Abstract
Template polymerization of a high internal phase emulsion (polyHIPE) is a relatively new method to produce tunable high-porosity scaffolds for tissue regeneration. This study focuses on the development of biodegradable injectable polyHIPEs with interconnected porosity that have the potential to fill bone defects and enhance healing. Our laboratory previously fabricated biodegradable polyHIPEs that cure in situ upon injection; however, these scaffolds possessed a closed-pore morphology, which could limit bone ingrowth. To address this issue, HIPEs were fabricated with a radical initiator dissolved in the organic phase rather than the aqueous phase of the emulsion. Organic-phase initiation resulted in macromer densification forces that facilitated pore opening during cure. Compressive modulus and strength of the polyHIPEs were found to increase over 2 weeks to 43±12 MPa and 3±0.2 MPa, respectively, properties comparable to cancellous bone. The viscosity of the HIPE before cure (11.0±2.3 Pa·s) allowed for injection and filling of the bone defect, retention at the defect site during cure under water, and microscale integration of the graft with the bone. Precuring the materials before injection allowed for tuning of the work and set times. Furthermore, storage of the HIPEs before cure for 1 week at 4°C had a negligible effect on pore architecture after injection and cure. These findings indicate the potential of these emulsions to be stored at reduced temperatures and thawed in the surgical suite before injection. Overall, this work highlights the potential of interconnected propylene fumarate dimethacrylate polyHIPEs as injectable scaffolds for bone tissue engineering.
Introduction
Emulsion templating is a relatively new technique for the fabrication of high-porosity scaffolds.1 High internal phase emulsions are characterized by an internal (droplet) phase volume fraction greater than 74%.2 Polymerization of the continuous phase locks in the emulsion geometry resulting in a highly porous foam (polyHIPE).3 Previously, polyHIPEs have been utilized as catalyst supports,4–6 solid-phase synthesis platforms,7 substrates for electrodes,8 and materials for cell culture.9 Within the past decade, researchers have investigated the use of polyHIPEs as tissue-engineered scaffolds to provide structural support and direct cell behavior for tissue regeneration.1,10 By varying compositional and processing variables, polyHIPEs with a range of porosities (75–99%), pore sizes (1–100 μm), interconnect sizes (0.2–30 μm), and compressive moduli (2 kPa–60 MPa) have been produced.10–24 These tunable characteristics illustrate the potential of polyHIPEs as high-porosity materials with suitable compressive properties for bone tissue engineering.
A unique characteristic of the polyHIPE system as a tissue-engineered graft is its mayonnaise-like viscosity before cure, which is suitable for injection and retention at the defect site. An injectable scaffold that fills irregularly shaped defects and integrates with host tissue can enhance healing and reduce inflammation.25 Initially, styrene-based polyHIPE systems were studied due to the established control of the interconnected pore architecture. Bioactive components were successfully introduced into styrene-based polyHIPEs to provide substrates that promoted osteoblast and neuronal cell adhesion and proliferation.12,26 Although these polyHIPEs are valuable as three-dimensional (3D) cell culture substrates, these materials are not biodegradable or injectable, which limits their use as tissue-engineered scaffolds. Biodegradability was introduced into polyHIPEs by the substitution of unsaturated polyesters into systems that still contained nonbiodegradable cross-linkers or diluents.10,20,27 Subsequently, fully biodegradable systems were achieved by eliminating styrene and utilizing polyurethane and fumarate chemistries.28,29 Although these compositions were biodegradable, the necessity of a toxic diluent and cure temperatures at or above 60°C rendered them noninjectable. An injectable polyHIPE system requires low viscosity macromers for HIPE formation without the need for a toxic diluent and reaction kinetics permitting cross-linking in situ. Recently, we developed the first biodegradable and injectable HIPE system based on propylene fumarate dimethacrylate (PFDMA) macromers.30 This was a notable advancement in polyHIPEs for bone tissue engineering; however, the lack of interconnectivity reduced the potential for cellular infiltration. Interconnected porosity is vital in tissue-engineered scaffolds to promote the transport of essential nutrients to cells. Recent work by Madden et al. highlighted the importance of interconnectivity for cell migration and nutrient/waste transport in scaffolds with small pore sizes. Human embryonic stem cell-derived cardiomyocytes seeded on bimodal scaffolds with interconnected pores ranging from 30 to 40 μm were viable for at least 2 weeks to a depth of 300 μm into the construct.31 Marcacci et al. reported that the porosity and interconnectivity were also of critical importance in the vascularization and healing of a bone segmental defect clinical trial.32 Therefore, interconnectivity is a key design criterion in the development of an injectable polyHIPE system to support full healing in vivo.
In the polyHIPE system, interconnects or windows between discrete pores form at the gel point from the rupture of the thin walls between droplets.11 This phenomenon is dependent on both wall thickness and macromer densification forces from volume contraction during conversion of monomer to polymer. Varying the wall thickness and densification forces can result in film breakage if the contractile forces are large enough to strain the film to failure. Diluents and monomers utilized in the majority of past polyHIPE systems most likely enhanced the interconnectivity by decreasing wall thickness and increasing the magnitude of densification forces, respectively. Recently, the Silverstein group published studies describing the effect of the locus of initiation on pore architecture and properties.33–36 The authors proposed that organic-phase initiation resulted in larger, spherical, interconnected pores and an increased loss modulus relative to aqueous-phase initiation.34 However, a mechanistic description of the effect of locus of initiation on the resulting macromer densification forces and the corollary effect on interconnect formation was not described.
In these studies, we improved upon our previous injectable PFDMA polyHIPE system to produce interconnected scaffolds that are both biodegradable and injectable with compressive properties comparable to cancellous bone. Characterization studies of pore architecture, injectability parameters, and compressive properties of PFDMA polyHIPEs initiated with the organic-phase soluble initiator benzoyl peroxide (BPO) were performed. A porcine femur model was utilized to determine the retention and integration of the injectable polyHIPE in a defect site. Human mesenchymal stem cell (hMSC) viability and morphology in contact with PFDMA polyHIPEs was assessed. The effects of precure, storage, injection on polyHIPE pore architecture, work time, and set time were also characterized. Finally, the resulting compressive moduli and strength values were evaluated over the course of 2 weeks. Overall, these studies highlight the potential of interconnected biodegradable PFDMA polyHIPEs as injectable bone grafts.
Materials and Methods
Materials
Polyglycerol polyricinoleate (PGPR 4125) was donated by Palsgaard. hMSCs were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White. All other chemicals were purchased and used as received from Sigma–Aldrich, unless otherwise noted.
PFDMA synthesis
PFDMA was synthesized in a two-step process adapted from Timmer et al.37 Briefly, propylene oxide was added dropwise to a solution of fumaric acid and pyridine in 2-butanone (2.3:1.0:0.033 mol) and refluxed at 80°C for 16–18 h. Residual propylene oxide and 2-butanone were removed by distillation; residual acidic byproducts and water were removed with washing, and the product was dried under vacuum. The diester bis(1,2 hydroxypropyl) fumarate product was then end-capped with methacrylate groups in an additional process with triethylamine and methacryloyl chloride. The molar ratios of the diester, methacryloyl chloride, triethylamine were 1:2.1:2.1, respectively. Hydroquinone was added at a molar ratio of 0.008:1 to diester to inhibit cross-linking during the synthesis. The reaction was maintained below −10°C to reduce undesirable side reactions and stirred vigorously overnight under a nitrogen blanket. Triethylamine was removed with a 5% hydrochloric acid wash after neutralization overnight with 2 M potassium carbonate. The PFDMA product was dried under vacuum, and the structure confirmed using H NMR (300 MHz, CdCl3) δ 1.33 (dd, 3H, CH3), 1.92 (s, 3H, CH3), 4.20 (m, 2H, -CH2-), 5.30 (m, 1H, -CH-), 5.58 (s, 1H, -C=CH2), 6.10 (s, 1H, -C=CH2), 6.84 (m, 2H, -CH=CH-). The integration ratio of methacrylate protons to fumarate protons in the 1H NMR spectra was used to confirm >90% functionalization for all macromers before polyHIPE fabrication.
PolyHIPE fabrication
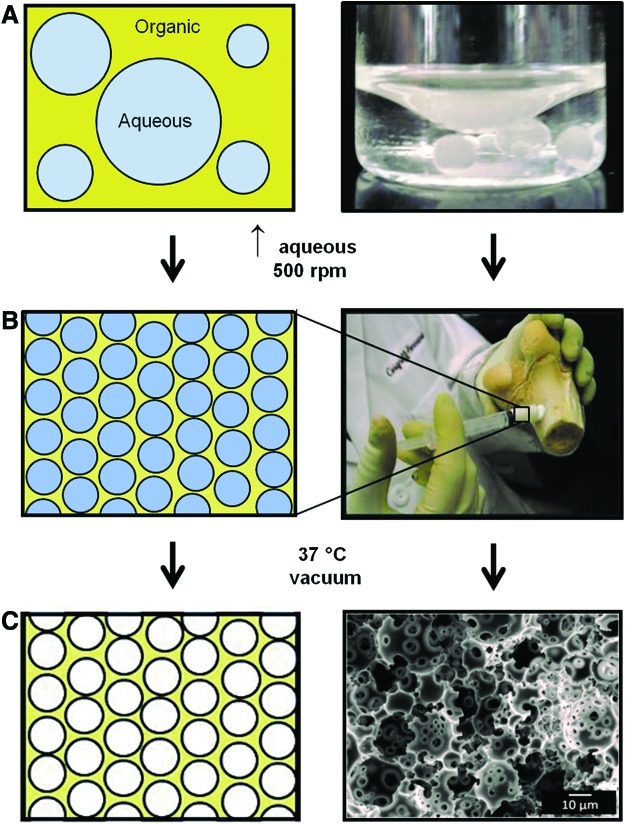
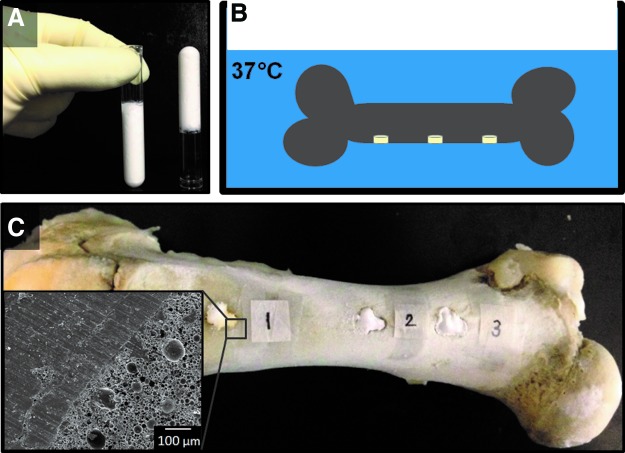
HIPEs were prepared using a FlackTek Speedmixer DAC 150 FVZ-K according to a protocol adapted from Moglia et al.30 PFDMA was first mixed with 5 wt% of the organic-phase soluble initiator, BPO, and 5 wt% of the surfactant, polyglycerol polyricenoleate (PGPR 4125–donated by Palsgaard) before emulsification. Once thoroughly mixed, the aqueous solution of calcium chloride (1 wt%) and deionized water was then added to the organic phase (75% v) in six additions and mixed at 500 rpm for 2.5 min each. HIPEs were transferred to a 37°C aluminum bead bath to facilitate cross-linking overnight. The polyHIPE fabrication process is illustrated in Figure 1. To evaluate the effects of locus of initiation on pore architecture, polyHIPEs were also fabricated with 5 wt% ammonium persulfate (APS), the aqueous-phase soluble initiator, added to the aqueous solution before emulsification with the organic phase. Initiator concentrations were calculated based on the organic phase weight and kept constant between the two systems. This results in a relatively higher initiator concentration in the organic phase (5 wt% BPO to total organic phase) compared to initiator concentration in the aqueous phase (1.66 wt% APS to total aqueous phase).
FIG. 1.
Schematic representation of the process to fabricate a polymerized high internal phase emulsion. (A) The emulsion is composed of a hydrophobic organic phase and an aqueous phase. (B) High internal phase emulsion (HIPE) is defined by the aqueous volume phase greater than 74% and exhibits a whipped mayonnaise consistency before cure. (C) Propylene fumarate dimethacrylate (PFDMA) cross-links at 37°C locking in the emulsion geometry creating a polymerization of HIPE (polyHIPE) with high porosity. Color images available online at www.liebertpub.com/tea
Pore and interconnect size characterization
SEM (JEOL 6500) was utilized to image all polyHIPEs and determine average pore and interconnect size for pretreatment and storage studies. PolyHIPEs were subjected to vacuum drying for 72 h to remove water before pore architecture characterization. Circular specimens from three separate polyHIPE specimens were sectioned into quarters, fractured at the center, coated with gold, and imaged in a rastor pattern yielding five images. Pore size measurements were completed on the first 10 pores that crossed the median of each 1000×magnification micrograph. Average pore sizes for each polyHIPE composition are reported (n=150). A statistical correction was calculated to account for nonperfect spherical pores, h2=R2 − r2, where R is the void diameter's equatorial value, r is the diameter value measured from the micrograph, and h is the distance from the center. The average diameter values were multiplied by this correction factor resulting in a more accurate description of pore diameter.38 To calculate interconnect sizes, three median lines were drawn on each image, and the first 10 interconnects located in pores crossing each of these lines were counted. Average interconnect sizes for each polyHIPE composition are reported (n=150).
Compressive testing
The change in polyHIPE compressive properties over time was investigated to determine the maximum values for a 5 wt% BPO polyHIPE. The PFDMA HIPE was split into five centrifuge tubes and cured at 37°C for 1, 3, 7, 10, and 14 days before mechanical testing. ASTM D1621-04a was utilized to determine the compressive modulus and strength of the polyHIPEs. Each polyHIPE specimen was sectioned into three disks with a 3:1 diameter to height ratio using an Isomet® saw (Buehler) and compressed using an Instron 3300 at a strain rate of 50 μm/s. The compressive modulus was calculated from the slope of the linear region after correcting for zero strain, and the compressive strength was identified as the stress at the yield point or 10% strain, whichever point occurred first. Reported moduli and strength data were averages of the three disks for each time point tested (n=15).
Pretreatment and storage studies
The potential of injectable PFDMA polyHIPEs to be stored at reduced temperatures and then thawed before implementation as a bone graft was evaluated. HIPEs fabricated with 5 wt% BPO were split into six 5-mL syringes and subjected to either 20°C or 37°C, stored for 1 week at 4°C, injected, and cured. HIPEs were also subjected to pretreatment heat conditions before storage to promote cross-linking and reduce cure time in vivo. The duration of pretreatment time was investigated for 1, 2, and 6 h at 20°C and 37°C. Time points for the pretreatment studies were determined as the hourly times that allowed for initial cross-linking before the gel point was reached. The specific storage and pretreatment conditions are detailed in Table 1. This study was repeated for a total of three polyHIPE specimens in each condition.
Table 1.
The Effect of Storage and Injection on polyHIPE Microarchitecture, Work Time, and Set Time
| Storage conditions | Injection conditions | Pretreatment time (h) | Pore size (μm) | Interconnect size (μm) | Work time (h) | Set time (h) |
|---|---|---|---|---|---|---|
| No storage | Not injected | — | 12±10 | 3±2 | — | 3.5±0.5 |
| Injected | — | 12±9 | 3±2 | — | 3.00±0.5 | |
| 1 week, 4°C | Injected | — | 12±9 | 3±2 | 2.25±0.25 | 3.00±0.75 |
| 1 at 20°C | 12±8 | 3±2 | — | 2.25±0.5 | ||
| 2 at 20°C | 11±8 | 3±2 | — | 2.25±0.25 | ||
| 1 at 37°C | 12±11 | 3±2 | 2.00±0.25 | 2.00±0.75 |
Work time was only determined for the storage control and pretreatment at 37°C composition to estimate the range of time the HIPE could be manipulated before injection.
polyHIPE, polymerization of a high internal phase emulsion.
Work and set time
The work time is defined by ISO1997 as the “period of time, measured from the start of mixing, during which it is possible to manipulate a dental material without an adverse effect on its properties.” The work time of the system was determined as the onset of an increase in storage modulus and measured via a dynamic time sweep test using a dynamic mechanical analyzer (DMA, RSAIII, TA Instruments) equipped with a parallel-plate compression clamp. Samples were compressed with a 1% cyclic strain at 1 Hz with measurements taken every 15 min at a constant temperature of 37°C. Work time was determined for three HIPE specimens each from the storage control and pretreatment at 37°C to estimate the range of time the HIPE could be manipulated before injection. The set time was approximated using the tack-free time, which is defined “as the time at which the material could be touched with a spatula with no adhesion of the spatula to the foam.”39 In this study, the HIPE was incubated at 37°C and adherence to a spatula was checked at 15-min intervals.
Integration with bone
The space-filling ability of a 10 wt% PGPR HIPE was evaluated utilizing an ex vivo porcine femur model. Cylindrical voids 1 cm in diameter and 0.5 cm in depth were created with a Dremel rotary tool in the femur and injected with the HIPE. After curing in the bead bath, the bone with polyHIPE was sectioned utilizing a low-speed Isomet® saw and imaged utilizing a scanning electron microscope (SEM) (JEOL 6500). The ability for injectable PFDMA polyHIPEs to fill the defect site without overflow or loss of material was evaluated utilizing an inverted porcine femur model. Three cylindrical voids with a diameter of 1 cm and depth of 0.5 cm were created with a Dremel rotary tool, injected with HIPE solution, and the bone with HIPE inverted in a 37°C water bath overnight. After overnight cure, each defect in the bone was cross-sectioned into three specimens utilizing a low-speed Isomet® saw and imaged utilizing the scanning electron microscope (JEOL 6500, n=9). All bones utilized in these studies were donated by the Rosenthal Meat Science and Technology Center at the Texas A&M University.
In vitro hMSC behavior
Investigation of hMSC viability and morphology was performed to assess initial stem cell response to injectable PFDMA polyHIPEs. Bone marrow-derived hMSCs were obtained as Passage 1 in a cryovial from the Center for the Preparation and Distribution of Adult Stem Cells. Cells were cultured in growth media containing 16.5% fetal bovine serum (Atlanta Biologicals), 1% l-glutamine (Life Technologies), and Minimum Essential Media α (MEM α; Life Technologies) to 80% confluency and utilized at Passages 5 and 6. The cytocompatibility of polyHIPE leachables was determined using a Transwell® (Corning) well plate system with polycarbonate membrane inserts. PolyHIPEs were fabricated as stated above utilizing either 5 wt% BPO or 2,2′-Azobis(2-methylpropionitrile) (AIBN), sectioned into 350-μm-thick wafers using the Isomet® saw, and cut with a razorblade to fit into the Transwell inserts. Specimens were sterilized for 3 h in 70% ethanol, washed four times with phosphate buffered saline (PBS), and incubated overnight in growth media at 5% CO2, 37°C. Cells were trypsinized with 0.25% Trypsin-EDTA (Life Technologies) and seeded at a density of 5000 cells/cm2 onto the polystyrene well plate. Viability at 72 h was assessed utilizing the Live/Dead assay kit (Molecular Probes). Rastor imaging (five images per specimen) was conducted on four specimens (n=20) utilizing a fluorescent microscope (Nikon Eclipse TE2000-S). hMSC adhesion and spreading was visibly assessed utilizing polyHIPEs fabricated with 5 wt% AIBN. One day before seeding, cells were stained with 10 μM CellTracker™ Orange (Molecular Probes) for 45 min in 37°C, 5% CO2, and replenished with growth media overnight. Specimens were sectioned and sterilized as stated previously and subjected to a wetting ladder and progressive solvent extraction with PBS. Wafers were incubated overnight with growth media supplemented with 40 wt% FBS to increase the amount of protein adsorption to provide increased integrin binding sites. Cells were seeded at a density of 250,000 cells/mL (∼7000 cells/mL of polyHIPE). hMSC behavior was observed using a fluorescent microscope (Nikon Eclipse TE2000-S).
Rheology
The zero shear viscosity of the HIPE before cure was measured using a rheometer with a cone-plate configuration (Physica MCR 301, Anton Paar, n=4). Temperature of the cone and plate was kept constant at 25°C.
Statistical analysis
The data are represented as mean±standard deviation for each composition. The Student's t-test was performed on the compressive data to determine any statistically significant differences between compositions. Tests for set time and compressive properties were carried out at a 90% (p<90%) and 95% confidence interval (p<0.05), respectively.
Results and Discussion
Locus of initiation
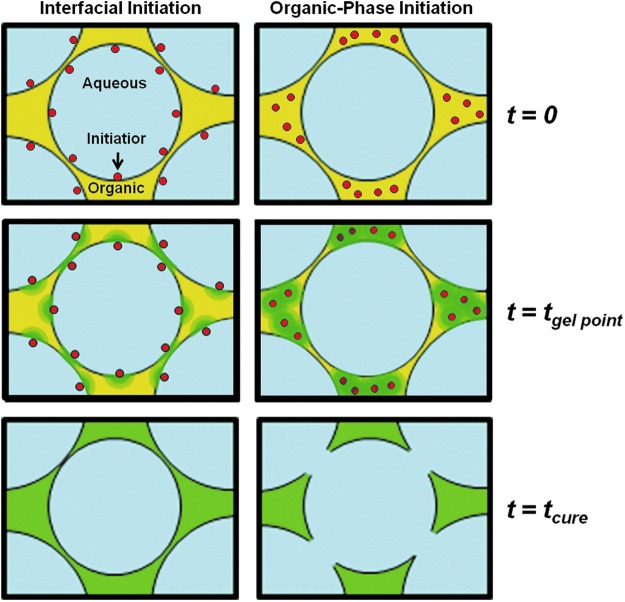
Previous PFDMA polyHIPEs were initiated with the aqueous-phase soluble initiator APS and resulted in closed pore foams. Interconnectivity was achieved in this study by switching the locus of initiation from the aqueous to organic phase using BPO as the radical initiator. The BPO concentration was chosen as the lowest amount that produced cured monoliths in the range of 2–3 h. Because the organic phase comprises a quarter of the total HIPE volume in these studies, the amount of BPO required to achieve roughly the same cure time among the two systems was one third the amount of APS utilized previously. This reduction in initiator concentration decreases the possibility that residual initiator leaches out of the material and has cytotoxic effect in vivo. The effect of the locus of initiation on interconnect formation is illustrated in Figure 2. Organic-phase initiation resulted in the formation of interconnects compared to the closed-pore morphology from aqueous-phase initiation in the PFDMA polyHIPE system. The effect of the locus of initiation on pore interconnectivity has been noted previously in the polyHIPE literature.33–36 However, a mechanistic understanding of the process has yet to be discussed. A probable description of interconnect formation based on the forces generated during the conversion from PFDMA macromer to cross-linked polymer is illustrated in Figure 3. The green gradient region illustrates the progression of cross-linking from initiation sites. It is proposed that the thin film between water droplets in the aqueous-phase initiation system is cross-linked resulting in a closed-pore architecture as evident by the location of the green region between pores. In organic-phase initiation, the green regions are located in the denser pockets where pores are not adjacent to each other. The force of densification upon conversion of macromer to polymer results in forces that pull and tear the thin film between the pores, which is shown as the yellow regions in the figure. This tearing results in interconnects or windows between the pores. With this understanding, future tuning of interconnect size and frequency may be achieved. Current work is focused on increasing densification, which is heavily dictated by the size/density of the monomer/macromer and altering cross-linking kinetics to further increase the interconnect size.
FIG. 2.
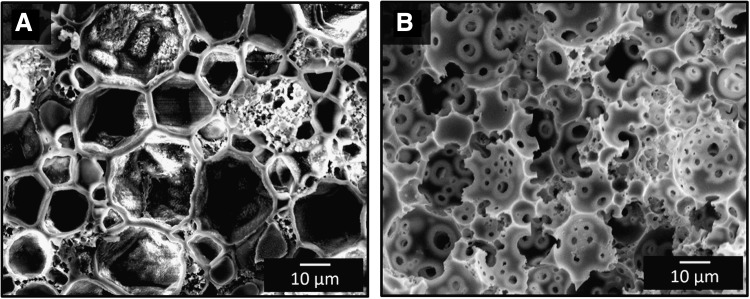
Scanning electron micrographs illustrate PFDMA polyHIPEs with (A) closed pore morphology from interfacial initiation and (B) open pore morphology from organic-phase initiation.
FIG. 3.
Schematic representation of the proposed mechanism for interconnect formation illustrating the effect of initiation loci on macromer densification forces during cure. Organic phase initiation results in sufficient densification forces as the macromer chains convert to polymer to tear the thin film between droplets and open pores. Red indicates initiator, yellow indicates macromer, and green indicates the loci of initiation and regions where cross-linking is occurring. Color images available online at www.liebertpub.com/tea
Compressive properties
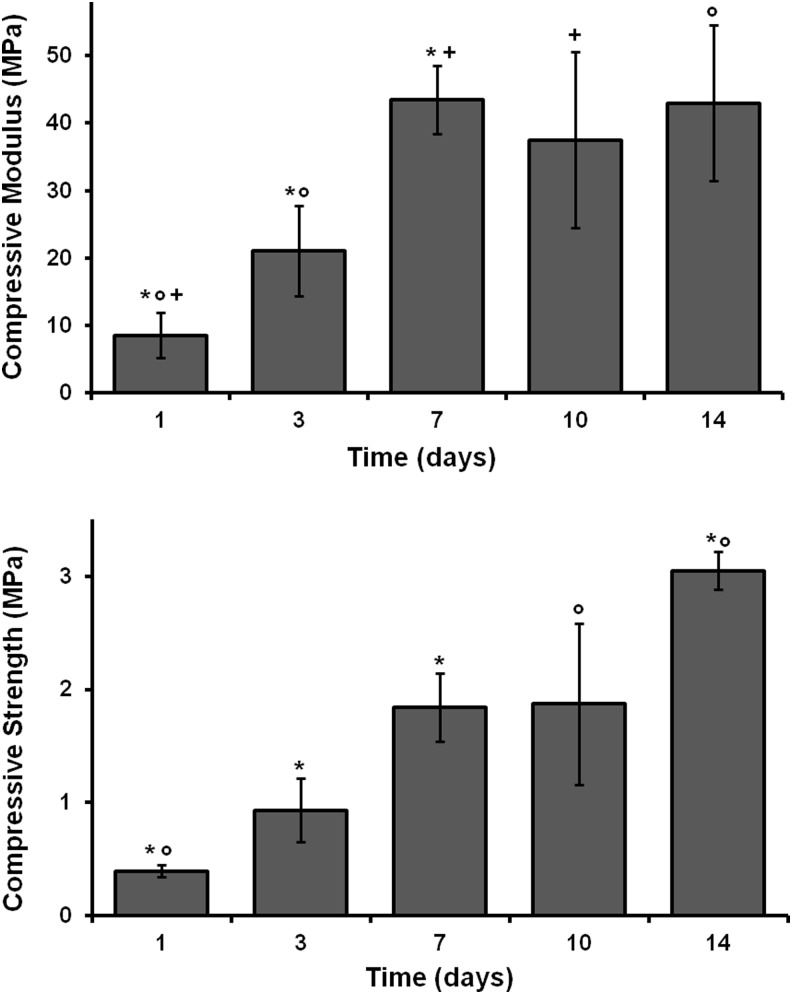
Compressive modulus and strength values were determined using an Instron mechanical testing apparatus on polyHIPEs cured through 2 weeks. A representative stress/strain plot for specimens with a yield point before or after 10% strain is shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea). PFDMA polyHIPEs initiated with 5 wt% BPO resulted in compressive modulus and strength values increasing through 2 weeks as illustrated in Figure 4. It is probable that cross-linking is continuing over time due to the relatively slow reaction kinetics at 37°C. Average values recorded at 2 weeks for compressive modulus and strength were 43±12 MPa and 3±0.2 MPa, respectively. These values approach those of human cancellous bone of 20–500 MPa40,41 for modulus and 2–30 MPa42,43 for strength. These data suggest that the compressive strength may continue to increase as indicated by the lack of a plateau in values. An ongoing study is being conducted to determine the compressive properties of these polyHIPEs over 12 months during storage at 37°C in PBS. ISO standard 5833 for bone cement states a requisite ≥70 MPa of compressive strength. However, materials with compressive properties on the low end of human cancellous bone have been shown to encourage new tissue growth in animal models in vivo.44,45 Therefore, it may not be necessary to meet these high compressive properties of bone cement in a tissue-engineered scaffold that will be remodeled to bone.
FIG. 4.
The effect of time on PFDMA polyHIPE compressive modulus and strength. *+°Indicate statistically significant differences between respective samples, p<0.05.
Pretreatment studies: effect on cure time
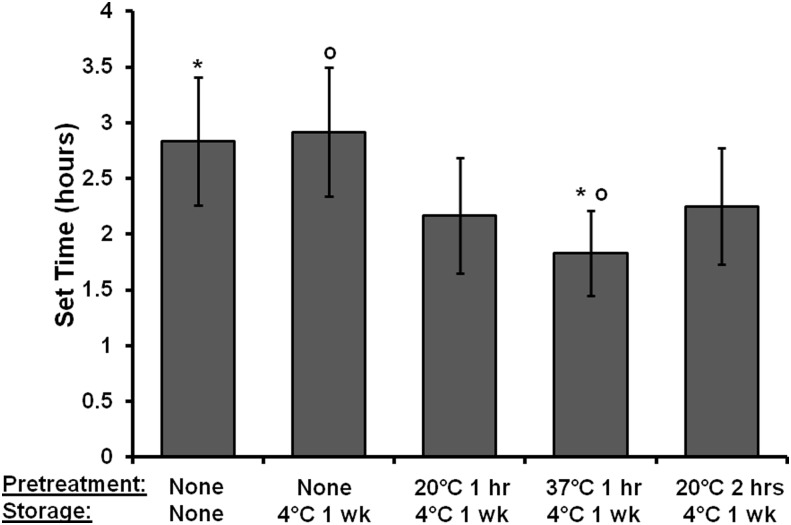
HIPEs were subjected to pretreatment conditions to promote cross-linking before injection for reduced cure time in vivo without the need to alter HIPE composition, such as initiator concentration. Work time is the time available to a surgeon after the onset of mixing to manipulate the material without detriment to its properties. The work time can be determined by the gel point in which the cross-linking in the system is sufficient to resist flow.39 The intersection of loss and storage modulus is often utilized to determine this gel point of cross-linkable polymers.46 However, in the HIPE system, the storage modulus was determined to be larger than the loss modulus throughout the duration of testing, which was attributed to the HIPE's viscoelastic behavior. Therefore, the onset of the increase in storage modulus determined with DMA was utilized as the work time in this study. PolyHIPE set time, or the time required for the material to cure to a solid monolith, was approximated using tack-free time as defined in the Materials and Methods section. Work and set time values as a function of pretreatment conditions are located in Table 1. Pretreatment for 1 h at 37°C decreased the work time but not statistically significantly compared to the storage control. However, the set time was statistically significantly decreased by pretreating at 37°C for 1 h, as shown in Figure 5. In these studies, pretreating at 20°C and 37°C had a modest effect on set time. BPO has an average 10 h half-life at 70°C in benzene.47 Therefore, at 37°C, only a fraction of BPO is dissociating into radicals, reducing the number of chains able to propagate, which results in a longer set time. Increasing the reaction kinetics in the system via aqueous phase temperatures during HIPE formation and cure temperatures is expected to increase the rate of BPO dissociation, thereby increasing the number of propagating chains and decrease set time. Studies are currently under way to increase the aqueous phase temperature upon the addition to the organic phase and pretreatment temperature to further decrease cure time in vivo. An additional benefit with increased pretreatment temperatures is the ability to decrease initiator concentration without the concern of an increased set time. Decreased initiator concentrations may be advantageous to avoid negative cytotoxic effects of the radical initiator.
FIG. 5.
The effect of pretreatment on polyHIPE set time. The specific pretreatment and storage conditions are listed in the first and second rows of the axis title, respectively. *°Indicates statistically significant differences between respective samples, p<0.10.
Storage studies: effect on HIPE stability
Storage studies were used to investigate the use of PFDMA polyHIPEs as off-the-shelf bone grafts. The envisioned deployment method includes HIPE fabrication and transfer to a sterile syringe, pretreatment to produce desired properties postinjection, storage at reduced temperatures, and injection after thawing in the surgical suite. To this end, it was essential to determine the effect of storage conditions on emulsion stability and resulting pore architecture. Storing PFDMA polyHIPEs at 4°C for 1 week had a negligible effect on the pore and interconnect size, as shown in Figure 6, indicating the potential of PFDMA polyHIPEs as off-the-shelf bone grafts. The values for pore and interconnect size are detailed in Table 1. A 1-year study is under way to determine any long-term effect of storage at 4°C on pore architecture. Additionally, all pretreatment conditions resulted in no statistically significant change in pore and interconnect sizes again noting the ability to pretreat, store, and inject these scaffolds without affecting the determined pore architecture. It is probable that the current pore sizes of injectable PFDMA polyHIPEs are too small for full bone healing in vivo. Previous work with ceramic and polymer scaffolds indicated a range of pore sizes from 75 to 400 μm was necessary for osteogenesis and increased bone formation.48–50 Previous polyHIPE studies by other investigators have shown a significant increase in pore size by heating the aqueous phase to temperatures ranging from 40°C to 80°C before the addition to the organic phase.12,38 We have recently replicated these results by increasing both aqueous phase and pretreatment temperature and are pursuing ways to homogenize the pore size.
FIG. 6.
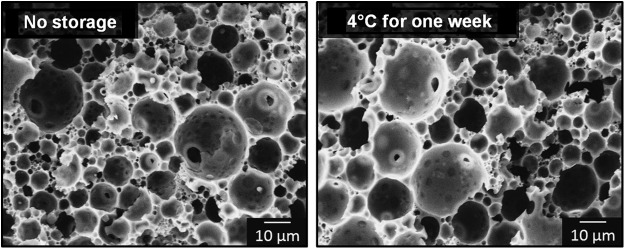
Scanning electron micrographs show minimal effect of 4°C storage for 1 week on polyHIPE pore architecture.
Integration with bone
Injectable scaffolds that integrate with native tissue are expected to enhance bone healing with minimal inflammation and scarring. To this end, the ability of a PFDMA HIPE to integrate with native tissue in a porcine femur defect was evaluated. Figure 7 illustrates the microscale integration of the HIPE in an ex vivo porcine femur defect evident as evidenced by the lack of gap between polyHIPE and cortical bone. Furthermore, the ability of the HIPE to resist flow and remain at a defect site throughout cure was investigated. This is important when comparing cure times of traditional bone cement to PFDMA polyHIPEs. The current set time of the injectable PFDMA polyHIPEs is roughly 8–12 times longer than the ISO 5833 standard requirement established for acrylic bone cements. It was hypothesized that the viscosity of the HIPE will be advantageous in preventing extravasation and result in the maintenance of the scaffold at the defect circumventing the need for a quick set time. The viscosity of HIPE before cure was 11.0±2.3 Pa·s, comparable to mayonnaise. Figure 8 illustrates the ability of PFDMA HIPE to resist flow when inverted in a clear tube. Additionally, the HIPE remains at the defect site in the porcine femur during cure when inverted in an aqueous environment. SEM analysis indicated the integration of HIPE into the bone with no evident gap after inversion in an aqueous environment overnight in all nine specimens evaluated. The inverted porcine femur study indicates that the relatively slow cure time of PFDMA polyHIPEs may not be an issue as the material remains in the defect throughout the duration of cure. Furthermore, Figure 9 illustrates indirect- and direct-contact studies of hMSCs with injectable polyHIPEs resulted in spread cells and good viability (90%) comparable to empty Transwell inserts at 72 h.
FIG. 7.
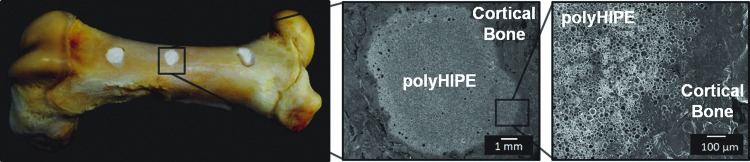
Injectable PFDMA HIPEs fill irregularly shaped defects created in a porcine femur and integrate with bone without shrinkage upon cure at 37°C. Color images available online at www.liebertpub.com/tea
FIG. 8.
The viscosity of a PFDMA HIPE before cure results in retention of the scaffold at the defect site during cure in an aqueous environment. (A) HIPE does not flow upon inversion in transparent tube. (B) Schematic representation illustrating the inversion of porcine femur with HIPE injected in 1×1×0.5 cm defects in 37°C water bath. (C) Digital and scanning electron micrographs showing good integration of HIPE with bone during inversion in water. Color images available online at www.liebertpub.com/tea
FIG. 9.
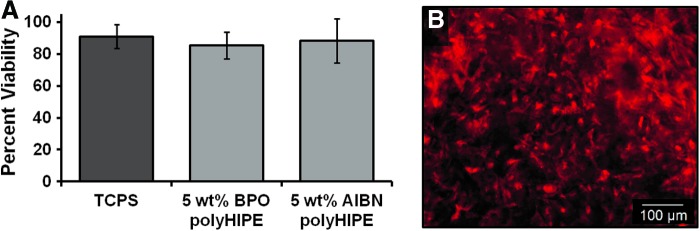
Seventy-two hour viability and morphology of human mesenchymal stem cells (hMSCs) in contact with polyHIPEs. (A) Live/Dead analysis of polyHIPEs in a Transwell insert. Viability of hMSCs in wells with polyHIPEs in the Transwell insert was statistically similar compared to empty inserts (∼90%). (B) hMSCs stained with CellTracker™ Orange were adhered and spread on polyHIPEs. Color images available online at www.liebertpub.com/tea
Conclusion
The focus of this work was to develop and characterize injectable polyHIPEs that had improved pore interconnectivity. The potential for these scaffolds to be utilized as tissue-engineered bone grafts was then evaluated by examining injection parameters, compressive properties, integration into bone defects, and 72 h hMSC viability and behavior. Interconnected pore architecture is necessary for cell viability and proliferation in tissue-engineered materials as previously discussed by Madden et al.31 and Marcacci et al.32 In this work, we highlight the ability to fabricate a biodegradable and injectable polyHIPE with interconnected porosity and investigate its use as an off-the-shelf bone graft. Previously, we created an injectable and biodegradable polyHIPE system by synthesizing the biodegradable macromer, PFDMA, which had appropriate viscosity and hydrophobicity for emulsification without toxic diluents. In this study, interconnectivity was introduced to these biodegradable injectable porous scaffolds by altering the phase in which radical initiation occurs. Organic-phase initiation resulted in macromer densification forces that facilitated pore opening. In addition to facilitating bone ingrowth, this interconnected porosity will also enable the PFDMA polyHIPEs to be pursued as rigid cell carriers through the encapsulation of hMSCs. The viscosity of the HIPE before cure permitted injection into a porcine bone defect, retention at the defect site during inversion in a water bath, and microscale integration of the polyHIPE with bone indicating complete space-filling of the defect. Pretreatment of the materials before injection allowed for tuning of both work and set times. Finally, the storage of the polyHIPEs for 1 week at 4°C had a negligible effect on pore architecture providing an initial indication of the ability of these materials to be utilized as off-the-shelf grafts. Overall, this work highlights the potential of interconnected PFDMA polyHIPEs as injectable scaffolds for bone tissue engineering.
Supplementary Material
Acknowledgments
The authors are grateful for the donated materials from Palsgaard and the Texas A&M University Rosenthal Meat Science and Technology Center. hMSCs were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447. Funding for this work was supported by the National Institutes of Health (R21 AR057531). Additionally, this material is based on the work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 2012115842. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Akay G., Downes S., and Price V.J.Microcellular polymers as cell growth media and novel polymers. European Patent EP1183328, 2000 [Google Scholar]
- 2.Lissant K.J.The geometry of high internal phase ratio emulsions. J Colloid Interface Sci 22,462, 1966 [Google Scholar]
- 3.Barby D., and Haq Z.Low density porous cross-linked polymeric materials and their preparation. European Patent 0,060,138 (to Unilever). 1982 [Google Scholar]
- 4.Ruckenstein E., and Hong L.Binding catalytic sites to the surface of porous polymers and some catalytic applications. Chem Mater 4,122, 1992 [Google Scholar]
- 5.Schoo H.F.M., Challa G., Rowatt B., and Sherrington D.C.Immobilization of flavin on highly porous polymeric disks: three routes to a catalytically active membrane. React Func Polym 16,125, 1992 [Google Scholar]
- 6.Ottens M., Leene G., Beenackers A.A.C.M., Cameron N., and Sherrington D.C.PolyHipe: a new polymeric support for heterogeneous catalytic reactions: kinetics of hydration of cyclohexene in two- and three-phase systems over a strongly acidic sulfonated PolyHipe. Ind Eng Chem Res 39,259, 2000 [Google Scholar]
- 7.Small P.W., and Sherrington D.C.Design and application of a new rigid support for high efficiency continuous-flow peptide-synthesis. J Chem Soc Chem Commun 1989. DOI: 10.1039/C39890001589 [DOI] [Google Scholar]
- 8.Brown I.J., Sotiropoulos S.Electrodeposition of Ni from a high internal phase emulsion (HIPE) template. Electrochim Acta 46,2711, 2001 [Google Scholar]
- 9.Umez-Eronini N.O., Collins A., and Neal D.E.Optimisation of bladder stromal culture on polyHIPE Eur Cell Mater 4,77, 2002 [Google Scholar]
- 10.Busby W., Cameron N.R., and Jahoda C.A.B.Emulsion-derived foams (polyHIPEs) containing poly(ɛ-caprolactone) as matrixes for tissue engineering. Biomacromolecules 2,154, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Cameron N.R., Sherrington D.C., Albiston L., and Gregory D.P.Study of the formation of the open-cellular morphology of poly(styrene/divinylbenzene) polyHiPE materials by cryo-SEM. Colloid Polym Sci 274,592, 1996 [Google Scholar]
- 12.Akay G., Birch M.A., and Bokhari M.A.Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 25,3991, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Hayman M.W., Smith K.H., Cameron N.R., and Przyborski S.A.Enhanced neurite outgrowth by human neurons grown on solid three-dimensional scaffolds. Biochem Biophys Res Commun 314,483, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Barbetta A., Dentini M., De Vecchis M.S., Filippini P., Formisano G., and Caiazza S.Scaffolds based on biopolymeric foams. Adv Funct Mater 15,118, 2005 [Google Scholar]
- 15.Barbetta A., Dentini M., Zannoni E.M., and De Stefano M.E.Tailoring the porosity and morphology of gelatin-methacrylate PolyHIPE scaffolds for tissue engineering applications. Langmuir 21,12333, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Cameron N.R., Barbetta A., and Cooper S.J.High internal phase emulsions (HIPEs) containing divinylbenzene and 4-vinylbenzyl chloride and the morphology of the resulting PolyHIPE materials. Chem Commun 221,2000, 2000 [Google Scholar]
- 17.Bokhari M.A., Birch M.A., and Akay G.Polyhipe polymer: a novel scaffold for in vitro bone tissue engineering. Exper Med Bio Tissue Eng Stem Cells Gene Therap 534,247, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Williams J.M., and Wrobleski D.A.Spatial distribution of the phases in water-in-oil emulsions. Open and closed microcellular foams from cross-linked polystryene. Langmuir 4,656, 1988 [Google Scholar]
- 19.Cameron N.R., and Sherrington D.C.Synthesis and characterisation of poly(aryl ether sulfone) polyHIPE materials. Macromolecules 30,1997 [Google Scholar]
- 20.Lumelsky Y., and Silverstein M.Biodegradable porous polymers through emulsion templating. Macromolecules 42,1627, 2009 [Google Scholar]
- 21.Youssef C., Backov R., Treguer M., Birot M., and Deleuze H.Preparation of amazingly hard polyHIPE material from a direct emulsion. Mater Res Soc Symp Proc 1269,1, 2010 [Google Scholar]
- 22.Lepine O.Preparation of macrocellular PU–PS interpenetrating networks. Polymer 46,9653, 2005 [Google Scholar]
- 23.Bokhari M., Carnachan R.J., Przyborskiac S.A., and Cameron N.R.Emulsion-templated porous polymers as scaffolds for three dimensional cell culture: effect of synthesis parameters on scaffold formation and homogeneity. J Mater Chem 17,4088, 2007 [Google Scholar]
- 24.Kovačič S., Štefanec D., and Krajnc P.Highly porous open-cellular monoliths from 2-hydroxyethyl methacrylate based high internal phase emulsions (HIPEs): preparation and void size tuning. Macromolecules 40,8056, 2007 [Google Scholar]
- 25.Jabbari E., Wang S., Lu L., Gruetzmacher J.A., Ameenuddin S., Hefferan T.E., Currier B.L., Windebank A.J., and Yaszemski M.J.Synthesis, material properties, and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules 6,2503, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayman M.W., Smith K.H., Cameron N.R., and Przyborskia S.A.Growth of human stem cell-derived neurons on solid three-dimensional polymers. J Biochem Biophys Methods 62,231, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Busby W., Cameron N.R., and Jahoda C.A.B.Tissue engineering matrixes by emulsion templating. Polym Int 51,871, 2002 [Google Scholar]
- 28.David D., and Silverstein M.Porous polyurethanes synthesized within high internal phase emulsions (HIPEs). J of Polym Sci A Polym Chem 47,5806, 2009 [Google Scholar]
- 29.Christenson E.M., Soofi W., Holm J.L., Cameron N.R., and Mikos A.G.Biodegradable fumarate-based polyHIPEs as tissue engineering scaffolds. Biomacromolecules 8,3806, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Moglia R.S., Holm J.L., Sears N.A., Wilson C.J., Harrison D.M., and Cosgriff-Hernandez E.Injectable PolyHIPEs as High-Porosity Bone Grafts. Biomacromolecules 12,3621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., and Ratner B.D.Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 107,15211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcacci M., Kon E., Moukhachev V., Lavroukov A., Kutepov S., Quarto R., Mastrogiacomo M., and Cancedda R.Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng 13,947, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Livshin S., and Silverstein M.S.Crystallinity and cross-linking in porous polymers synthesized from long side chain monomers through emulsion templating. Macromolecules 41,3930, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Gitli T., and Silverstein M.S.Bicontinuous hydrogel-hydrophobic polymer systems through emulsion templated simultaneous polymerizations. Soft Matter 4,2475, 2008 [Google Scholar]
- 35.Gurevitch I., and Silverstein M.S.Polymerized pickering HIPEs: effects of synthesis parameters on porous structure. J Polym Sci A Polym Chem 48,1516, 2010 [Google Scholar]
- 36.Gurevitch I., and Silverstein M.S.Nanoparticle-based and organic-phase-based AGET ATRP polyHIPE synthesis within pickering HIPEs and surfactant-stabilized HIPEs. Macromolecules 44,3398, 2011 [Google Scholar]
- 37.Timmer M.D., Horch A.R., Ambrose C.G., and Mikos A.G.Effect of physiological temperature on the mechanical properties and network structure of biodegradable poly(propylene fumarate)-based networks. J Biomat Sci Polym Ed 14,369, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Carnachan R.J., Bokhari M., Przyborski S.A., and Cameron N.R.Tailoring the morphology of emulsion-templated porous polymers. Soft Matter 2,608, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Dumas J.E., Zienkiewicz K., Tanner S.A., Prieto E.M., Bhattacharyya S., and Guelcher S.A.Synthesis and characterization of an injectable allograft bone/polymer composite bone void filler with tunable mechanical properties. Tissue Eng Part A 16,2505, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Karageorgiou V., and Kaplan D.Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26,5474, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Coombes A.G.A., and Meikle M.C.Resorbable synthetic polymers as replacements for bone graft. Clin Mater 17,35, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Svaldi-Muggli D., Burkoth A.K., and Anseth K.S.Crosslinked polyanhydrides for use in orthopaedic applications: degradation behavior and mechanics. J Biomed Mater Res 46,271, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Muggli D.S., Burkoth A.K., and Anseth K.S.Crosslinked polyanhydrides for use in orthopedic applications: degradation behavior and mechanics. J Biomed Mater Res 46,271, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Temenoff J.S., and Mikos A.G.Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials 21,2405, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Rai B., Oest M.E., Dupont K.M., Ho K.H., Teoh S.H., and Guldberg R.E.Combination of platelet-rich plasma with polycaprolactone-tricalcium phosphate scaffolds for segmental bone defect repair. J Biomed Mater Res A 81,888, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Winter H.H.Can the gel point of a cross-linking polymer be detected by the G′–G″ crossover? Polym Eng Sci 27,1698, 1987 [Google Scholar]
- 47.Brandrup J., Immergut E.H., and Grulke E.A.Polymer Handbook, 4th edition. New York: John Wiley, 1999 [Google Scholar]
- 48.Hulbert S.F., Young F.A., Mathews R.S., Klawitter J.J., Talbert C.D., and Stelling F.H.Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res 4,433, 1970 [DOI] [PubMed] [Google Scholar]
- 49.Tsuruga E., Takita H., Itoh H., Wakisaka Y., and Kuboki Y.Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem 121,317, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Cestro H.J., Salyer K.E., and Toranto I.R.Bone growth into porous carbon, polyethylene, and polypropylene prostheses. J Biomed Mater Res 9,1, 1975 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.