulation was normalized to DNA content. There was no significant difference in the DNA content of RA, NA, and FI pellets at day 0 or at day 21.
FIG. 2.
Comparison of RA, NA, and FI cells in a (A) colony forming unit fibroblasts assay stained with crystal violet, (B) osteogenic assay stained with Alizarin red, (C) chondrogenic assay stained with Alcian blue or quantified via DNA and DMMB assays (* represents a significant difference, p<0.05). Color images available online at www.liebertpub.com/tea
IFP cells that do not rapidly adhere are more proliferative and produce greater amounts of cartilaginous matrix after hydrogel encapsulation
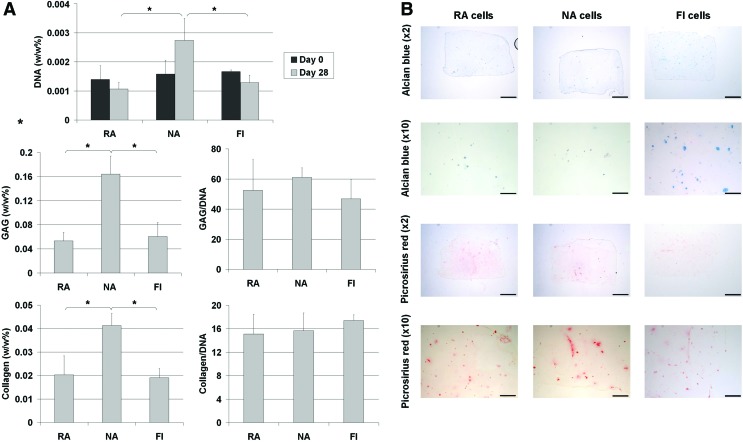
Having demonstrated that FI IFP derived cells possess a capacity to undergo chondrogenesis in a pellet culture system, we next sought to determine their ability to generate cartilaginous tissue in a 3D hydrogel suitable for cartilage tissue engineering applications. To this end, FI RA, NA, and FI IFP cells were embedded into agarose hydrogels at a seeding density of 5×106 cells per mL. After 28 days in culture, it can be seen from the DNA data that there was an increase in the number of cells in the NA constructs, but no dramatic change in total cell number in the RA or FI constructs (Fig. 3A). There was a statistically significant difference in the total amount of DNA present in the NA group compared to the other groups at the end of the culture period, although no difference between the RA and FI groups. Total sGAG and collagen accumulation were also significantly higher in the NA seeded hydrogels compared to RA and FI hydrogels, although these differences were not significant when sGAG and collagen levels were normalized to DNA content (Fig. 3A). Histological staining with alcian blue and picro-sirius red showed a small amount of sGAG and collagen accumulation localized to the pericellular region of certain cells. The low absolute amounts sGAG and collagen within the hydrogels was probably due primarily to the low cell seeding density used in this study, as sGAG synthesis on a per cell basis (sGAG/DNA) was not dramatically lower than that observed in pellet culture (Fig. 2C) and was within the range typically reported for this cell type.
FIG. 3.
(A) Biochemical analysis of DNA, sGAG and collagen and (B) histological staining for sGAG and collagen in RA, NA, and FI seeded agarose hydrogels after 0 and 28 days in culture (scalebar=1 mm, * represents a significant difference, p<0.05). Color images available online at www.liebertpub.com/tea
Cartilaginous tissues can be engineered by combining FI IFP cells with a growth factor delivery hydrogel
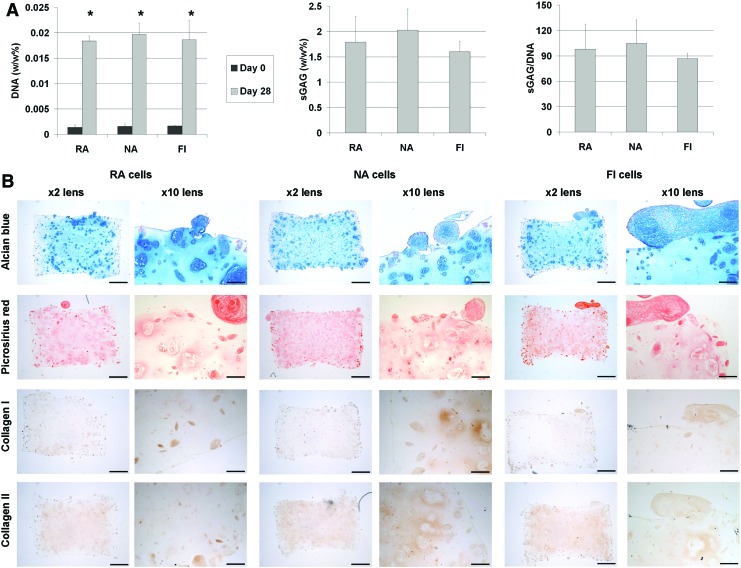
The previous study demonstrates that a subpopulation of FI stromal cells from IFP tissue will undergo chondrogenesis after hydrogel encapsulation and stimulation with TGF-β3. To translate this finding into a single stage therapy for cartilage repair requires a mechanism to control the release of TGF-β3 in vivo. With this aim in mind, the final phase of this study explored if robust chondrogenesis could be initiated by combining FI IFP cells with a growth factor delivery hydrogel. Serum was added to the media to promote the proliferative stem cell phenotype that is typically observed in the early stages of repair within cartilage defects.24 After 28 days in culture, there was a steep rise in the DNA content of growth factor delivery hydrogels seeded with both NA and RA cells (Fig. 4A). The level of sGAG within all three types of cell seeded growth factor delivery hydrogels were similar, approximately 2% of tissue wet weight. Due to presence of gelatin microspheres in the hydrogels, collagen could not be quantified accurately using our biochemical assay. Histological staining showed sGAG and collagen had accumulated throughout the hydrogels, with regions of intense staining observed in regions where clusters of cells were present (Fig. 4B). These clusters formed both inside the hydrogel and around the surface of the construct. Immuno-staining for collagen types I and II appeared to show more collagen type II present in the hydrogels than collagen type I (Fig. 4B).
FIG. 4.
(A) Biochemical analysis of DNA and sGAG, (B) histological staining for sGAG and collagen and immunohistochemical staining for type I and type II collagen in RA NA and FI seeded growth factor delivery hydrogels after 0 and 28 days in culture (scalebar ×2=1 mm, ×10=200 μm, * represents a significant difference between day 0 and day 28 samples, p<0.05). Color images available online at www.liebertpub.com/tea
Discussion
While it is well established that traditionally expanded IFP derived MSCs are very promising for cartilage tissue engineering and regenerative medicine applications,19,25–32 the results of this study provide support for the concept of seeding FI IFP derived cells into a growth factor delivery hydrogel for use as an in-theatre, single stage therapy for articular cartilage repair. The study also demonstrates that simple methods to isolate different subpopulations of cells, such as rapid or delayed adherence to a specific substrate, might be useful for isolating cells with an altered capacity to differentiate along a specific pathway. FI IFP cells that rapidly adhered to cell culture plastic were found to display both a unique differentiation capacity and surface marker profile to cells that did not adhere within the same timeframe. In spite of this, all three cell populations examined (RA, NA and FI cells) demonstrated a comparable capacity to generate cartilaginous tissue once encapsulated into a growth factor delivery hydrogel and maintained in culture conditions that promoted cell proliferation. This result raises questions about the benefit of selecting FI IFP cells based on adherence to a specific substrate for single stage cartilage repair applications.
The majority of cells in all subpopulations were CD90 and CD44 positive, while only a small proportion of cells were CD14 positive. Such an expression of cell surface molecules is consistent with that of mesenchymal stem/progenitor cells,33,34 although clearly only a small percentage of all subpopulations were true progenitors given that only 5–10% of the cells in the different groups demonstrated a colony forming capacity. The RA cell population had a higher proportion of colony forming cells and a greater osteogenic capacity than NA cells, which may indicate a higher number of MSCs within the RA subpopulation. The most noticeable difference between the RA, FI, and NA subpopulations was the presence of more CD90 positive cells in the RA and FI groups compared to the NA group. CD90, also called thy-1, is a cell surface glycoprotein and is a commonly used stem cell marker associated with cell adhesion. It is therefore, perhaps unsurprising that a higher percentage of the RA cell population were CD90 positive compared to the NA population. The RA cells also displayed an enhanced osteogenic capacity and a diminished chondrogenic capacity compared to NA cells, which can be related to their differential expression of cell surface markers. It has previously been shown that CD90 enriched cells isolated from adipose tissue were highly osteogenic but demonstrated poor chondrogenic potential.35 A similar study also demonstrated that CD90 positive cells isolated from adipose derived stem cells display an enhanced osteogenic capacity.36
The NA subpopulation, which demonstrated an enhanced capacity to generate cartilage-like tissue, had a lower percentage of both CD44 and CD90 positive cells than the RA subpopulation. The correlation of increased chondrogenesis with a reduction in the percentage of CD90 positive cells is consistent with the findings of Adesida et al. (2012), who found a similar correlation for bone marrow stromal cells cultured under hypoxic conditions.18 In contrast, the CD90+ subpopulation of synovial fluid derived progenitor cells has been shown to have an increased chondrogenic potential compared to the CD90− cells.37 CD44 is another cell marker commonly associated with MSCs (although it may be absent in mesenchymal progenitor cells38 and it can also be a marker of other cells types e.g., T cells39). It has been shown that CD44 is expressed at significantly higher levels in chondrocytes with a higher chondrogenic capacity.40 This suggests that the enhanced chondrogenic capacity of the NA subpopulation is unrelated to the fact that it has a lower percentage of CD44+ cells. An alternative explanation for the enhanced chondrogenic capacity of the NA group might be the presence of other NA or slowly adhering cell types within this subpopulation that may be enhancing either the proliferation or chondrogenic differentiation of the progenitor cells that do not rapidly adhere and remain in this subpopulation (and/or potentially suppressing their osteogenic potential). CD14 is a marker associated with macrophages which have previously been shown to affect MSCs by inhibiting osteogenesis41 and promoting the secretion of various factors, including IL-6.42 IL-6 has been shown to inhibit differentiation of MSCs while maintaining their proliferation.43 While the number of CD14+ positive cells in the NA subpopulation was not significantly higher than in the RA subpopulation (1.8% of RA cells, 3.06% of NA cells; p=0.162), we cannot rule out the possibility that higher numbers of contaminating cell types, such as macrophages in the NA subpopulation could be secreting factors that diminish the overall osteogenic potential of this group. A similar mechanism could explain the greater levels of proliferation observed in the NA seeded agarose hydrogels when compared to the RA seeded hydrogels and indeed the FI seeded hydrogels, as a relatively higher ratio of such contaminating cell types to putative progenitor cells will exist in the NA subpopulation. Furthermore, macrophages have also been shown to release TGF-β1,44 a growth factor which is known to promote a chondrogenic phenotype, although CD14-negative isolation has also been shown to enhance chondrogenesis in culture expanded synovial fibroblasts.41 Further work is required to confirm what role the different cell types play in determining the overall proliferative and differentiation capacity of the different subpopulations.
It should be noted that the chondrogenic capacity of the FI subpopulations, as measured by sGAG/DNA, was at least 35% lower compared with IFP cells that were first expanded to passage 2 using traditional approaches22 before pellet formation (data not shown). This is perhaps unsurprising given that only up to 10% of the FI subpopulations are colony forming cells (Fig. 2A), suggesting that a significant proportion of the cells within these groups are not progenitor cells and do not have the ability to rapidly divide and undergo chondrogenesis. In the context of developing a single stage therapy for articular cartilage repair using FI IFP derived cells, this highlights the importance of developing a scaffold or hydrogel that can promote the proliferation of the limited number of chondroprogenitor cells actually seeded onto the scaffold at the time of implantation.
The NA cells were found to proliferate after hydrogel encapsulation, as evidenced by an increase in the DNA content of these constructs, while no significant change in the DNA content of the RA constructs was observed over time. sGAG and collagen synthesis, on a per cell basis, was comparable in all groups, resulting in overall higher levels of sGAG and collagen accumulation in the NA constructs. In spite of this, the absolute levels of cartilaginous extracellular matrix accumulation within the constructs was low, relative to native articular cartilage, in a large part due to the relatively low cell seeding density of 5×106 cells per mL used in this study compared to more traditional in vitro cartilage tissue engineering strategies that typically use between 20 and 60×106 cells per mL of hydrogel.45–47 Given that the average volume of a human cartilage defect is approximately 550 mm3,48 and that the average number of stromal cells that can be isolated from a freshly digested human fat pad is on average approximately 4 million (ranging between 0.55 and 16 million cells),3 the seeding density used in this study is probably at the higher end of what is feasible assuming that the majority of the fat pad would have to be preserved during biopsy in a clinical setting. Therefore, the success of the proposed therapy is based on the assumption that the in vivo environment within a cartilage defect (and as discussed above the cues provided by a supporting scaffold or hydrogel) would promote proliferation of the implanted IFP cells. Indeed, such proliferation is observed in the early stages of repair in full thickness cartilage defects.24 When we attempted to mimic such conditions in vitro through the addition of serum to the media, dramatic increases in cartilage matrix accumulation were observed. This was due primarily to an increase in cell proliferation within the hydrogels, rather than a dramatic increase in matrix synthesis on a per cell basis (compare Fig. 3A and Fig. 4A).
Another impact of the addition of serum to the media was that we no longer observed any differences in the proliferative capacity (or in the total matrix producing capacity) of the RA, NA, and FI populations. We speculate that this is due to the potent mitotic cues provided by the serum overriding any inherent differences between the different populations. This would suggest that there is little to benefit from the use of plastic adhesion as a mechanism to isolate chondro-progenitor cells for single stage cartilage regeneration strategies. An obvious advantage of using nonfractionated FI cells over NA or RA cells is that a larger number of cells would be available for transplantation and the processing time would be reduced since the adhesion step would be removed from the procedure. However, there may be other benefits to isolating specific subpopulations based on their ability to selectively adhere to a given substrate that cannot be evaluated based on the in vitro assays utilised in this study. The NA subpopulation appeared to possess a diminished osteogeneic capacity. It has been demonstrated that even chondrogenically primed MSCs have a tendency to undergo ossification and produce mineralized tissue when implanted subcutaneously or into cartilage defects.49–51 By reducing the osteogenic potential of a population of cells before implantation, it may be that the risk of calcification and endochondral ossification would be reduced; however, in vivo studies are required to confirm this. Likewise, plastic adhesion could also be employed to help select for a more osteogenic subpopulation of cells, which could be advantageous if attempting to regenerate bone, for example, in the case of an osteochondral defect. Further, in vivo studies are required to elucidate if there is any benefit to isolating subpopulations of FI IFPs by means of selective adherence to plastic for cartilage repair applications. Furthermore, it should be noted that several other factors that could affect the fractionalization of the initial FI cell population, such as the cell concentration, adhesion time, and brand/type of culture plastic used have not yet been fully investigated.
In conclusion, the combination of a growth factor delivery hydrogel containing FI chondroprogenitor cells represents a promising new treatment option for the repair of articular cartilage defects. The slow release of growth factors from microspheres within the hydrogel enabled a chondrogenic phenotype to be established and maintained within the construct; thus, allowing the formation of cartilaginous tissue. This approach could potentially be used as part of an in-theatre, single stage therapy for cartilage defect repair.
Acknowledgment
This research was funded by a European Research Council Starter Grant (StemRepair–Project number 258463).
Disclosure Statement
No competing financial interests exist.
References
- 1.Ochi M., Uchio Y., Kawasaki K., Wakitani S., and Iwasa J.Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br 84,571, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Jurgens W.J., Kroeze R.J., Bank R.A., Ritt M.J., and Helder M.N.Rapid attachment of adipose stromal cells on resorbable polymeric scaffolds facilitates the one-step surgical procedure for cartilage and bone tissue engineering purposes. J Orthop Res 29,853, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Jurgens W.J., van Dijk A., Doulabi B.Z., Niessen F.B., Ritt M.J., van Milligen F.J., et al. . Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy 11,1052, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Gomm J.J., Browne P.J., Coope R.C., Liu Q.Y., Buluwela L., and Coombes R.C.Isolation of pure populations of epithelial and myoepithelial cells from the normal human mammary gland using immunomagnetic separation with Dynabeads. Anal Biochem 226,91, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Schmitz B., Radbruch A., Kummel T., Wickenhauser C., Korb H., Hansmann M.L., et al. . Magnetic activated cell sorting (MACS)—a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. Eur J Haematol 52,267, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Yanada S., Ochi M., Adachi N., Nobuto H., Agung M., and Kawamata S.Effects of CD44 antibody—or RGDS peptide—immobilized magnetic beads on cell proliferation and chondrogenesis of mesenchymal stem cells. J Biomed Mater Res A 77,773, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Elliott S.R., Macardle P.J., and Zola H.Removal of erythroid cells from umbilical cord blood mononuclear cell preparations using magnetic beads and a monoclonal antibody against glycophorin A. J Immunol Methods 217,121, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Chang Y.J., Tseng C.P., Hsu L.F., Hsieh T.B., and Hwang S.M.Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int 30,495, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Tondreau T., Lagneaux L., Dejeneffe M., Delforge A., Massy M., Mortier C., et al. . Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy 6,372, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Colter D.C., Class R., DiGirolamo C.M., and Prockop D.J.Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A 97,3213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedenstein A.J., Gorskaja J.F., and Kulagina N.N.Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4,267, 1976 [PubMed] [Google Scholar]
- 12.Ahearne M., and Kelly D.J.A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed Mater 8,035004, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Fan H., Zhang C., Li J., Bi L., Qin L., Wu H., et al. . Gelatin microspheres containing TGF-beta3 enhance the chondrogenesis of mesenchymal stem cells in modified pellet culture. Biomacromolecules 9,927, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Park J.S., Yang H.N., Woo D.G., Jeon S.Y., and Park K.H.Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials 32,1495, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Ahearne M., Buckley C.T., and Kelly D.J.A growth factor delivery system for chondrogenic induction of infrapatellar fat pad-derived stem cells in fibrin hydrogels. Biotechnol Appl Biochem 58,345, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., and Burdick J.A.Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32,6425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinardell T., Buckley C.T., Thorpe S.D., and Kelly D.J.Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med 5,673, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Adesida A.B., Mulet-Sierra A., and Jomha N.M.Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther 3,9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan W.S., Adesida A.B., and Hardingham T.E.Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther 9,R55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley C.T., Vinardell T., and Kelly D.J.Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage 18,1345, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Meyer E.G., Buckley C.T., Thorpe S.D., and Kelly D.J.Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech 43,2516, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Buckley C.T., and Kelly D.J.Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J Mech Behav Biomed Mater 11,102, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ignat'eva N.Y., Danilov N.A., Averkiev S.V., Obrezkova M.V., Lunin V.V., and Sobol’ E.N.Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem+ 62,51, 2007 [Google Scholar]
- 24.Shapiro F., Koide S., and Glimcher M.J.Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75,532, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Dragoo J.L., Samimi B., Zhu M., Hame S.L., Thomas B.J., Lieberman J.R., et al. . Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br 85,740, 2003 [PubMed] [Google Scholar]
- 26.Lee S.Y., Nakagawa T., and Reddi A.H.Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun 376,148, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Buckley C., Mulhall K., and Kelly D.J.Combining BMP-6, TGF-ß3 and hydrostatic pressure stimulation enhances the functional development of cartilage tissues engineered using human infrapatellar fat pad derived stem cells. Biomater Sci 1,745, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Buckley C.T., Downey R., Mulhall K.J., and Kelly D.J.The role of environmental factors in regulating the development of cartilaginous grafts engineered using osteoarthritic human infrapatellar fat pad-derived stem cells. Tissue Eng Part A 18,1531, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopa S., Colombini A., de Girolamo L., Sansone V., and Moretti M.New strategies in cartilage tissue engineering for osteoarthritic patients: infrapatellar fat pad as an alternative source of progenitor cells. J Biomater Tiss Eng 1,40, 2011 [Google Scholar]
- 30.O'HEireamhoin S., Buckley C.T., Jones E., McGonagle D., Mulhall K.J., and Kelly D.J.Recapitulating aspects of the oxygen and substrate environment of the damaged joint milieu for stem cell-based cartilage tissue engineering. Tissue Eng Part C Methods 19,117, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Toghraie F.S., Chenari N., Gholipour M.A., Faghih Z., Torabinejad S., Dehghani S., et al. . Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 18,71, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Wickham M.Q., Erickson G.R., Gimble J.M., Vail T.P., and Guilak F.Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res 412,196, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. . Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rada T., Reis R.L., and Gomes M.E.Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev 7,64, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Chung M.T., Liu C., Hyun J.S., Lo D.D., Montoro D.T., Hasegawa M., et al. . CD90 (Thy-1) positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19,989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krawetz R.J., Wu Y.E., Martin L., Rattner J.B., Matyas J.R., and Hart D.A.Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One 7,e43616, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes M., Lund T., Lenvik T., Aguiar D., Koodie L., and Verfaillie C.M.Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 98,2615, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Huet S., Groux H., Caillou B., Valentin H., Prieur A.M., and Bernard A.Cd44 Contributes to T-Cell Activation. J Immunol 143,798, 1989 [PubMed] [Google Scholar]
- 40.Grogan S.P., Barbero A., Diaz-Romero J., Cleton-Jansen A.M., Soeder S., Whiteside R., et al. . Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum 56,586, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Bilgen B., Ren Y., Pei M., Aaron R.K., and Ciombor D.M.CD14-negative isolation enhances chondrogenesis in synovial fibroblasts. Tissue Eng Part A 15,3261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton K., Banerjee D., and Glod J.Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS One 7,e35036, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pricola K.L., Kuhn N.Z., Haleem-Smith H., Song Y., and Tuan R.S.Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem 108,577, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagnik D.R., Evans B.J., Florey O., Mason J.C., Landis R.C., and Haskard D.O.Macrophage release of transforming growth factor beta1 during resolution of monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 50,2273, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Mauck R.L., Seyhan S.L., Ateshian G.A., and Hung C.T.Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 30,1046, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Thorpe S.D., Nagel T., Carroll S.F., and Kelly D.J.Modulating gradients in regulatory signals within mesenchymal stem cell seeded hydrogels: a novel strategy to engineer zonal articular cartilage. PLoS One 8,e60764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang A.H., Stein A., Tuan R.S., and Mauck R.L.Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A 15,3461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu C.R., Szczodry M., and Bruno S.Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev 16,105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickhut A., Pelttari K., Janicki P., Wagner W., Eckstein V., Egermann M., et al. . Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol 219,219, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Mueller M.B., and Tuan R.S.Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58,1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinardell T., Sheehy E.J., Buckley C.T., and Kelly D.J.A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18,1161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]