Abstract
Epidemic modeling suggests that a major scale-up in HIV treatment could have a dramatic impact on HIV incidence. This has led both researchers and policymakers to set a goal of an “AIDS-Free Generation.” One of the greatest obstacles to achieving this objective is the number of people with undiagnosed HIV infection. Despite recent innovations, new research strategies are needed to identify, engage, and successfully treat people who are unaware of their infection.
Introduction
Successful treatment has long been known to block the progression of HIV infection to AIDS and increase survival.1–3 Recent epidemic modeling suggests that a major scale-up of antiretroviral therapy (ART) could also have a dramatic impact on HIV-1 incidence.4 These observations have led both researchers and policymakers to set a goal of an “AIDS-Free Generation.”
The mathematical models of treatment as prevention are supported by a recent UNAIDS report that new HIV infection rates fell 50% or more in 25 countries during the past decade, most of which are African nations that have been scaling up ART.2 A careful ecologic analysis in rural KwaZulu-Natal has provided even stronger evidence of an association between increased ART coverage and a lower risk of HIV acquisition.5 Community randomized trials starting this year in sub-Saharan Africa will examine the population level impact of expanded ART on HIV incidence when offered in combination with other prevention interventions.6
At the same time, HIV prevention efforts in more resource-rich settings have encountered a number of obstacles. For example, despite increases in testing and uptake of ART, HIV incidence among men who have sex with men (MSM) has increased.7–9 More than 80% of new infections in this highly affected population appear to be attributable to men who are unaware of their HIV infection.8,9 To address this critical issue, we reviewed recent innovations, identified remaining gaps, and considered research priorities required to overcome these challenges.
Epidemiology of People Unaware of Their HIV Infection
In higher income countries, it is estimated that approximately 20–30% of people living with HIV infection are undiagnosed.10,11 In resource-limited settings, this proportion is estimated to exceed 60%.12–14 There is also considerable variability across different populations in the mean time to diagnosis and number of susceptible partners, and the frequency and type of unprotected sexual/injecting drug use acts.15
In the United States, adolescents (13–24 years) are more likely to be undiagnosed than other age groups (58.9%, compared to 31.5% for 25–34 years and 18% or less for older people), males more than females, and heterosexual males more than MSM.11 The proportion of undiagnosed cases is also higher for Asian/Pacific Islanders and American Indian/Alaskan natives, followed by black/African Americans, Hispanic/Latinos, and whites.11
People who are undiagnosed are estimated to have a transmission rate three to seven times higher than those who are aware of their infection, depending on the number of at-risk sexual partners and the prevalence of linkage to care, retention, and viral suppression among diagnosed persons.16 As a result, 44–66% of all new infections in the United States may be attributable to people who are unaware of their status.16,17 This proportion will increase as more diagnosed persons are started on ART and viral suppression is successfully maintained.
Among undiagnosed persons, those with acute HIV infection are likely to have the highest risk of transmission due to higher viral loads and the dominance of variants that closely resemble the transmitted/founder virus.18–21 An increased rate of risk taking near the time of infection and other behavioral dynamics may also play important roles.22,23 In addition, the transmission risk may be amplified by concomitant sexually transmitted infections, which are relatively common during this period.24–26 The acute phase of infection is comparatively brief, however, and there is still no consensus on its overall impact.20,27,28
In the United Kingdom, HIV incidence among MSM has not declined in the past decade despite a substantial increase in HIV testing, a reduction in the mean time to diagnosis, and an increase in ART uptake.29 These improvements have occurred in parallel with a modest (26%) rise in condomless sex.9 One analysis indicates that the source of nearly half of all new infections is undiagnosed men during their primary infection (median 0.48; 5–95% centiles, 0.34–0.62).9 Estimates for other sources are undiagnosed men after primary infection, 0.34 (0.22–0.46), diagnosed ART naive, 0.10 (0.04–0.19), and diagnosed ART experienced, 0.07 (0.02–0.17). These data emphasize that as ART uptake increases, earlier diagnosis and treatment are necessary to further reduce the number of new infections.9,20
Insights from Mathematical Modeling
Mathematical models developed over the past decade have clearly indicated that new HIV infections can be substantially reduced if testing and ART can be massively scaled up.4,30–32 It has been repeatedly noted, however, that outcomes are sensitive to changes in the level of HIV risk behaviors.31,33,34 The results of these models are also highly dependent on a number of testing and treatment parameters. For example, to achieve a 60% reduction in HIV incidence in a given population, one model indicates that testing must identify 60% of new cases within 1 year of infection, 90% of those diagnosed must be started on ART (regardless of CD4 cell count), 87% must achieve viral suppression within 6 months, and 99% must be retained on ART [Novel calculation using the Eaton model described in reference #4,T. Hallett (timothy.hallett@imperial.ac.uk), personal communication with David Burns, June 18, 2013]. This model also assumes that viral suppression will be associated with a 98% reduction in onward transmission, which is similar to what was observed in a randomized clinical trial that enrolled HIV serodiscordant heterosexual couples in nine countries worldwide,35 but substantially greater than the 26–92% reduction observed in observational cohort studies in more “real world” settings.36–38 Reliable estimates for other partnerships, e.g., MSM and injecting drug users, are not yet available.39,40
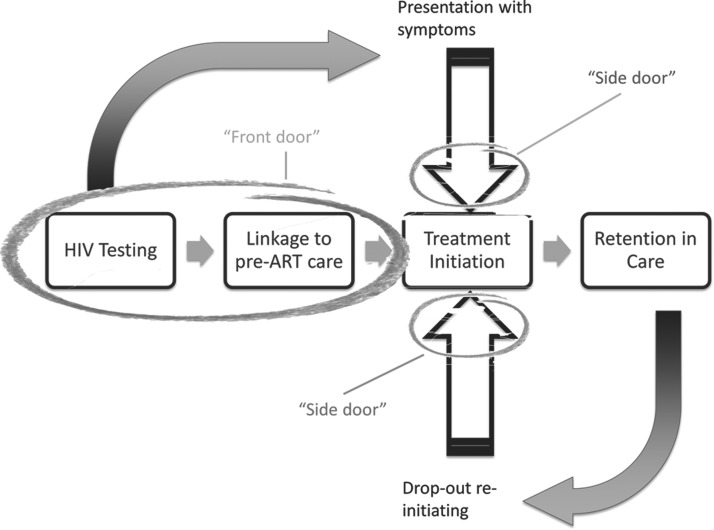
To reach the HIV incidence targets predicted by current models, improvement is needed at all steps in the testing and treatment continuum.41,42 This is complicated by the fact that the “continuum” is not a strictly ordered sequence of events (Fig. 1).43 Some newly diagnosed persons decline HIV care or are linked to care but decline treatment until symptoms occur; others achieve some or all of these milestones but later drop out of treatment. Considerable effort is required to maximize the proportion of people who initiate ART, adhere to the dosing regimen, and remain in care. In addition, increased case finding and earlier diagnosis and treatment are imperative.
FIG. 1.
Nonlinear nature of the HIV treatment continuum. Some persons will fail to test, test but decline linkage to care, or link but decline treatment until symptoms occur. Others will achieve some or all of these milestones but later drop out of care and treatment. Entry is therefore constantly occurring not only through the “front door” but also through at least two “side” doors.43
Other insights from mathematical modeling can be described using the basic reproduction number, R0, the number of new infections, on average, caused by one infected individual in a susceptible population.44,45 If R0 is greater than 1, an epidemic will continue to spread. The primary goal of HIV prevention programs is therefore to reduce R0 to<1.
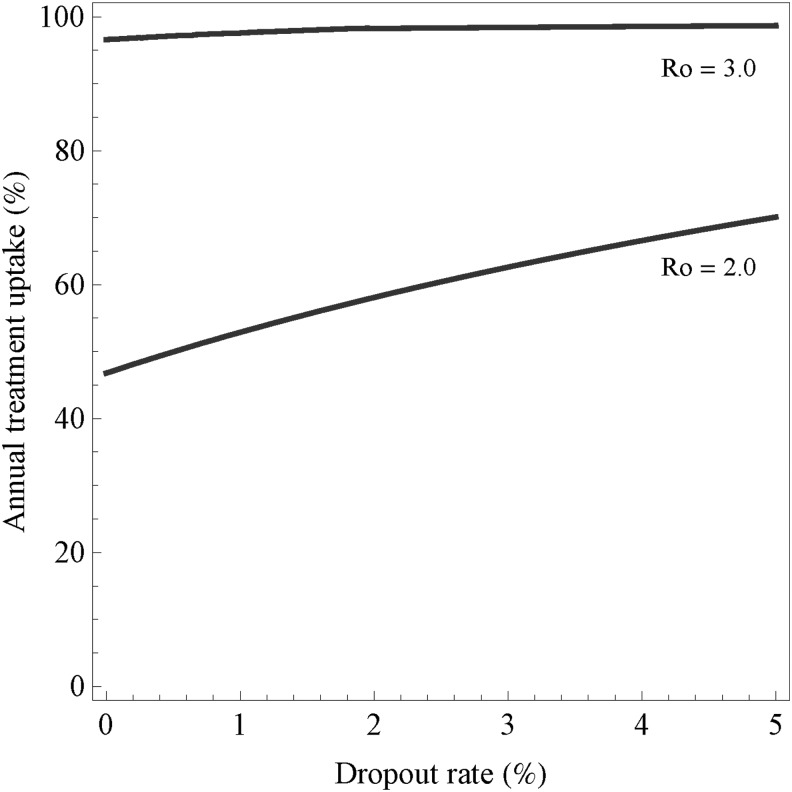
For a given infectious agent in a particular population and setting, R0 varies with the frequency and type of contact between an infectious case and a susceptible partner (e.g., vaginal, oral, receptive anal, insertive anal), the risk of transmission per encounter, and the duration of infectiousness.45 Both regular and casual partners must be considered, together with the sexual networks to which they are linked. For example, modeling indicates that for MSM in Amsterdam, in the absence of ART R0 exceeds 1 and then rises rapidly when the proportion of men in regular partnerships who also have casual partners surpasses one third (≥0.34).46 Other modeling indicates that if R0 is >3 during the initial phase of the epidemic, elimination by “test-and-treat” strategies is unlikely unless other effective interventions are added (Fig. 2).47
FIG. 2.
Elimination threshold as a function of the annual treatment uptake and dropout rate for various values of the basic reproduction number R0. The two curves describe the threshold for R0=2.0 and for R0=3.0. For parameter combinations above the lines, elimination is possible; for combinations below the curve it is not possible.47
Methods for Finding and Engaging Undiagnosed Persons
Phylogenetics
HIV phylogenetic data have become increasingly valuable as a prevention tool. In many resource-rich settings, viral sequencing is performed for most newly diagnosed cases in order to monitor drug resistance and guide treatment. The more closely related two sequences are the more likely the cases are linked epidemiologically.48 Although a transmission pair can never be known with certainty (given the possibility of a common source for both, or an intervening partner closely spaced in time), closely linked cases can be identified and transmission clusters described.49–51 To the extent that demographic, behavioral, and other correlates are also available, a profile of persons most at risk of HIV infection in a given population can be constructed. This information can then be used to design and target prevention interventions.52
Phylogenetic analysis can also be used to estimate R0.53,54 Using the sequences identified, a phylogenetic tree is constructed to describe the evolutionary history of the virus in the population. The time of entry of the virus into the population, the initial exponential growth rate of the epidemic, the generation time distribution, and R0 can all be estimated. Greater discrimination will be possible as whole genome sequencing becomes more affordable and as the sampling fraction is increased.49,52 As with all epidemiologic analyses, the analytic methods should take into account the sampling strategy used.
To protect confidentiality, all possible identifiers must be carefully delinked from phylogenetic data sets. Messaging should make clear that these data can never be used to determine person-to-person transmission. The United Nations Global Commission on HIV and the Law and others have made declarations that address this issue.55,56
Network research
Social and sexual network research can also provide extremely valuable information for finding and engaging undiagnosed persons in HIV treatment and prevention. Sociocentric study designs are usually preferable to egocentric designs, given that they aim to provide information regarding all members of a network rather than being limited to the contacts of the individuals interviewed.57,58 An individual's involvement in a particular network may have a positive or negative impact on their health depending on the nature of the network and his or her position within it. For example, those at or near the center of a health information network may experience various benefits, whereas a person who is highly connected within a sexual network is likely to be at greater risk of HIV and other infections.57
Various methods are available for collecting and analyzing network data. Partner notification (contact tracing) programs provide demographic, behavioral, and partnership data that can be used to identify partner characteristics associated with undiagnosed HIV infection.59 Respondent-driven sampling (RDS) is often used to sample MSM, injecting drug users, and other “hidden” populations.60–63 “Seeds” are given coupons to share with members of their network, and this process is repeated through several cycles. RDS is analytically unbiased under a limited number of assumptions; ongoing methodological research is examining limitations under other conditions and how best to estimate variance.62,64
An advantage of network research over phylogenetic studies is that it can provide information on both infected “cases” and uninfected “controls” in the network. Analyses comparing these two groups can yield insights that may not be evident from studies limited to HIV-positive people.
Combined methods
Data from epidemiologic, phylogenetic, and social and sexual network research can be used in a complementary manner with mathematical modeling and other methods. For example, the rates of unprotected anal intercourse reported by men in a prospective cohort study in Amsterdam during the period 1984–2009 have been compared with an independent estimate of risk behavior among MSM in the Netherlands during the same period derived from mathematical modeling.65 The model incorporated multiple parameters including annual HIV diagnoses, stage of infection, and ART. The proportion of HIV-negative men reporting unprotected anal intercourse—which dropped precipitously between 1984 and 1986, rose sharply and then more gradually after 1996, and plateaued from 2005 onward—coincided very closely with similar changes in the modeled transmission rates for the same period. It was concluded that the beneficial impact of enhanced testing and ART on HIV incidence was offset by increased risk behavior among untreated and susceptible individuals.
Epidemiologic, phylogenetic, and clinical data have been combined to examine the risk of HIV transmission among MSM followed at a sexual health clinic in southern England between 2000 and 2006.66 Younger age, recent HIV infection, coinfection with another sexually transmitted infection, and HIV viral load in the source partner were associated with an increased risk of transmission.
Another particularly innovative strategy has been to combine geospatial, phylogenetic, and population-based cohort data.67 Using probabilistic transmission models, spatial clustering statistics, and phylogenetic analyses, the investigators found that a substantial proportion of new transmissions in a rural African setting came from outside the community, followed by onward transmission within households (more than 1/3 of new infections) and small community networks.67 This implies that successful treatment as prevention within households requires rapid diagnosis and initiation of ART by newly infected partners. In this study, a large proportion of extracommunity partners came from regional urban centers and fishing villages along Lake Victoria.
Other combined methods research suggests that the pattern of risk-taking over time can also influence the rate of spread of HIV-1 infection.23,68 One model indicates that when higher contact rates occur frequently in an episodic manner, the size and duration of acute infection outbreaks increase.23 Cohort data suggest that this type of risk dynamic may be more characteristic of certain populations, such as urban MSM.69
Interventions and strategies
A number of innovative strategies to find undiagnosed persons and engage them in care and treatment are in various stages of development. Examples include a “peer-driven” social network strategy that is being examined in New York City communities known to have a high prevalence of undiagnosed heterosexuals,70,71 a combined behavioral, psychosocial, epidemiologic, and phylogenetic approach being used to characterize HIV transmission clusters among MSM in San Francisco and to evaluate the impact of specific risk-reduction interventions,72 and various innovative strategies to find and engage people with acute HIV infection.73–75
Special attention is needed for adolescents and young adults, given their disproportionately high rates of undiagnosed HIV infection.11 Some innovative strategies in development include online messages promoting testing and linkage to care, educational materials presented in a mobile phone game format, and virtual “safe spaces” where young people can interact with online avatars to role play communicating with a provider, taking an HIV test, and receiving test results.76–78 These interventions are being tailored according to gender and sexual identity.
Priorities for Future Research
After reviewing these areas of research, an expert working group convened by the National Institutes of Health and the Bill and Melinda Gates Foundation discussed which recent innovations deserved further development, identified remaining gaps, and made recommendations for prioritizing future research. Research methodologies that were felt to be particularly promising included phylogenetic studies, geospatial analysis, social and sexual network studies, mathematical modeling, and combined studies that included these and other related areas of prevention research. Although the group focused on sexual transmission of HIV, the same methods can and should be applied to transmission via injecting drug use. The “modes of transmission” model is a useful tool for setting local/national priorities.15,79
One of the most important overall priorities identified was new strategies to increase the yield of HIV testing programs. Further studies should determine the optimal use of physical venues, internet sites, and other electronic social media. In addition to advancing earlier diagnosis, expanded testing can be expected to reduce stigma and increase utilization of HIV treatment and prevention services. There is evidence that expanded testing is also cost-effective.80
To reach largely “hidden” at-risk groups such as young MSM in the United States, enhanced partner referral services and other innovative strategies must be developed. Greater involvement of peer advocates may prove to be crucial. Incentives should be linked to care, treatment, and viral suppression, not just testing, so that all steps in the treatment continuum are reinforced.
In resource-limited settings, where the number and proportion of undiagnosed people are greatest,12,13 simply scaling up “test-and-treat” programs must be the first priority. Home-based counseling and testing has shown promise, yielding up to 90% acceptance.81 Point of care CD4+ and viral load testing may facilitate linkage to care, retention, ART initiation, and successful viral suppression.82,83 Although side effects and high pill burdens remain significant barriers to ART uptake in some settings, improvement is expected if newer, better tolerated, long-acting combination formulations can be introduced.84,85 Uptake can also be expected to increase if ongoing studies unequivocally show that earlier ART provides a long-term immune system benefit, reduces viral reservoirs, and increases the potential for cure or functional cure.86
In all populations and settings, a combination of research methods will be required to develop strategies that are both effective and cost-efficient. One approach that warrants further examination is the use of anonymized, phylogenetic-based techniques to identify acute transmission clusters and launch an intensive public health effort targeting those at greatest risk. A combination of partner referral services, respondent-driven sampling, and venue (“hot spot”)-based sampling and testing may be most successful at reaching these target populations. Greater community participation and ownership are clearly needed to make these methods more acceptable, accessible, and effective.
Other priorities are efficient inferential procedures and more high-quality data to improve our mathematical models. For example, current data for the risk behaviors of a given population may underestimate the actual overall risk, given that particularly high-risk members may be less likely to enroll in research studies. A shortage of high-quality data can also make it more difficult to estimate correlations between crucial parameters in the model, increasing uncertainty regarding their impact on major outcomes.
The “behavioral economics” of HIV testing, care, and treatment is another important area for further research. A growing body of evidence indicates that conditional economic incentives can improve health-related measures, including adherence to ART.87–89 For other behaviors that are largely automatic, interventions must target the stimuli that produce them.89 In some cases this may be as simple as resetting default choices (e.g., opt-out versus opt-in), but a number of other options are available and should be explored further.87
Conclusions
Epidemic modeling suggests that a major scale-up in HIV treatment could have a dramatic impact on HIV incidence, leading both researchers and policymakers to set a goal of an “AIDS-Free Generation.” One of the greatest obstacles to achieving this objective in both domestic and international settings is the number of people who are unaware of their HIV infection. Further research is required to develop effective strategies to identify these persons and engage them in HIV care, treatment, and prevention programs.
This research will require a combination of methods, including epidemiologic, phylogenetic, network, and clinical studies; geospatial analysis; mathematical modeling; behavioral economics; implementation science; and cost-effective analysis. Although some of this work can be done by small groups of investigators working in their individual areas of expertise, multidisciplinary teams will also be required. As new strategies are developed, further research will be needed to rigorously assess acceptability and efficacy in target populations as well as adherence and overall effectiveness in real world settings.
Acknowledgments
NIH-BMGF Finding Persons Unaware of their HIV Infection Workshop participants: Susannah Allison (NIMH), Ruanne Barnabas (University of Washington), Linda-Gail Bekker (Desmond Tutu HIV Center), Chris Beyrer (Johns Hopkins University), Alison Brown (Public Health England), David N. Burns (NIAID), Susan Buchbinder (San Francisco Department of Public Health), Connie Celum (University of Washington), Grace Chow (NIAID), Myron S. Cohen (University of North Carolina at Chapel Hill), Nicole Crepaz (CDC), Katherine Davenny (NIDA), Rebecca Delcarmen-Wiggins (NIMH), Victor DeGruttola (Harvard University), Carl Dieffenbach (NIAID), Chris Duncombe (BMGF), Emily Erbelding (NIAID), Vanessa Elharrar (NIAID), Elizabeth H. Flanagan (NIAID), Andrew D. Forsyth (DHHS), Christophe Fraser (Imperial College London), Christopher M. Gordon (NIMH), Alan Greenberg (George Washington University), Marya Gwadz (New York University), Tim Hallett (Imperial College London), Stéphane Helleringer (Columbia University), Lisa Hightow-Weidman (University of North Carolina at Chapel Hill), Bill Kapogiannis (NICHD), Mirjam Kretzschmar (RIVM), Ann Kurth (New York University), Sonia Lee (NICHD), Susan Little (University of California at San Diego), Cherlynn Mathias (NIAID), Molly McNairy (Columbia University), William C. Miller (University of North Carolina at Chapel Hill), Audrey Pettifor (University of North Carolina at Chapel Hill), Christopher D. Pilcher (University of California at San Francisco), Dianne Rausch (NIMH), Robert H. Remien (Columbia University), Scott D. Rhodes (Wake Forest University), Travis Sanchez (Emory University), Usha Sharma (NIAID), Hans Spiegel (HJF-DAIDS), Tanja Stadler (Swiss Federal Institute of Technology), Joanne Stekler (University of Washington), Michael Stirratt (NIMH), Hong-Ha Truong (University of California at San Francisco), Fulvia Veronese (NIAID), Erik Volz (University of Michigan), Lance Weindhardt (University of Wisconsin at Milwaukee), Carolyn F. Williams (NIAID), Sheryl Zwerski (NIAID).
The topics reviewed and discussed, and the final recommendations, were the joint effort of all participants. Other opinions expressed in this article are the responsibility of the writing group and should not be attributed to their institutions or agencies. D.N.B. wrote the first draft and E.H.F. coordinated the revisions. All authors (including V.D.G., C.D.P., M.K., C.M.G., C.D., and M.S.C.) contributed to and approved the final version.
Special thanks go to Heather Thompson (BMGF) and John Wroblewski (HJF-DAIDS Contractor) for their support in coordinating the workshop; to Kimberly Powers (University of North Carolina at Chapel Hill) for reviewing the manuscript; and to Lester Freeman (HJF-DAIDS Contractor) for assistance with the submission process.
The Finding Persons Unaware of their HIV Infection Workshop was sponsored by the Division of AIDS of the National Institute of Allergy and Infectious Diseases, and the National Institute of Mental Health, National Institutes of Health, and the Bill and Melinda Gates Foundation. This research was supported in part by the National Institutes of Health (Christopher D. Pilcher, R34 MH096606; Victor DeGruttola, R37 A151164; and Myron S. Cohen, UMI A1068619 and P30 A150410).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Schwarcz L, Chen M-J, Vittinghoff E, et al. : Declining incidence of AIDS-defining opportunistic illnesses: Results from 16 years of population-based AIDS surveillance. AIDS 2013;27(4):597–605 [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS: World AIDS Day Report 2012 www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf Accessed July15, 2013
- 3.Walensky RP, Paltiel AD, Losina E, et al. : The survival benefits of AIDS treatment in the United States. J Infect Dis 2006;194(1):11–19 [DOI] [PubMed] [Google Scholar]
- 4.Eaton JW, Johnson LF, Salomon JA, et al. : HIV treatment as prevention: Systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 2012;9:e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanser F, Baernighausen T, Grapsa E, et al. : High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013;339(6122):966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boily MC, Masse B, Alsallaq R, et al. : HIV treatment as prevention: Considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. PLoS Med 2012;9(7):e1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PS, Hamouda O, Delpech V, et al. : Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol 2009;19(6):423–431 [DOI] [PubMed] [Google Scholar]
- 8.Bezemer D, de Wolf F, Boerlijst MC, et al. : A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS 2008;22(9):1071–1077 [DOI] [PubMed] [Google Scholar]
- 9.Phillips AN, Cambiano V, Nakagawa F, et al. : Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: Analysis of an extensively documented epidemic. PLoS One 2013;8(2):e55312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamers FF. and Phillips AN: Diagnosed and undiagnosed HIV-infected populations in Europe. HIV Med 2008;9(Suppl 2):6–12 [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Rhodes PH, Hall IH, et al. : Prevalence of undiagnosed HIV infection among persons aged ≥13 years–National HIV Surveillance System, United States, 2005–2008. MMWR Morb Mortal Wkly Rep 2012;61(Suppl):57–64 [PubMed] [Google Scholar]
- 12.Anand A, Shiraishi RW, Bunnell RE, et al. : Knowledge of HIV status, sexual risk behaviors and contraceptive need among people living with HIV in Kenya and Malawi. AIDS 2009;23(12):1565–1573 [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS: World AIDS Day Report 2011 www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/jc2216_worldaidsday_report_2011_en.pdf Accessed June6, 2013
- 14.Cherutich P, Bunnell R, and Mermin J: HIV testing: Current practice and future directions. Curr HIV/AIDS Rep 2013;10(2):134–141 [DOI] [PubMed] [Google Scholar]
- 15.Gouws E. and Cuchi P: Focusing the HIV response through estimating the major modes of HIV transmission: A multi-country analysis. Sex Transm Infect 2013;88(Suppl 2):i76–i85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall HI, Holtgrave DR, and Maulsby C: HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS 2012;26(7):893–896 [DOI] [PubMed] [Google Scholar]
- 17.Marks G, Crepaz N, and Janssen RS: Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006;20(10):1447–1450 [DOI] [PubMed] [Google Scholar]
- 18.Wawer MJ, Gray RH, Sewankambo NK, et al. : Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005;191(9):1403–1409 [DOI] [PubMed] [Google Scholar]
- 19.Pilcher CD, Joaki G, Hoffman IF, et al. : Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007;21(13):1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powers KA, Ghani AC, Miller WC, et al. : The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: A modelling study. Lancet 2011;378(9787):256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Z-M, Keele BF, Qureshi H, et al. : SIVmac251 Is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS Res Hum Retroviruses 2011;27(12):1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH. and Koopman JS: HIV transmissions by stage in dynamic sexual partnerships. J Theor Biol 2012;298:147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam SJ, Zhang X, Romero-Severson EO, et al. : Detectable signals of episodic risk effects on acute HIV transmission: Strategies for analyzing transmission systems using genetic data. Epidemics 2013;5(1):44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erbelding EJ, Chung SE, Kamb ML, et al. : New sexually transmitted diseases in HIV-infected patients: Markers for ongoing HIV transmission behavior. J Acquir Immune Defic Syndr 2003;33:247–252 [DOI] [PubMed] [Google Scholar]
- 25.Kalichman SC, Pellowski J, and Turner C: Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: Systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011;87:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathela P, Braunstein S, Shepard C, et al. : Population-based HIV incidence among men diagnosed with infectious syphilis, 2000–2011. Paper presented at the 20th Meeting of the International Society for Sexually Transmitted Diseases Research, July14–17, 2013, Vienna, Austria [Google Scholar]
- 27.Cohen MS, Shaw GM, McMichael AJ, et al. : Acute HIV-1 infection. N Engl J Med 2011;364:1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MS, Dye C, Fraser C, et al. : HIV treatment as prevention: Debate and commentary—will early infection compromise treatment-as-prevention strategies? PLoS Med 2012;9:e1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birrell PJ, Gill ON, Delpech VC, et al. : HIV incidence in men who have sex with men in England and Wales 2001–2010: A nationwide population study. Lancet Infect Dis 2013;13(4):313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blower SM, Gershengorn HB, and Grant RM: A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 2000;287(5453):650–654 [DOI] [PubMed] [Google Scholar]
- 31.Velasco-Hernandez JX, Gershengorn HB, and Blower SM: Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis 2002;2(8):487–493 [DOI] [PubMed] [Google Scholar]
- 32.Granich RM, Gilks CF, Dye C, et al. : Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet 2009;373(9657):48–57 [DOI] [PubMed] [Google Scholar]
- 33.Xiridou M, Geskus R, de Wit J, et al. : Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS 2004;18(9):1311–1320 [DOI] [PubMed] [Google Scholar]
- 34.Delva W, Wilson DP, Abu-Raddad L, et al. : HIV treatment as prevention: Principles of good HIV epidemiology modelling for public health decision-making in all modes of prevention and evaluation. PLoS Med 2012;9(7):e1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen MS, Chen YQ, McCauley M, et al. : Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia Z, Mao Y, Zhang F, et al. : Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–2011): A national observational cohort study. Lancet 2013;382(9899):1195–1203 [DOI] [PubMed] [Google Scholar]
- 37.He N, Duan S, Ding Y, et al. : Antiretroviral therapy reduces HIV transmission in discordant couples in rural Yunnan, China. PloS One 2013;8(11):e77981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnell D, Baeten JM, Kiarie J, et al. : Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet 2010;375(9731):2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson DP: Evidence is still required for treatment as prevention for riskier routes of HIV transmission [letter]. AIDS 2010;24(18):2891–2892 [DOI] [PubMed] [Google Scholar]
- 40.Muessig KE, Smith MK, Powers KA, et al. : Does ART prevent HIV transmission among MSM? AIDS 2012;26:2267–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention: Today's HIV epidemic www.cdc.gov/nchhstp/newsroom/docs/HIVFactSheets/TodaysEpidemic-508.pdf Accessed July16, 2013
- 42.Kranzer K, Lawn SD, Johnson LF, et al. : Community viral load and CD4 count distribution among people living with HIV in a South African township: Implications for treatment as prevention. J Acquir Immune Defic Syndr 2013;63:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallett TB. and Eaton JW: A side door into care cascade for HIV-infected patients? J Acquir Immune Defic Syndr 2013;63(Suppl 2):S228–S232 [DOI] [PubMed] [Google Scholar]
- 44.May RM. and Anderson RM: Transmission dynamics of HIV infection. Nature 1987;326(6109):137–142 [DOI] [PubMed] [Google Scholar]
- 45.Garnett GP: The basic reproductive rate of infection and the course of HIV epidemics. AIDS Patient Care STDS 1998;12(6):435–449 [DOI] [PubMed] [Google Scholar]
- 46.Kretzschmar M. and Carael M: Is concurrency driving HIV transmission in sub-Saharan African sexual networks? The significance of sexual partnership typology. AIDS Behav 2012;16(7):1746–1752 [DOI] [PubMed] [Google Scholar]
- 47.Kretzschmar M, van der Loeff M, Birrell P, et al. : Prospects of elimination of HIV with test-and-treat strategy. Proc Natl Acad Sci USA 2013;110(39):15538–15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hue S, Clewley JP, Cane PA, et al. : HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS 2004;18:719–728 [DOI] [PubMed] [Google Scholar]
- 49.Volz EM, Koopman JS, Ward MJ, et al. : Simple epidemiological dynamics explain phylogenetic clustering of HIV from patients with recent infection. PLoS Comput Biol 2012;8(6):e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldous JL, Pond SK, Poon A, et al. : Characterizing HIV transmission networks across the United States. Clin Infect Dis 2012;55(8):1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stadler T. and Bonhoeffer S: Uncovering epidemiological dynamics in heterogeneous host populations using phylogenetic methods. Philos Trans R Soc Lond B Biol Sci 2013;368:20120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner B, Wainberg MA, and Roger M: Phylogenetic inferences on HIV-1 transmission: Implications for the design of prevention and treatment interventions. AIDS 2013;27(7):1045–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stadler T, Kouyos R, von Wyl V, et al. : Estimating the basic reproductive number from viral sequence data. Mol Biol Evol 2012;29(1):347–357 [DOI] [PubMed] [Google Scholar]
- 54.Volz EM, Koelle K, and Bedford T: Viral phylodynamics. PLoS Comput Biol 2013;9(3):e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Commission on HIV and the Law: Global Commission on HIV and the Law: Rights, Risk, Health www.hivlawcommission.org/resources/report/FinalReport-Risks,Rights&Health-EN.pdf Accessed July15, 2013
- 56.Bernard E, Azad Y, Vandamme A, et al. : The use of phylogenetic analysis as evidence in criminal investigation of HIV transmission. www.nat.org.uk/Media%20library/Files/PDF%20Documents/HIV-Forensics.pdf Accessed October21, 2013 [DOI] [PubMed]
- 57.Smith KP. and Christakis NA: Social networks and health. Annu Rev Sociol 2008;34:405–429 [Google Scholar]
- 58.Helleringer S, Mkandawire J, Kalilani-Phiri L, et al. : Cohort profile: The Likoma Network Study (LNS). Int J Epidemiol 2013; [Epub ahead of print]; DOI: 10.1093/ije/dyt001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoots BE, MacDonald PDM, Hightow-Weidman LB, et al. : Developing a predictive model to prioritize human immunodeficiency virus partner notification in North Carolina. Sex Transm Dis 2012;39(1):65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salganik MJ. and Heckathorn DD: Sampling and estimation in hidden populations using respondent-driven sampling. Sociol Methodol 2004;34:193–239 [Google Scholar]
- 61.Kimbrough LW, Fisher HE, Jones KT, et al. : Accessing social networks with high rates of undiagnosed HIV infection: The Social Networks Demonstration Project. Am J Public Health 2009;99(6):1093–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bengtsson L, Lu X, Nguyen QC, et al. : Implementation of web-based respondent-driven sampling among men who have sex with men in Vietnam. PLoS One 2012;7:e49417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCoy SI, Shiu K, Martz TE, et al. : Improving the efficiency of HIV testing with peer recruitment, financial incentives, and the involvement of persons living with HIV infection. J Acquir Immune Defic Syndr 2013;63(2):e56–e63 [DOI] [PubMed] [Google Scholar]
- 64.Lu X, Bengtsson L, Britton T, et al. : The sensitivity of respondent-driven sampling. J R Stat Soc Ser A Stat Soc 2012;175:191–216 [Google Scholar]
- 65.van Sighem A, Jansen I, Bezemer D, et al. : Increasing sexual risk behaviour among Dutch men who have sex with men: Mathematical models versus prospective cohort data. AIDS 2012;26(14):1840–1843 [DOI] [PubMed] [Google Scholar]
- 66.Fisher M, Pao D, Brown AE, et al. : Determinants of HIV-1 transmission in men who have sex with men: A combined clinical, epidemiological and phylogenetic approach. AIDS 2010;24(11):1739–1747 [DOI] [PubMed] [Google Scholar]
- 67.Grabowski MK, Lessler J, Redd AD, et al. : Frequent viral introductions sustain local HIV epidemics in rural Rakai, Uganda. Paper presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia, 2013 [Google Scholar]
- 68.Rocha LE. and Blondel VD: Bursts of vertex activation and epidemics in evolving networks. PLoS Comput Biol 2013;9:e1002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero-Severson E, Alam S, Volz E, et al. : Heterogeneity in number and type of sexual contacts in a gay urban cohort. Stat Commun Infect Dis 2012;4(4):1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gwadz M: Peer-driven intervention to seek, test and treat heterosexuals at high risk for HIV. www.cduhr.org/projects/description.aspx?projectID=72 Accessed June6, 2013
- 71.Jenness SM, Neaigus A, Murrill CS, et al. : Estimated HIV incidence among high-risk heterosexuals in New York City, 2007. J Acquir Immune Defic Syndr 2011;56(2):193–197 [DOI] [PubMed] [Google Scholar]
- 72.Truong H: HIV transmission cluster analysis to inform prevention. http://projectreporter.nih.gov/project_info_description.cfm?aid=8263303&icde=16571418&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC Accessed June6, 2013
- 73.Remien R: Structural intervention to increase screening and testing for acute HIV infection. http://projectreporter.nih.gov/project_info_description.cfm?aid=8434183&icde=16568781&ddparam=&ddvalue=&ddsub=&cr=3&csb=default&cs=ASC Accessed June6, 2013
- 74.Pilcher C, Louie B, Facente S, et al. : Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PloS One 2013;8(12):e80629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christopoulos KA, Zetola NM, Klausner JD, et al. : Leveraging a rapid, round-the-clock HIV testing system to screen for acute HIV infection in a large urban public medical center. J Acquir Immune Defic Syndr 2012;62:e30–e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hightow-Weidman LB, Fowler B, Kibe J, et al.: HealthMpowerment.org: Development of a theory-based HIV/STI website for young black MSM. AIDS Educ Prev 2011;23(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hightow-Weidman LB, Pike E, Fowler B, et al.: HealthMpowerment.org: Feasibility and acceptability of delivering an Internet intervention to young black men who have sex with men. AIDS Care 2012;24(7):910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muessig KE, Pike EC, LeGrand S, et al. : Mobile phone applications for the care and prevention of HIV and other sexually transmitted diseases: A review. J Med Internet Res 2013;15(1):19–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Case KK, Ghys PD, Gouws E, et al. : Understanding the modes of transmission model of new HIV infection and its use in prevention planning. Bull World Health Organ 2012;90:831a–838a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lucas A. and Armbruster B: The cost-effectiveness of expanded HIV screening in the United States. AIDS 2012;27:795–801 [DOI] [PubMed] [Google Scholar]
- 81.van Rooyen H, Barnabas RV, Baeten JM, et al. : High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2013;64(1):e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jani IV, Sitoe NE, Alfai ER, et al. : Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: An observational cohort study. Lancet 2011;378(9802):1572–1579 [DOI] [PubMed] [Google Scholar]
- 83.Jani I, Meggi B, Vubil A, et al. : Evaluation of point-of-care nucleic acid testing for HIV viral load and early infant diagnosis in primary health clinics: Mozambique. Paper presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia, 2013 [Google Scholar]
- 84.Heffron R, Ngure K, Mugo N, et al. : Willingness of Kenyan HIV-1 serodiscordant couples to use antiretroviral-based HIV-1 prevention strategies. J Acquir Immune Defic Syndr 2012;61(1):116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanna DB, Buchacz K, Gebo KA, et al. : Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 2013;56(8):1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker BD. and Hirsch MS: Antiretroviral therapy in early HIV infection. N Engl J Med 2013;368(3):279–281 [DOI] [PubMed] [Google Scholar]
- 87.Dolan P, Hallsworth M, Halpern D, et al. : Influencing behaviour: The mindspace way. J Econ Psychol 2012;33(1):264–277 [Google Scholar]
- 88.Galarraga O, Genberg BL, Martin RA, et al. : Conditional economic incentives to improve HIV treatment adherence: Literature review and theoretical considerations. AIDS Behav 2013;17(7):2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marteau TM, Hollands GJ, and Fletcher PC: Changing human behavior to prevent disease: The importance of targeting automatic processes. Science 2012;337(6101):1492–1495 [DOI] [PubMed] [Google Scholar]