Abstract
Both basal and submucosal gland (SMG) duct stem cells of the airway epithelium are capable of sphere formation in the in vitro sphere assay, although the efficiency at which this occurs is very low. We sought to improve this efficiency of sphere formation by identifying subpopulations of airway basal stem cells (ABSC) and SMG duct cells based on their aldehyde dehydrogenase (ALDH) activity. ALDHhi ABSCs and SMG duct cells were highly enriched for the population of cells that could make spheres, while the co-culture of ALDHhi differentiated cells with the ALDHhi ABSCs increased their sphere-forming efficiency. Specific ALDH agonists and antagonists were used to show that airway specific ALDH isozymes are important for ABSC proliferation. Pathway analysis of gene expression profiling of ALDHhi and ALDHlo ABSCs revealed a significant upregulation of the arachidonic acid (AA) metabolism pathway in ALDHhi ABSCs. We confirmed the importance of this pathway in the metabolism of proliferating ALDHhi ABSCs using bioenergetics studies as well as agonists and antagonists of the AA pathway. These studies could lead to the development of novel strategies for altering ABSC proliferation in the airway epithelium.
Introduction
The mouse proximal airway epithelium is maintained and repaired after injury by the action of at least two distinct epithelial progenitor cell populations, airway basal stem cells (ABSCs) of the surface epithelium and the duct cells of the submucosal glands (SMG) [1–5]. These progenitor cells are capable of self-renewal and of differentiating into the mature cell types of the airway to ensure efficient mucociliary clearance. Our understanding of these progenitor cell populations has increased greatly, thanks in large part to an in vitro sphere-forming assay that is used to assess the proliferation and differentiation potential of these progenitor cells [1–3,5]. These studies showed that ABSCs and SMG duct cells are capable of forming clonal spheres while non-ABSCs and non-duct cells do not. However, the very low incidence of sphere formation in this assay (range 0.6%–1%, average 0.75%±0.13% in our hands, 3% in others' hands [5], 10%–70% in other organs including the brain, prostate, and breast [6]) prompted us to try to find a marker to enrich for the subpopulations of ABSCs and duct cells with the ability to form spheres.
Aldehyde dehydrogenase (ALDH) activity has been shown in other tissues, such as hematopoietic tissue [7,8] and breast tissue [9], to delineate stem cell subpopulations with greater proliferative capacity and potentially a cancer stem cell phenotype [9–11]. In the lungs, ALDH1A1 and ALDH3A1 expression was found in normal airways and high expression of ALDH1A1 and ALDH3A1 was found in non-small cell lung cancer (NSCLC) [12]. Further, ALDH1A1 expression was found to correlate with poorer prognosis in NSCLC and to mark a subpopulation of tumor cells [13].
There are more than 19 different isozymes of ALDH [14–16], and we hypothesized that functionally they play a crucial role in protecting the airways from aldehydes derived from endogenous and exogenous sources [17]. As the airways are constantly exposed to air pollution, which is a major source of exogenous aldehydes, we reasoned that the cells of the airway epithelium would need to be enriched in ALDH to protect the body from toxic aldehyde effects [17]. We further speculated that cells with the greatest ability to withstand toxic aldehyde exposure would be the cells most likely to survive and proliferate for repair after injury.
Here, we identified high ALDH activity as a marker that enriches for proliferating ABSCs and SMG duct cells. We performed gene expression profiling of ALDHhi and ALDHlo ABSCs and non-ABSCs and found that one of the most significant differences was in the arachidonic acid (AA) metabolism pathway. We confirmed the importance of this pathway in selective proliferation of ALDHhi ABSCs using bioenergetics studies and inhibition and activation of the pathway. Our work suggests that mechanistically, the ability of proliferating ABSCs to metabolize AA as an energy source is important when metabolic substrates are in short supply after airway injury.
Materials and Methods
Mice
Eight to ten week-old wild-type C57BL/6 and β-actin red fluorescent protein (RFP) (C57BL/6-Tg[ACTbERFP]1Nagy/J) mice were used for these experiments. Mice were housed and bred under the regulation of the Division of Laboratory Animal Medicine at the University of California, Los Angeles.
Fluorescence-activated cell sorting based on ALDH activity, sphere formation assay, and quantification of sphere number and size
Mouse tracheal epithelial cells were collected and sorted into ABSCs and non-ABSCs and SMG duct and non-duct cells as described previously [1,3]. Sorting was further performed based on the ALDH activity of airway epithelial cells using the Aldefluor® kit (Stem Cell Technologies) and was performed at the concentration of 1×106 cells/mL Aldefluor assay buffer, per the manufacturer's recommendations. Eight to ten tracheas were used per isolation and unless stated otherwise, 50,000 cells were seeded per transwell. Sphere formation efficiency was calculated as a percentage of sphere number to number of seeded cells. Sphere numbers and diameters were visually counted and measured from digital images from all transwells 2 weeks after seeding. At this time point, a colony of cells with a diameter of >50 μm is considered a sphere. All experimental wells were run in triplicates and every experiment was repeated at least twice.
In vitro sphere cultures of wild type with RFP+ cell populations
Fluorescence-activated cell sorting (FACS)-sorted cells were resuspended in mouse tracheal epithelial cells (MTEC)/Plus media [18], and mixed 1:1 with growth factor–reduced Matrigel (BD Biosciences). Sorted RFP+ ALDHlo ABSCs, ALDHlo non-ABSCs, and ALDHhi non-ABSCs were co-cultured with wild-type ALDHhi ABSCs (50,000 cells/transwell). The number of spheres per well was counted on day 7 and 14 of culture. RFP+ spheres were detected with an inverted fluorescent microscope (Zeiss Axiovert).
Tracing the mitotically active ABSCs by labeling with the red fluorescent cell membrane linker PKH26
Primary sorted ABSCs were stained with PKH-26, a fluorescent dye with long aliphatic tails for incorporation in the cell membrane, using the PKH26 Red Fluorescent Cell Linker Kit (Sigma) according to the manufacturer's instructions. Two weeks later, spheres were digested and sorted based on PKH26 fluorescence and ITGA6 immunostaining.
Treating with ALDH agonists and antagonists
Sorted ABSCs or SMG duct cells were cultured on matrigel and treated with the broad-spectrum ALDH inhibitor, diethylaminobenzaldehyde (DEAB) (100–200 μM) or with specific ALDH1A1, ALDH2, and ALDH3 inhibitors, Acrolein (3 μM), Daidzin (100 μM), and AA (100 μM) or with agonists of ALDH2, and ALDH3; Alda-1(100 μM), and Alda-89 (100 μM) (kind gift of Dr. Mochly-Rosen) at day 0 of in vitro culture or at day 7. Spheres were imaged and collected at days 14 and/or 21 of culture.
Quantitative real-time PCR
Total RNA was extracted from sorted cell populations using the RNeasy kit (Qiagen). cDNA was synthesized from all RNA samples using TaqMan reverse transcription reagents according to the manufacturer's protocol (Applied Biosytems). Quantitative real-time PCR (QPCR) was performed using a TaqMan Fast Universal PCR master mix according to the manufacturer's protocol (Applied Biosystems) and analyzed with the Step One real-time PCR system (Applied Biosystems) using predesigned primer/probe mixtures for Gapdh, Aldh1a1, Aldh2, and Aldh3a1 (Applied Biosystems).
Western blots
Cell lysates obtained from sorted cells containing equivalents of total protein (10 μg) were resolved on a 12% sodium dodecyl sulfate-polyacrylamide gel, followed by transfer to nitrocellulose membranes (Bio-Rad). Membranes were blocked in skimmed milk in PBS buffer for 60 min followed by incubation with rabbit ALDH1A1, rabbit ALDH2 (Abcam), rabbit ALDH3A1 (Abgent), or rabbit anti-β-actin (Rockland Immunochemicals) (1:500 to 1:1,000 dilutions). Membranes were then washed and incubated with the appropriate horseradish peroxidase–coupled secondary antibodies (Bio-Rad) and the immunocomplexes were visualized using SuperSignal West Pico Chemiluminescent System (Thermo Scientific). The bands were quantified by densitometric scanning using ImageLab software Version 3.0 Build 11 (Bio-Rad).
RNA extraction and library manufacturing for RNA sequencing
RNA was extracted from sorted cells using the RNeasy Micro Kit (Qiagen). The cDNA was generated and amplified using the Ovation RNA-Seq V2 System (NuGEN). The resulting cDNA was sheared to 140–180 bp using the Covaris Focused-ultrasonicator with the following settings, duty cycle: 10%; intensity: 5; cycles per burst: 200; total time: 6 min. The size range of the sheared cDNA was visualized and confirmed with bioanalyzer analysis prior to library construction. The sequencing libraries were prepared using the Encore Library System (NuGEN). The average sizes of the libraries were estimated with a bioanalyzer, and the concentrations of the libraries were measured on the Qubit™ fluorometer (Invitrogen).
RNA-sequencing read mapping and expression quantification
We first filtered the raw reads to remove low quality reads and reads containing sequencing adapters. Then, we aligned the filtered raw reads (single end, 50 bp in length) to the reference mouse genome (University of California, Santa Cruz release mm9) with the gapped aligner Tophat [19] (version 1.3.0) allowing up to two mismatches. The mouse gene model annotation (version of Mus_musculus.NCBIM37.63) was downloaded from the Ensembl database (ftp://ftp.ensembl.org/pub/current_gtf/mus_musculus/) and supplied to Tophat. Only uniquely aligned reads were considered for further analysis. Altogether, about 386 million reads were uniquely mapped and used in this study. The expression abundance of each individual gene and transcript was quantified by Cufflinks [20] (version 1.0.3) in the Fragments Per Kilobase of exon per Million (FPKM) unit fragments mapped together with confidence intervals. Cufflinks ran in the default parameters except that the annotated gene set was supplied using the–G option. We also measured the raw read count for each gene and transcript using customized scripts written in Perl.
Bioinformatic analysis
We performed the differential expression analysis using the R package, DESeq [21]. With this test, raw read counts were employed to model the negative binomial distribution of expression abundances of all genes and transcripts. We filtered out low expressing genes and transcripts by only keeping those that had at least one count per million in the samples. The multiple testing errors were corrected by the false discovery rate. In addition to the cutoff adjusted P-value<0.05, we also adopted an additional cutoff set as the expression ratio of above two-fold changes in expression values. In summary, we considered genes as differentially expressed genes if (1) the adjusted P-value was <0.05 and (2) the expression ratio between two conditions was above two-fold.
Extracellular flux bioenergetic assay
Assays were performed in accordance with manufacturer's instructions (Seahorse Bioscience) and described previously [22]. Briefly, 40,000 sorted cells were seeded in extracellular flux 96-well cell culture microplates (Seahorse Bioscience) in 80 μL of MTEC plus cell growth medium (with 0.01 μM retinoic acid) and then incubated at 37°C/5% CO2 for ∼24 h. Assays were initiated by replacing the cell growth medium from each well with 200 μL of Krebs-Henseleit buffer assay medium (Seahorse Bioscience) supplemented with 0.5 mM l-carnitine. The microplates were incubated at 37°C for 60 min to equilibrate the temperature and pH of the media before measurement. A Seahorse Bioscience instrument (model XF96) was used to measure the rate of change of dissolved O2 and pH in the media. Briefly, freshly prepared bovine serurm albumin-complexed palmitate/AA was injected at a final concentration of 20 μM in low glucose minimal medium supplemented with carnitine and the palmitate/AA oxidation rate was calculated as the increase in oxygen consumption above baseline. Oxygen consumption rate (OCR) was measured simultaneously for ∼2 min in repeated cycles, to obtain a basal average. All OCR values were normalized to protein concentration using the Protein Assay reagent (Qubit flurometer; Invitrogen) in all experiments. All wells were run in triplicates. The media formulations of the high glucose (25 mM), low glucose (5.56 mM), and no glucose medium were equal (Invitrogen 11995, 11885 and 11966). The very low glucose (2.78 mM) medium was prepared by mixing equal volumes of low and no glucose media.
Immunostaining of spheres
After 14–21 days of in vitro culture, matrigel discs containing the spheres were embedded in Histogel and then in paraffin for sectioning. Sections were processed and immunofluorescence was performed as previously described [1]. Primary antibodies used were Rabbit Keratin 5 (1:200; Covance), Goat polymeric immunoglobulin receptor (pIgR) (1:100; R&D Systems), Rabbit ALDH1/2 (1:100; Santa Cruz), Rabbit ALDH1A1 and Rabbit ALDH2 (1:100; Abcam), Rabbit ALDH3A1 (1:50; Abgent), Rabbit Keratin 14 (1:200; Epitomics), and Rat Ki67 (1:100; Dako). To detect mucus secretions in spheres, Alcian blue and Periodic Acid Schiff staining was performed, as previously described [1].
Lipoxygenase inhibition
Sorted ABSCs (60,000–80,000 cells per well) were cultured in Transwell membrane inserts (0.4 μm pore size; Corning) on matrigel and MTEC/Plus media and treated with the specific lipoxygenase 12e and 15 inhibitor, ethyl 3,4-dihydroxybenzylidenecyanoacetate (DHBLCA; Sigma) using concentrations of 5, 10, 15, or 20 μM for 14 days. Spheres were imaged, embedded, and sectioned. Immunofluorescence staining using a 1:200 dilution of anti-Ki67 (Dako), anti-Keratin 5 (Covance), and anti-pIgR (R&D Systems) was performed as previously described [1].
Statistical analysis
Data are presented as average +/− SEM. The two-tailed student's t-test was used for comparisons, with P<0.05 considered statistically significant.
Results
ALDH activity enriches for proliferating ABSCs and SMG duct cells of the airway epithelium
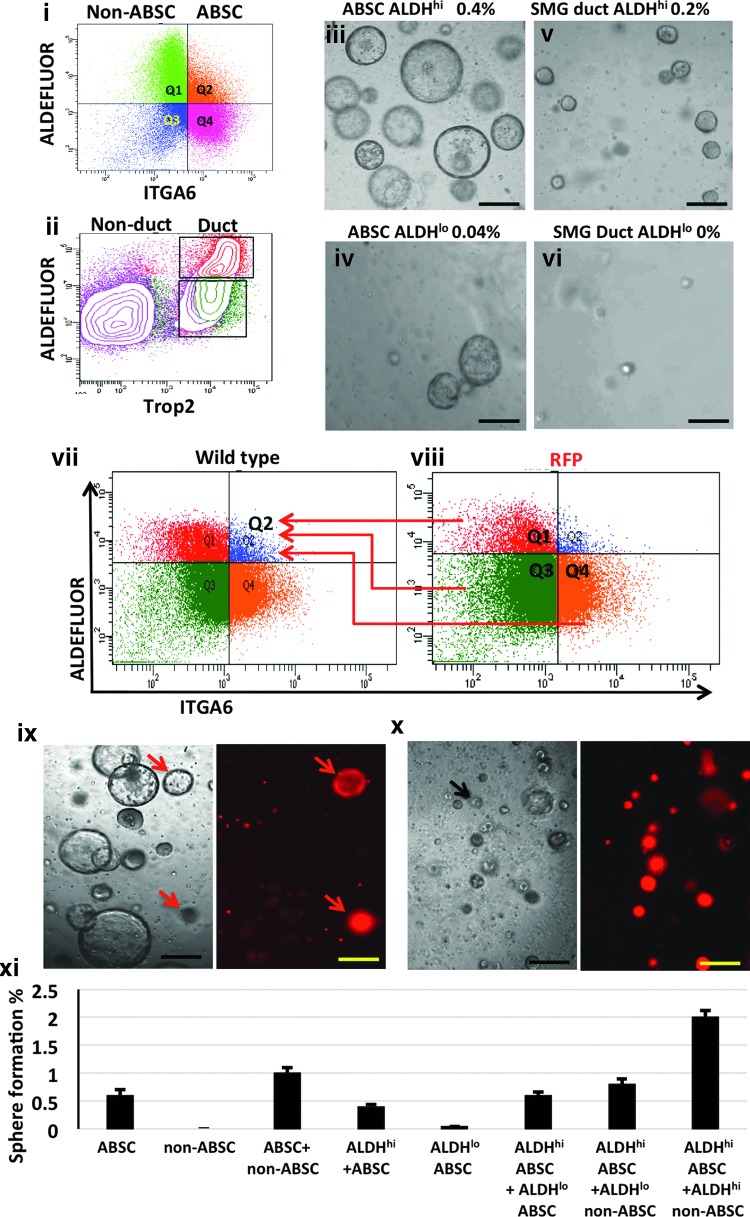
Airway surface epithelial ABSCs and SMG duct cells of the mouse trachea demonstrate self-renewal and differentiation properties in vitro in the sphere formation assay [1,5]. We use the sphere formation assay as a surrogate to examine ABSC proliferation, and serial propagation of single cells from spheres allows an assessment of self-renewal [6]. The percentage of cells that give rise to spheres in our assay is 0.4%±0.2% for Trop2+ sorted SMG duct cells and 0.6%±0.4% for Itga6+/Trop2+ sorted ABSCs. We hypothesized that high ALDH activity would enrich for the progenitor cells within the ABSC and SMG duct cell populations. We therefore sorted ALDHhi and ALDHlo populations (Fig. 1i, ii) and cultured them in the sphere formation assay. The ALDHhi ABSC population had a 10-fold higher sphere-forming efficiency than the ALDHlo ABSC population (Fig. 1iii, iv), while the ALDHlo SMG duct cell population was essentially unable to form spheres (Fig. 1v, vi). However, culturing the ALDHhi ABSCs or ALDHhi SMG duct cells alone resulted in lower sphere-forming efficiency compared with culturing ALDHhi and ALDHlo populations together (0.2% for duct cells down from 0.4%, and 0.4% for ABSCs down from 0.6%, P<0.001).
FIG. 1.
ALDHhi airway basal stem cells (ABSC) and duct cell populations are enriched for the sphere-forming airway epithelial cells. ABSC (i) and submucosal gland (SMG) duct (ii) cells were sorted based on their aldehyde dehydrogenase (ALDH) expression, using the Aldefluor assay. ALDHhi ABSCs showed 10 times higher sphere formation efficiency (iii) compared with ALDHlo ABSCs (iv). ALDH high (v) and low (vi) cells within the SMG duct cell population showed the same phenomenon. ALDHhi ABSCs (Q2), ALDHlo ABSCs (Q4), ALDHhi non-ABSCs (Q1), and ALDHlo non-ABSCs (Q3) were sorted from red fluorescent protein (RFP) mice. Then cells from RFP Q1, 3 and 4 were co-cultured with cells from wild-type Q2 (vii, viii). (ix) Shows that the coculture of wild-type ALDHhi ABSCs with RFP ALDHhi non-ABSCs caused marked increase in sphere-formation efficiency with almost all spheres originating from the wild-type ALDHhi ABSCs. Very few spheres originated from the ALDHhi non-ABSCs (red arrows). (x) Shows similar results to ix with the coculture of RFP ALDHhi ABSCs with wild-type ALDHhi non-ABSCs. In this experiment, one sphere originated from the wild-type ALDHhi non-ABSC population (black arrow). (xi) Bar graph showing the sphere formation efficiency from the different populations. Scale bar=100 μm.
ALDHhi non-ABSCs of the airway epithelium promote ALDHhi ABSC proliferation and sphere formation
We hypothesized that the ALDHlo ABSC population of the airway epithelium might contain an essential cell population required for supporting the sphere-forming progenitor cells within the ALDHhi ABSC population. To examine this, we sorted airway epithelial cells into four populations based on low and high expression of Itga6 and ALDH activity, and then cultured the sorted populations independently or together to determine their sphere forming potential. Sorted total ABSCs had a sphere-forming efficiency of 0.6%. While the ALDHhi ABSCs were the only population of cells capable of independent sphere formation, the efficiency decreased to 0.4%. When we co-cultured the four populations, the efficiency increased to 1% (P<0.05). This implies that there is a subpopulation of cells other than the ALDHhi ABSCs that promotes its proliferation to form spheres. To identify this subpopulation, we sorted ALDHlo ABSCs, ALDHlo non-ABSCs, and ALDHhi non-ABSCs from mice that ubiquitously express RFP and co-cultured each subpopulation separately with ALDHhi ABSCs sorted from wild-type mice (Fig. 1vii, viii). We then assessed the sphere-forming efficiency of the different cell populations by their expression of RFP (Fig. 1ix–xi). We found that the presence of ALDHlo ABSCs or ALDHlo non-ABSCs in culture with the ALDHhi ABSCs resulted in a modest increase in sphere-forming efficiency compared with culturing ALDHhi ABSCs alone under equal seeding densities (increased from 0.4%±0.04% to 0.6%±0.06% (P<0.005) (Fig. 1xi). However, the presence of ALDHhi non-ABSCs in culture with the ALDHhi ABSCs resulted in a marked increase in sphere-forming efficiency compared with culturing ALDHhi ABSCs alone [increased from 0.4%±0.04% to 2%±0.12% (P<0.0005), Fig. 1ix–xi]. In all co-culture experiments, ALDHhi ABSCs remained the sphere-forming population of cells, as the majority of spheres that formed were RFP negative (Fig. 1ix–xi). These data indicate that the ALDHhi ABSC population is highly enriched for a progenitor population and that their regenerative capacity is increased by the addition of non-sphere forming cells, which are highly enriched within the ALDHhi non-ABSC population.
High ALDH expression does not select for the proliferating ABSC populations with serial propagation of spheres
We wanted to examine whether high ALDH expression would continue to mark for the ABSCs after serial propagation of spheres in vitro. First, we wanted to demonstrate that ABSCs are the cells that are responsible for the self-renewal and propagation of spheres with serial passaging. We therefore fluorescently labeled sorted ABSCs with the PKH-26 membrane linker dye to track the serial dilution of the dye with serial mitotic events [23]. After visually confirming homogenous PKH staining of all cells, we cultured the labeled ABSCs for 2 weeks before dispersing the spheres into single cell suspensions and sorting them into Itga6hi/PKHlo, Itga6hi/PKHhi (ABSCs with and without frequent mitoses) and Itga6lo/PKHlo, Itga6lo/PKHhi (non-ABSCs with and without frequent mitoses). When these four populations were re-cultured, Itga6hi/PKHlo cells (ABSCs with frequent mitoses) had significantly higher sphere-forming efficiency than other cell populations (P<0.05) indicating that the ABSCs that were mitotically active continued to be the sphere-forming cells with serial passaging in our in vitro assay and that more quiescent cells did not make spheres (Supplementary Fig. S1i, ii; Supplementary Data are available online at www.liebertpub.com/scd). Then, to determine whether ALDH activity continued to mark mitotically active sphere-forming cells with serial passaging, we sorted passaged Itga6hi ABSCs into ALDHhi and ALDHlo populations and re-cultured them in the sphere assay. While ALDH activity marked the sphere-forming cells of the airway epithelium, there were no differences in the sphere-forming potential between ALDHhi and ALDHlo cells in the passaged cell cultures (Supplementary Fig. S1iii, iv).
Identification and validation of ALDH isoforms that are highly expressed in proximal airway epithelium
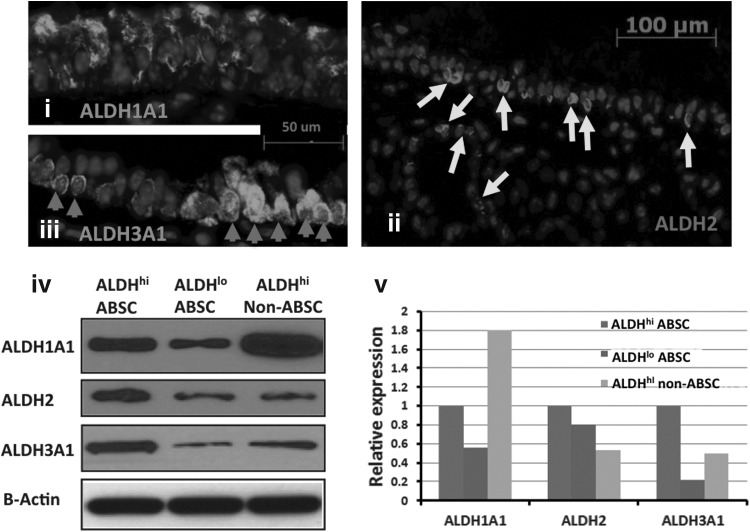
To identify which ALDH isozymes are important in the airway epithelium, we examined gene expression profiles of ALDHhi non-ABSCs, ALDHhi ABSCs, and ALDHlo ABSCs using high-throughput RNA sequencing (RNA-seq) (Table 1). Differentially expressed candidate genes from the RNA-seq data were validated by QPCR, (Supplementary Fig. S2). The RNA-seq data showed that Aldh1a1, Aldh2, Aldh3a1, and Aldh1a7 were the most highly expressed ALDH isozymes in mouse airways and lung, which is similar to what we found in our previous RNA microarray studies [1] and in a previously published study [24]. Because Aldh1a7 has no corresponding human gene, we restricted our further studies to Aldh1a1, Aldh2, and Aldh3a1. We performed immunostaining for these three ALDHs in mouse tracheal sections and found that all proximal airway epithelial cells expressed these three ALDH isoforms although ALDH2 and ALDH3A1 had higher expression in ABSCs and SMG duct cells while ALDH1A1 had higher expression in non-ABSCs (Fig. 2i–iii). We also compared protein and RNA expression of Aldh1a1, Aldh2, and Aldh3a1 in ALDHhi ABSC, ALDHlo ABSC, and ALDHhi non-ABSC populations. We confirmed that the protein and gene expression of these three ALDH isoforms were higher in the ALDHhi cells than the ALDHlo cells (Fig. 2iv, v).
Table 1.
Relative Gene Expression (RPKM) of ALDH Isozymes from RNA-Seq of Subpopulations of Mouse Airway Epithelial Cells
| Gene | Non-basal ALDHhi | Non-basal ALDHhi | Basal ALDHhi | Basal ALDHhi | Basal ALDHlo | Basal ALDHlo |
|---|---|---|---|---|---|---|
| Aldh1a1 | 1863.88 | 1621.72 | 851.606 | 765.868 | 327.942 | 275.356 |
| Aldh3a1 | 115.371 | 133.11 | 35.8555 | 67.7412 | 54.0426 | 51.4267 |
| Aldh1a7 | 74.8274 | 54.5831 | 39.3715 | 33.0375 | 27.9833 | 18.2261 |
| Aldh2 | 52.4179 | 69.4437 | 39.9574 | 31.7235 | 30.4623 | 35.5069 |
| Aldh3a2 | 39.0079 | 33.9991 | 115.14 | 29.953 | 16.3966 | 22.787 |
| Aldh6a1 | 14.2279 | 9.24365 | 14.8621 | 18.8626 | 17.3809 | 17.7573 |
| Aldh16a1 | 14.1157 | 16.6422 | 14.8939 | 9.55912 | 7.451 | 4.86497 |
| Aldh18a1 | 9.84418 | 11.2891 | 7.62724 | 2.78219 | 4.88272 | 2.31466 |
| Aldh3b1 | 9.8247 | 3.14901 | 3.34813 | 2.88716 | 1.26869 | 1.76851 |
| Aldh7a1 | 8.43542 | 11.1004 | 8.5909 | 15.6174 | 11.0085 | 35.9611 |
| Aldh9a1 | 5.7111 | 8.55623 | 6.00172 | 5.67304 | 7.47862 | 4.16696 |
| Aldh4a1 | 4.41157 | 7.01552 | 3.01155 | 5.91411 | 3.17983 | 6.53516 |
| Aldh5a1 | 4.34505 | 4.95532 | 3.63737 | 2.37622 | 1.84831 | 1.72413 |
| Aldh1l1 | 3.71107 | 3.0356 | 1.3466 | 1.69656 | 1.02243 | 0.957952 |
| Aldh3b2 | 0.876781 | 10.3994 | 5.80983 | 1.31737 | 0.995072 | 1.16484 |
| Aldh1a3 | 0.574336 | 0.190996 | 0.451274 | 0.322906 | 0.0961869 | 0.0623636 |
| Aldh1a2 | 0.461689 | 0.129546 | 0.36822 | 0.250303 | 0 | 0 |
| Aldh1l2 | 0.395049 | 2.0586 | 0.0325728 | 0.218406 | 0.233396 | 0.468432 |
| Aldh1b1 | 0.324425 | 0.0910306 | 0.181121 | 0.0947076 | 0.0550123 | 0.076091 |
| Aldh8a1 | 0.132232 | 0.0185515 | 0 | 0 | 0.0771887 | 0 |
Two samples of each population are represented from two different sorts.
ALDH, aldehyde dehydrogenase; RNA-seq, RNA sequencing.
FIG. 2.
Expression of ALDH1A1, ALDH2, and ALDH3A1 in the airway epithelium. Immunofluorescent staining of mouse trachea for ALDH1A1 (i), ALDH2 (ii), and ALDH3A1 (iii). Arrows point to positively stained basal and submucosal gland duct cells. (iv) Western blot for ALDH1A1, ALDH2, and ALDH3A1 on protein collected from the fluorescence-activated cell sorted ALDHhi and ALDHlo ABSC and ALDHhi non-ABSC (differentiated) cell populations. (v) Densitometry quantification of the bands in (iv). The expression levels of ALDH1A1, ALDH2, and ALDH3A1 in the ALDHlo ABSCs and ALDHhi non-ABSCs are shown relative to their expression in ALDHhi ABSCs. Protein levels were normalized with B-Actin.
Effect of inhibition and induction of the ALDH isoforms on ABSC sphere formation, proliferation, and differentiation capabilities
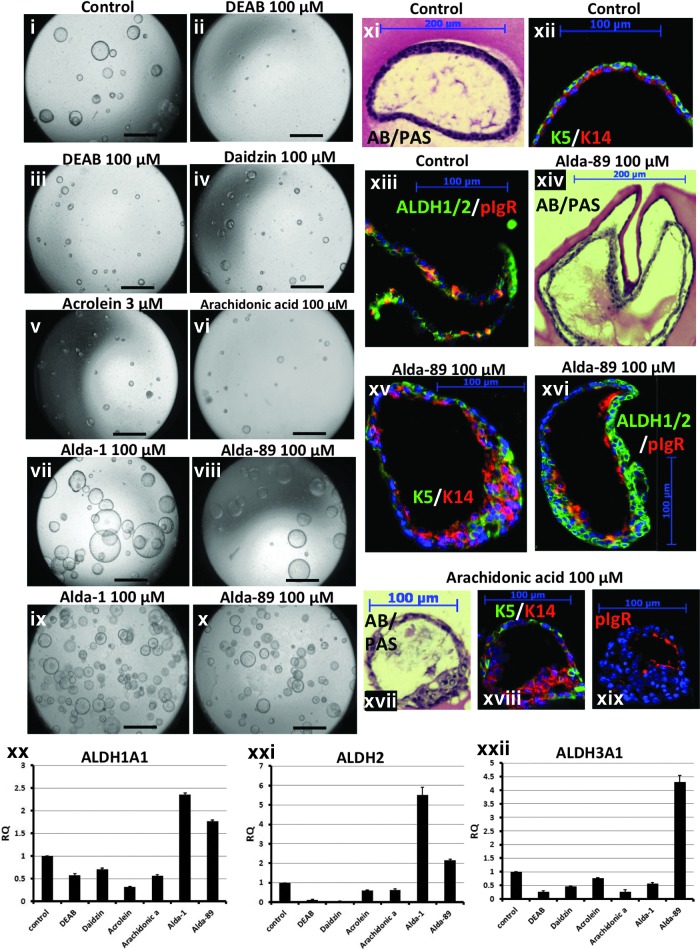
To examine the role of ALDH activity in sphere formation, we treated sorted ABSCs or SMG duct cells with the broad-spectrum ALDH inhibitor, DEAB (100–200 μM), at day 0 of in vitro culture (before sphere formation occurs) or at day 7 of culture, after spheres had already formed but were still small in size and undergoing rapid proliferation [1]. We found that treatment at day 0 markedly diminished sphere formation (Fig. 3ii), while treatment at day 7 (Fig. 3iii), after spheres had already formed, resulted in a decrease in sphere size compared with control (Fig. 3i–iii, Supplementary Table S1 and Supplementary Fig. S3).
FIG. 3.
Effect of inhibition and induction of the ALDH isoforms on stem/progenitor cell proliferation and differentiation capabilities. (i–x) Images taken 14 days after seeding of ABSCs at equal densities. (i) Vehicle-treated. (ii) 100 μM diethylaminobenzaldehyde (DEAB) at culture day 0. (iii–viii) 100 μM DEAB, daidzin, acrolein, arachidonic acid (AA), Alda-1, and Alda-89 at culture day 7. (ix, x) 100 μM Alda-1 and Alda-89 at culture day 0, Scale bar=500 μm. (xi, xiv, xvii) Alcian blue/Periodic Acid Schiff staining, (xii, xv, xviii) immunofluorescent staining for Keratin 5 and 14 and (xiii, xvi, xix) immunofluorescent staining for polymeric immunoglobulin receptor (pIgR) and ALDH1/2 from vehicle-treated control, Alda-89-treated well and AA treated well, respectively. (xx–xxii) Quantitative real-time PCR (QPCR) assessment of the effect of each agonist and antagonist on relative gene expression of Aldh1a1, Aldh2, and Aldh3a1.
To further assess the effect of individual ALDH isozymes on ABSC sphere formation and proliferation, we similarly treated sorted cells in culture with specific ALDH2, ALDH1A1, and ALDH3 inhibitors namely, daidzin [25], acrolein [26] and high dose AA [27], respectively. These three agents had similar effects to DEAB, that is, treatment at day 0 markedly diminished sphere formation (data not shown), while treatment at day 7 after spheres had already formed resulted in a decrease in sphere size (Fig. 3iv–vi, Supplementary Table S1 and Supplementary Fig. S3).
To directly examine the effect of ALDH inhibition on cell proliferation, we immunostained sections of spheres that were previously treated with ALDH inhibitors for proliferating cell nuclear antigen and Ki-67 expression and counted the number of proliferating cells. We found that ALDH inhibition significantly reduced the number of proliferating cells within spheres (Supplementary Table S1 and Supplementary Fig. S3ii). We used trypan blue exclusion to confirm that all ALDH antagonists used at the specified concentrations had no effect on cell death compared to the vehicle treated control (data not shown).
To examine the effects of agonists of these ALDH isoenzymes on sphere formation and proliferation, we used Alda-1 and Alda-89, two recently developed small molecule agonists for ALDH2 and ALDH3, respectively [28,29]. Treating sorted ABSCs at day 7 with Alda-1 or Alda-89 produced significantly larger spheres compared to controls with the average sphere diameter increasing from the untreated average size of 110±34 μm to 132±42 μm and 128.5±54 μm (increases of 19.7% and 16.5% respectively (P<0.004 and P<0.005 respectively) (Fig. 3vii, viii and Supplementary Fig. S3), while treatment with these agonists at day 0 produced larger numbers of spheres and larger diameter spheres compared to untreated controls (Fig. 3ix, x).
We next examined whether altering ALDH levels in growing spheres affected the differentiation ability of ABSCs. Compared to untreated controls, all ALDH agonists and antagonists, in spite of affecting ABSC proliferation in the sphere assay, did not affect differentiation toward the secretory lineage as both mucus and serous secretions were detectable 2 weeks after treatment (Fig. 3xiv–xvi represent agonists and xvii-xix represent antagonists).
To confirm the direct inhibitory or inductive effect of these antagonists and agonists on ABSCs in our assays, Aldh1a1, Aldh2, and Aldh3a1 expression in the treated or control spheres were examined using QPCR. Alda-1 and Alda-89 significantly induced Aldh2 and Aldh3a1 expression, respectively. However, Alda-1 and Alda-89 treatment also moderately induced Aldh1a1 expression. The antagonists acrolein, daidzin, and high concentrations of AA, were the most efficient in reducing the expression of Aldh1a1, Aldh2, and Aldh3a1, respectively. However, the expression of each gene was also variably reduced by all the other inhibitors (Fig. 3xx–xxii), indicating a lack of specificity for induction of each isozyme, at the concentrations used. Taken together, these results demonstrate that ALDH activity is not only a marker of the proliferative subpopulation of ABSCs, but also functionally important in sphere formation and cell proliferation in the sphere assay.
ALDHhi ABSCs have a few key gene expression profile differences when compared to the ALDHlo ABSCs
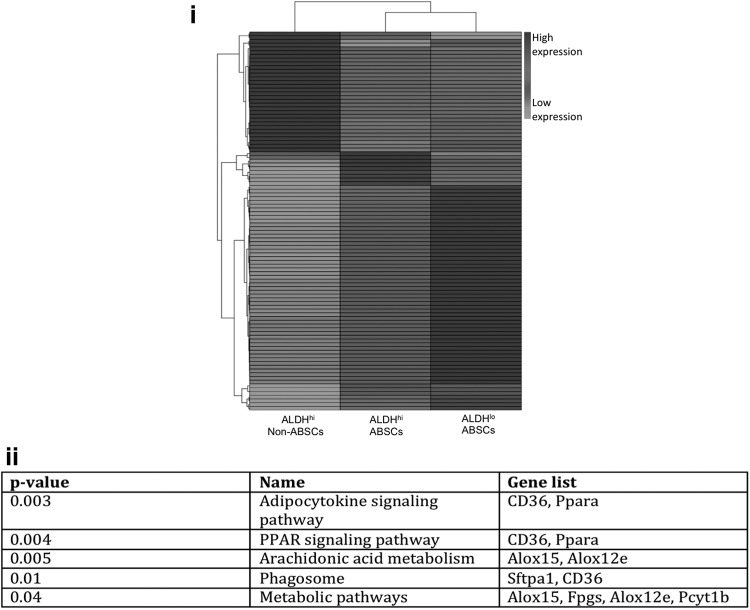
Analysis of the RNA-seq data comparing differentially expressed genes between ALDHhi and ALDHlo ABSCs revealed that ∼200 genes were significantly differentially expressed between the two cell populations, despite the fact that functionally only the sorted ALDHhi cells could produce spheres in culture (Fig. 4i. Gene Expression Omnibus accession number pending). Kegg pathway analysis of the RNA-seq data revealed that two of the pathways that were most significantly upregulated in the sphere-forming ALDHhi ABSCs versus non-sphere-forming ALDHlo non-ABSCs were the PPAR signaling pathway (Cd36, Pparα), and the AA metabolism pathway (lipoxygenases 15 and 12e) (Fig. 4ii).
FIG. 4.
Analysis of RNA-sequencing (RNA-seq) data from sorted ALDHhi ABSCs, ALDHlo ABSCs, and ALDHhi non-ABSCs. (i) Heatmap demonstrating the top 100 differentially expressed genes between ALDHhi and ALDHlo subpopulations. (ii) Table demonstrating Kegg pathway analysis of RNA-seq data for the pathways that are most significantly upregulated in the ALDHhi ABSC sphere-forming cells versus ALDHlo non-sphere-forming cells.
Differential metabolism of AA by subpopulations of ABSCs
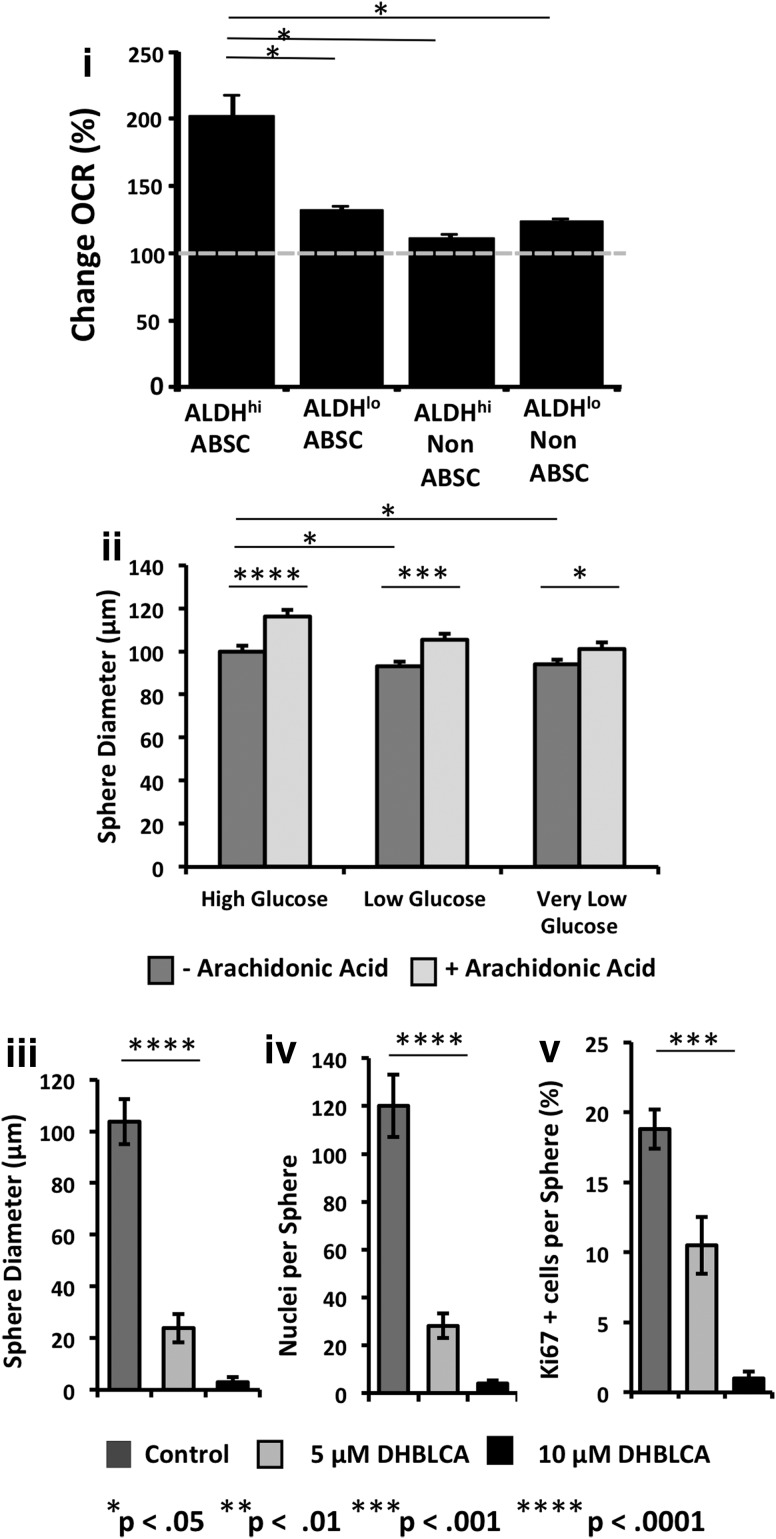
Based on the RNA-seq data, we sought to determine whether ALDHhi ABSCs are more efficient than ALDHlo ABSCs in utilizing AA as a metabolic substrate. We therefore sorted ALDHhi ABSC, ALDHlo ABSC, ALDHhi non-ABSC, and ALDHlo non-ABSC subpopulations and measured the change in their OCR after incubating cells with an empirically set standard concentration of the fatty acid, Palmitate (20 μM) or the same concentration of AA using the XF96 Seahorse bioenergetic flux analyzer. In the presence of AA, the OCR values of ALDHhi ABSCs increased two-fold (200%±6.4% P<0.01) relative to the basal respiration OCR (Fig. 5i). However, ALDHlo ABSCs showed only a mild increase (31%±3.9% P<0.2) in OCR as a result of AA metabolism. In contrast, the differentiated non-ABSC subpopulation, which is highly metabolic due to production of mucus and serous secretions and cilia motility, did not utilize AA as a substrate for oxidative phosphorylation (Fig. 5i). These data suggest that the subpopulation of ABSCs with ALDHhi activity might be better equipped to utilize AA as a substrate compared to the ALDHlo ABSCs and non-ABSCs.
FIG. 5.
AA metabolism in airway epithelial cell subpopulations. (i) Airway epithelial cells were sorted into ALDHhi ABSC, ALDHlo ABSC, ALDHhi non-ABSC, and ALDHlo non-ABSC subpopulations and cultured with AA as the only substrate. The change in oxygen consumption rate (OCR) was measured as a result of AA metabolism by each subpopulation. Dotted line shows % basal respiration before injection of AA. Data are expressed as mean±S.D. (n=3), (ii) Spheres grown under conditions of normal, low, or very low glucose concentrations showed an increase in stem/progenitor cell proliferation with larger sphere diameter with the addition of AA (P<0.0001 for high glucose, P<0.001 for low glucose, P<0.05 for very low glucose). Spheres exposed to low and very low glucose showed a decrease in sphere size compared to spheres grown in high glucose medium (P<0.05). (iii–v) Specific inhibition of lipoxygenases-12e and −15 with ethyl 3,4-dihydroxybenzylidenecyanoacetate (DHBLCA) resulted in a dose-dependent decrease in ABSC proliferation with a decrease in sphere diameter, nuclei per sphere, and reduced Ki67 expression (P<0.0001 and P<0.001 respectively).
The ability of ABSCs to utilize AA as a source of energy in the presence or absence of glucose
We hypothesized that during airway injury when glucose levels are low due to an interruption of blood supply or inflammation, progenitor cells would require an alternative energy source to proliferate rapidly and repair the airway. We therefore tested whether ABSCs would have the ability to utilize AA as an energy source when glucose is limited. The Dulbecco's modified Eagle's medium/F12 cell culture medium that we routinely use to grow airway cells in vitro has a supraphysiologic glucose concentration of 25 mM. Culturing the cells with a normoglycemic glucose concentration (5.56 mM) in the medium will expose cells to increasing hypoglycemia until the next medium change. To examine the effects of varying concentrations of glucose on ABSC sphere formation, we cultured sorted ABSCs with the usual high glucose medium until spheres were visible in all wells, then continued culture using the same high glucose (25 mM) medium, low glucose (5.56 mM), or very low glucose (2.78 mM) medium for seven more days. We found that spheres exposed to low and very low glucose had a statistically significant decrease in sphere size compared with spheres grown in high glucose medium (Fig. 5ii, P<0.05). The lower glucose medium had no effect on sphere-forming efficiency or on differentiation of cell types in the spheres. Prior to testing the ability of ABSCs to uilitze AA as a metabolic substrate when glucose levels are low, we examined a dose course of AA to identify the optimum concentration at which AA causes a positive OCR using the XF96 Seahorse bioenergetic flux analyzer. We found that 60 μM or less of AA produced induction in OCR while 100 μM or more induced a down turn of OCR (data not shown). Therefore, we repeated the high, low, and very low glucose treatment experiment described above with AA supplementation. We found that 60 μM AA supplementation increased the average sphere diameter in the high, low, and very low glucose treatments (Fig. 5ii) (P<0.0001, P<0.001, and P<0.05 respectively). Thus, AA is an additional substrate for ALDHhi ABSCs that may be especially important when glucose is limited.
Inhibition of lipoxygenases-12e and -15 prevents ABSC sphere formation and proliferation
We found that ALDHhi ABSCs have increased lipoxygenase 12e and 15 gene expression and utilitze AA as a metabolic substrate more efficiently than the ALDHlo ABSC population. Therefore, to further examine the role of AA metabolism in ABSC sphere formation and proliferation, we inhibited lipoxygenases-12e (Alox12e or 8-LOX) and -15 (Alox15), the two lipoxygenases that were significantly upregulated in our RNA-seq data. We used the specific lipoxygenase 12e and 15 inhibitor, DHBLCA in the sphere-forming assay. We performed a dose course and found that at a concentration of 20 μM DHBLCA, there was complete inhibition of sphere formation, although the cells were viable (data not shown). At lower DHBLCA concentrations, sphere formation did occur but the spheres were smaller in size and fewer in number (Fig. 5iii, iv, P<0.0001, and data not shown). To evaluate the effect of DHBLCA on cell proliferation, treated spheres were embedded, sectioned, and immunostained for Ki67. We found decreased cell proliferation when spheres were treated with 5 and 10 μM DHBLCA (Fig. 5v, P<0.001 and Supplementary Fig. S4).
Discussion
Here, we show that high ALDH activity is a marker that enriches for the population of ABSCs that proliferate to generate spheres in vitro. In addition to being a marker, the ALDH activity within the ABSCs is functionally important for their ability to proliferate to form spheres. One mechanism for this appears to be the ability of ALDHhi ABSCs to utilize AA more efficiently than other cell populations in the airway epithelium.
Aldehydes are derived from endogenous (eg, lipid peroxidation) and exogenous sources (eg, smoke) that form DNA adducts in cells and are therefore toxic to DNA [17]. ALDHs provide an efficient system for removal of aldehydes, which is critical to protect cells. As smoke is a well-known source of aldehydes, the airway epithelial cells are required to efficiently remove aldehydes to avoid their toxicity. We therefore speculate that ALDHs play an important role in protecting the airway epithelium, in addition to being markers of proximal ABSC populations. This is supported by the results of our in vitro experiments in which global ALDH inhibition interfered with ABSC proliferation. Further, ABSC proliferation increased when cultured with ALDH agonists, suggesting a functional role for ALDHs in ABSC repair after injury.
There are 19 different ALDH isozymes [14–16], which likely speak for their importance in the body, but here we identified and characterized the expression pattern of the three most highly expressed ALDH isozymes in the airway epithelium. In mouse tracheal sections, we found expression of ALDH2 and ALDH3A1 in ABSCs and SMG duct cells and expression of ALDH1A1 in non-ABSCs. Of note, none of the chemical or small molecule agonists [30,31] and antagonists [25,26,32] that we used to specifically perturb ALDH1A1, ALDH2, or ALDH3A1 isozyme levels were completely specific for these isoforms, suggesting overlapping roles. In addition, it is possible that the agonists and antagonists that we used to target specific ALDH isoforms may have exerted their effect on specific ALDH isozymes early on in the time course of the in vitro cultures and other ALDH isozymes may have been upregulated or downregulated to compensate for the lack of ALDH activity. It is also possible that these ALDH isozyme specific agonists and antagonists have dose-dependent effects on specificity that we did not test for in our system.
We used the “Aldefluor fluorescent reagent system” to detect ALDH activity in airway epithelial cells. Although ALDH1A1 is considered to be the main isozyme detected by Aldefluor and inhibited by DEAB, it has been recently shown that Aldefluor and DEAB are not specific for isoform ALDH1A1, as they can also detect and inhibit other isoforms like ALDH1A2 and ALDH2 [32]. In addition, the full range of ALDH isozymes that oxidize Aldefluor are as yet unknown. Our data also show that within ABSCs, ALDH2 and ALDH3A1, in addition to ALDH1A1, oxidize Aldefluor.
Despite the fact that multiple ALDH isozymes appear to have similar functions, it is important to note that mutations in the human ALDH2 gene are well described and in addition to suffering from flushing with alcohol, these patients are known to be at higher risk for squamous cancers, especially oropharyngolaryngeal, esophageal, gastric and lung cancer [33–37]. We speculate that because the airway epithelium is exposed to a large amount of exogenous aldehydes from smoke, loss of ALDH2 function may lead to aberrant repair and this together with the reduced ability to clear toxic aldehydes may predispose to lung cancer.
While high Aldefluor activity was important for determining which population of freshly sorted cells was capable of sphere formation, this was not the case with serial propagation of the spheres. The inability of high Aldefluor activity to select for ABSCs with self-renewal capacity during serial propagation in the sphere assay is consistent with observations in skeletal muscle progenitors, mesenchymal stem cells, and endothelial cells showing that Aldh expression is occasionally uncoupled from functional activity when cells are propagated in culture [38]. This may reflect the change in cellular microenvironment in the in vitro culture system and/or the reduction in aldehyde exposure in culture.
The concept of a “niche” cell in the epithelium that provides environmental cues and possibly paracrine factors has been established in the colon, where Paneth cells have been found to promote gut stem cell sphere formation [39]. Here, we found that a cell in the ALDHhi non-ABSC population promotes sphere formation. This is further supported by our previous studies where we found that the sphere-forming efficiency of ABSCs was 2.1%±0.6% [1]. However, since we have become stricter with our gating system for FACS, the sphere-forming efficiency in our current experiments has decreased. It is possible that in our earlier gating strategy, some non-ABSCs were included in the sort (including the niche cells that we are describing here) and enhanced the sphere formation that we previously described [1].
AA is a polyunsaturated fatty acid that is present in cell plasma membranes and is a lipid second messenger involved in cell signaling and inflammation. AA is derived from membranes by the action of Phospholipase A2 (PLA2) and is a precursor of the eicosanoids. It has been proposed that cellular injury to the airway results in prolonged activation of PLA2, resulting in hydrolysis of plasma membrane phospholipids and release of AA [40]. In our RNA-seq comparison of ALDHhi and ALDHlo ABSCs, we found upregulation of lipoxygenases-12e and -15, implying that AA may be an important energy source for ALDHhi ABSCs. We showed that the addition of AA to ABSCs enhanced proliferation and this could be blocked with lipoxygenase inhibition. This suggests that following severe airway injury, when the blood supply may be interrupted and glucose supply is low, ALDHhi ABSCs may utilize AA in the local environment to facilitate proliferation for repair. Interestingly, the overexpression of lipoxygenase-15 in transgenic mice resulted in hyperplasia of prostate basal cells [41]. Further, lipoxygenases-12e and 15 have been linked to epithelial proliferation in corneal repair after injury [42]. In addition, asthmatics have been found to have high lipoxygenase levels in their airways and leukotriene inhibitors are clinically used in these patients to reduce inflammation, as well as reduce bronchial and vascular constriction. In light of our studies, we speculate that some of the epithelial cell proliferation and remodeling seen in asthma may arise from abnormally high lipoxygenase expression in ABSCs. This is supported by the fact that mice lacking lipoxygenase-12/15 have reduced airway epithelial proliferation in addition to reduced inflammation [43].
Conclusion
We have shown that ALDHs play a functional role in ABSC proliferation and perturbation of these enzyme levels alters proliferation in the sphere assay. In addition, we found that the specific isozymes of ALDH, ALDH1A1, ALDH2, and ALDH3A1 are highly enriched in the airway epithelium. Mechanistically, when comparing transcriptional profiles between ALDHhi and ALDHlo ABSC populations, we found that the AA metabolism pathway, specifically via lipoxygenases-12e and -15, is upregulated in the ALDHhi ABSCs and that this promotes proliferation of these cells. We speculate that AA metabolic pathway plays a critical role in repair of the airway epithelium after injury.
Supplementary Material
Acknowledgments
We would like to acknowledge the UCLA Broad Stem Cell Research Center High-Throughput Sequencing Core Resource and the UCLA Broad Stem Cell Research Center FACS Core Resource. We would like to thank Dr. Mochly-Rosen for the Alda-1 and Alda-89 reagents. Acknowledgment of grants, equipment, or drugs for research support: CIRM RN2-00904-1, R01 HL094561, American Thoracic Society/COPD Foundation ATS-06-065, the Concern Foundation, the UCLA Jonsson Comprehensive Cancer Center Thoracic Oncology Program/Lung Cancer SPORE, the University of California Cancer Research Coordinating Committee, and the Gwynne Hazen Cherry Memorial Laboratories (BG).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisht B, Ooi AT, et al. (2011). Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 29:1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisht B, Nickerson DW. and Gomperts BN. (2012). Isolation and in vitro characterization of Basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med 1:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegab AE, Ha VL, Attiga YS, Nickerson DW. and Gomperts BN. (2012). Isolation of basal cells and submucosal gland duct cells from mouse trachea. J Vis Exp e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KU, Reynolds SD, Watkins S, Fuchs E. and Stripp BR. (2004). Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 164:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH. and Hogan BL. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106:12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastrana E, Silva-Vargas V. and Doetsch F. (2011). Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8:486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N. and Lako M. (2004). Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells 22:1142–1151 [DOI] [PubMed] [Google Scholar]
- 8.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, Eades WC, Creer MH. and Nolta JA. (2006). Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood 107:2162–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA. and Bonnet D. (2005). Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells 23:752–760 [DOI] [PubMed] [Google Scholar]
- 11.Marcato P, Dean CA, Giacomantonio CA. and Lee PW. (2011). Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 10:1378–1384 [DOI] [PubMed] [Google Scholar]
- 12.Patel M, Lu L, Zander DS, Sreerama L, Coco D. and Moreb JS. (2008). ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer 59:340–349 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, et al. (2010). Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res 70:9937–9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida A, Rzhetsky A, Hsu LC. and Chang C. (1998). Human aldehyde dehydrogenase gene family. Eur J Biochem 251:549–557 [DOI] [PubMed] [Google Scholar]
- 15.Sophos NA. and Vasiliou V. (2003). Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact 143–144:5–22 [DOI] [PubMed] [Google Scholar]
- 16.Vasiliou V. and Nebert DW. (2005). Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics 2:138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien PJ, Siraki AG. and Shangari N. (2005). Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol 35:609–662 [DOI] [PubMed] [Google Scholar]
- 18.You Y, Richer EJ, Huang T. and Brody SL. (2002). Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 283:L1315–L1321 [DOI] [PubMed] [Google Scholar]
- 19.Trapnell C, Pachter L. and Salzberg SL. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ. and Pachter L. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S. and Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol 11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ. and Auwerx J. (2008). Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358 [DOI] [PubMed] [Google Scholar]
- 23.Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP. and Pelicci PG. (2009). The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138:1083–1095 [DOI] [PubMed] [Google Scholar]
- 24.Alnouti Y. and Klaassen CD. (2008). Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci 101:51–64 [DOI] [PubMed] [Google Scholar]
- 25.Keung WM. and Vallee BL. (1993). Daidzin: a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc Natl Acad Sci U S A 90:1247–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren S, Kalhorn TF. and Slattery JT. (1999). Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein. Drug Metab Dispos 27:133–137 [PubMed] [Google Scholar]
- 27.Canuto RA, Maggiora M, Trombetta A, Martinasso G. and Muzio G. (2003). Aldehyde dehydrogenase 3 expression is decreased by clofibrate via PPAR gamma induction in JM2 rat hepatoma cell line. Chem Biol Interact 143–144:29–35 [DOI] [PubMed] [Google Scholar]
- 28.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J. and Cardoso WV. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136:2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA. and Rajagopal J. (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136:1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banh A, Xiao N, Cao H, Chen CH, Kuo P, Krakow T, Bavan B, Khong B, Yao M, et al. (2011). A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res 17:7265–7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanna M, Chen CH, Kimble-Hill A, Parajuli B, Perez-Miller S, Baskaran S, Kim J, Dria K, Vasiliou V, Mochly-Rosen D. and Hurley TD. (2011). Discovery of a novel class of covalent inhibitor for aldehyde dehydrogenases. J Biol Chem 286:43486–43494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B. and Chang LJ. (2012). The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact 195:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katada C, Muto M, Nakayama M, Tanabe S, Higuchi K, Sasaki T, Azuma M, Ishido K, Katada N, et al. (2012). Risk of superficial squamous cell carcinoma developing in the head and neck region in patients with esophageal squamous cell carcinoma. Laryngoscope 122:1291–1296 [DOI] [PubMed] [Google Scholar]
- 34.Oyama T, Nagayoshi H, Matsuda T, Oka M, Isse T, Yu HS, Pham TT, Tanaka M, Kagawa N, Kaneko K. and Kawamoto T. (2010). Effects of acetaldehyde inhalation in mitochondrial aldehyde dehydrogenase deficient mice (Aldh2-/-). Front Biosci 2:1344–1354 [DOI] [PubMed] [Google Scholar]
- 35.Park JY, Matsuo K, Suzuki T, Ito H, Hosono S, Kawase T, Watanabe M, Oze I, Hida T, et al. (2010). Impact of smoking on lung cancer risk is stronger in those with the homozygous aldehyde dehydrogenase 2 null allele in a Japanese population. Carcinogenesis 31:660–665 [DOI] [PubMed] [Google Scholar]
- 36.Lewis SJ. and Smith GD. (2005). Alcohol, ALDH2, and esophageal cancer: a meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev 14:1967–1971 [DOI] [PubMed] [Google Scholar]
- 37.Matsuo K, Oze I, Hosono S, Ito H, Watanabe M, Ishioka K, Ito S, Tajika M, Yatabe Y, et al. (2013). The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis 34:1510–1515 [DOI] [PubMed] [Google Scholar]
- 38.Balber AE. (2011). Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells 29:570–575 [DOI] [PubMed] [Google Scholar]
- 39.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M. and Clevers H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings BS, McHowat J. and Schnellmann RG. (2000). Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther 294:793–799 [PubMed] [Google Scholar]
- 41.Suraneni MV, Schneider-Broussard R, Moore JR, Davis TC, Maldonado CJ, Li H, Newman RA, Kusewitt D, Hu J, Yang P. and Tang DG. (2010). Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence. Oncogene 29:4261–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenchegowda S, Bazan NG. and Bazan HE. (2011). EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Invest Ophthalmol Vis Sci 52:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson CK, Claesson HE, Rydell-Tormanen K, Swedmark S, Hallgren A. and Erjefalt JS. (2008). Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am J Respir Cell Mol Biol 39:648–656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.