Abstract
We have previously shown that an HIV vaccine regimen including three HIV-DNA immunizations and a single HIV-modified vaccinia virus Ankara (MVA) boost was safe and highly immunogenic in Swedish volunteers. A median 38 months after the first HIV-MVA vaccination, 24 volunteers received 108 plaque-forming units of HIV-MVA. The vaccine was well tolerated. Two weeks after this HIV-MVA vaccination, 18 (82%) of 22 evaluable vaccinees were interferon (IFN)-γ enzyme-linked immunospot (ELISpot) reactive: 18 to Gag and 10 (45%) to Env. A median minimal epitope count of 4 to Gag or Env was found in a subset of 10 vaccinees. Intracellular cytokine staining revealed CD4+ and/or CD8+ T cell responses in 23 (95%) of 24 vaccinees, 19 to Gag and 19 to Env. The frequency of HIV-specific CD4+ and CD8+ T cell responses was equally high (75%). A high proportion of CD4+ and CD8+ T cell responses to Gag was polyfunctional with production of three or more cytokines (40% and 60%, respectively). Of the Env-specific CD4+ T cells 40% were polyfunctional. Strong lymphoproliferative responses to Aldrithiol-2 (AT-2)-treated subtype A, B, C, and A_E virus were demonstrable in 21 (95%) of 22 vaccinees. All vaccinees developed binding antibodies to Env and Gag. Neutralizing antibodies were detected in a peripheral blood mononuclear cell (PBMC)-based assay against subtype B and CRF01_AE viruses. The neutralizing antibody response rates were influenced by the vaccine dose and/or mode of delivery used at the previous HIV-MVA vaccination. Thus, a second late HIV-MVA boost induced strong and broad cellular immune responses and improved antibody responses. The data support further exploration of this vaccine concept.
Introduction
By the end of 2011, UNAIDS estimated that 34 million people (31.4 million to 35.9 million) were living with human immunodeficiency virus type 1 (HIV-1) infection worldwide. A slowing of the rate of new infections to 2.5 million (2.2 million to 2.8 million), 20% less than in 2001, was described, as was a decrease in the number of people dying of AIDS-related causes, 1.7 million (1.5 million to 1.9 million), representing a 24% decline in AIDS-related mortality compared to 2005.1 Nonetheless, a safe and effective HIV vaccine remains an important goal and offers the best hope for control of the pandemic.2 An ideal HIV vaccine would prevent acquisition of infection and control viral replication.
Preclinical studies in nonhuman primates have shown that vaccine regimens expressing simian immunodeficiency virus (SIV) Env, Gag, and Pol antigens reduced infection risk and that Env was required for protection against acquisition of infection.3–5 Env-specific antibodies were suggested to be critical for blocking acquisition.5 CD8+ T lymphocytes can mediate control of viral replication in HIV-infected individuals and SIV-infected monkeys.6 Preclinical vaccine trials have shown an association between Gag-specific cellular immune responses and control of viremia in vaccinated monkeys following virus challenge.6 The importance of HIV-specific CD4+ T cell responses in early viral control in acute HIV infection was recently shown.7 Thus, both cell-mediated and antibody-mediated HIV-specific immune responses would contribute to an effective vaccine, with functional antibodies to inhibit viral replication at the site of infection and cytotoxic cells directed against the HIV-infected cells.
Several HIV vaccine strategies have been tested in clinical phase I/II trials, among them the prime-boost vaccination regimen using DNA priming and recombinant virus-based vaccines such as adenovirus or recombinant modified vaccinia virus Ankara (MVA) for boosting, live recombinant prime/protein boost, or DNA prime/protein boost.1,8 The first phase III trial of a prime-boost regimen using a canary pox (ALVAC) prime and an envelope protein boost (AIDSVax) showed a modest 31.2% efficacy in low-incidence Thai heterosexuals, without effect on HIV viral RNA load and CD4 count in infected individuals.9 In the immune-correlates analysis, binding IgG antibodies to variable regions 1 and 2 (V1V2) of HIV-1 (envelope) proteins correlated inversely with the rate of HIV-1 infection and binding of plasma anti-Env IgA antibodies correlated directly with risk of infection. Further analyses suggested that Env-specific IgA antibodies might interfere with IgG effector functions and weaken the benefit of potentially protective antibodies.10 Analysis of the T cell response in the RV 144 vaccinees, although modest in frequency compared to the humoral immune response, confirmed HIV gp120 V2 specificity and revealed CD4+ T cell polyfunctionality and cytolytic capacity.11
A phase I HIV-vaccine study (HIVIS01/02) was performed in Stockholm, Sweden to assess different modes of administering an HIV DNA vaccine candidate (plasmid DNA containing HIV env of subtypes A, B, and C, gag A and B, and rtB, HIV-DNA) boosted with heterologous HIV-1 recombinant MVA containing env, gag, and pol genes of CRF01_AE (HIV-MVA).12 Following three HIV-DNA immunizations and a single HIV-MVA vaccination a total of 37 (97%) of 38 were responders. Thirty-four (89%) of 38 vaccinees exhibited HIV-specific interferon (IFN)-γ enzyme-linked immunospot (ELISpot) responses, 32 to Gag and 24 to Env. A lymphoproliferative assay (LPA) response was noted in 35 (92%) of 38 vaccinees and HIV-specific CD4+ and CD8+ T cells with proliferative capacity were induced.13 However, anti-gp160 antibodies were detected in only one (3%) of 38 vaccinees.12
Three years (median 38 months, range 33–40 months) after the last vaccination 24 volunteers from the HIVIS01/02 trial were rerecruited to receive a second HIV-MVA vaccination. Here, we describe the safety of administering a second HIV-MVA vaccination and present a comprehensive analysis of the cellular and humoral immune responses present at the time of and induced by the late HIV-MVA vaccination.
Materials and Methods
Study design
A phase I/II trial (HIVIS01/02) was conducted in 2005–2006 in Stockholm, Sweden.12 The volunteers in the HIVIS01/02 trial received four different treatment arms of HIV-DNA vaccination using a Bioject 2000 device either intradermally (id) or intramuscularly (im) and with or without granulocyte-macrophage colony-stimulating factor (GM-CSF). Three HIV-DNA vaccinations were given followed by a single HIV-MVA boosting vaccination 6 months later (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). The HIV-MVA vaccinations were given either as 107 pfu id or 108 pfu im by needle injection in the left deltoid muscle.
Three years (median 38, range 33–40 months) after the last vaccination 24 volunteers remaining from the HIVIS01/02 trial received a second HIV-MVA vaccination of 108 pfu of HIV-1 MVA given by needle and syringe into the left deltoid muscle. Of the 24 volunteers, 13 had previously received 108 pfu of HIV-1 MVA im and 11 had received 107 pfu of HIV-1 MVA id.
Clinical trials registration: www.controlled-trials.com/ISRCTN32604572.
Vaccine
MVA-Chang Mai double recombinant (CMDR) (HIV-MVA) has been described elsewhere.14 The construct expresses HIV-1 subtype E Env and subtype A Gag/Pol from Thai isolates CM235 and CM240, respectively, both under the control of the early/late mH5 promoter. The cytoplasmic tail of Env was truncated and the RNaseH and integrase genes were entirely deleted. The active site of RT contains a mutation that abolishes enzymatic activity. The vaccine was produced by the WRAIR Pilot Bio production facility, Forest Glen, MD.
Clinical and laboratory safety assessments
Safety evaluations, which included physical examinations and laboratory tests, were performed before as well as 2 weeks and 3 months after the vaccination. A 12-lead electrocardiogram (ECG) was administered before and 2 weeks after the HIV-MVA vaccination.
Safety laboratory tests included a complete blood count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, creatinine, fasting blood glucose, and complete urine analysis including a pregnancy test for female participants. The hematology and biochemistry tests were performed at Karolinska University Hospital, Huddinge, Sweden. T-lymphocyte subset determinations were performed using FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Diagnostic HIV serological testing was performed using IMxHIV-1/HIV-2 III Plus (Abbott) and Enzygnost HIV Integral II (Dade Behring, Marburg, Germany) enzyme-linked immunoassays (ELISAs). Samples that were reactive by ELISA were tested by Western blot analysis (HIV-1 western blot, Diagnostic Biotechnology). The Centers for Disease Control and Prevention criteria were used, which require a reactivity to at least two of the following antigens for a positive classification: p24, gp41, and gp120/160. Infection was ruled out by HIV RNA detection using the Roche Amplicor HIV-1 Monitor v.1.5 RNA-PCR kit (Roche Diagnostic Systems).
Cellular immunogenicity assessment
Blood collection and cell preparation
Whole blood samples for analysis of immune responses were collected in cell preparation tubes (CPT Vacutainer tubes; BD) containing sodium heparin as anticoagulant. Peripheral blood mononuclear cells (PBMCs) were processed and stored as described previously.13,15
ELISpot assays
IFN-γ ELISpot was performed on fresh PBMCs using the h-IFN-γ ELISpotPLUS kit and a two-step detection system according to the manufacturers' instructions (Mabtech, Nacka, Sweden) as described previously.12 The HIV-specific peptide pools used are described in Table 1.
Table 1.
HIV-Specific Peptide Pools Used in ELISpot and Intracellular Cytokine Staining Assays
| Peptide pool ID | Protein | Peptide number | Subtype |
|---|---|---|---|
| Gag Ia | p17 | 1–26 | B |
| Gag IIa | p24 | 27–71 | A |
| Gag WRb,c | p6, p7, p17, p24 | 1–160 | A |
| Env Ia | gp120, including V1 and V2 | 1–50 | A/B |
| Env IIa | gp120, including V3-V5 | 51–100 | A |
| Env IIIa | gp41 | 101–169 | B |
| Env WRb,c,d | Env | 1–177 | E |
| Pol WRb,c | Pol | 1–146 | A |
HIV-1 vaccine clade A- and B-specific peptides corresponding to the HIV-DNA prime.
All peptides were 15-mers with 10 amino acid (aa) overlap except in Walter Reed Army Institute of Research (WR) peptide pools, which had peptides with 11 aa overlap.
HIV-1 vaccine-specific peptides corresponding to the MVA-CMDR boost.
Not used in ELISpot testing.
For epitope mapping, a matrix-based strategy was applied. Pools of overlapping peptide sets matching the insert sequences of MVA-CMDR were used. Ninety-five peptides for CM240 Gag were divided into 20 matrix pools, while 138 peptides for CM235 Env were divided into 20 matrix peptide pools. Each matrix peptide pool contained 10–12 peptides to permit enumeration of the number of epitopes targeted in the ELISpot assay format. The IFN-γ ELISpot assay was performed as described above using PBMCs collected at baseline (prior to receiving any vaccination) and 2 or 4 weeks after the HIV-MVA boosting vaccination. Thawed and rested PBMCs were stimulated with each of the matrix peptide pools (1 μg/ml) in single wells. The results were expressed as minimum epitope counts.
Eight-color intracellular cytokine staining (ICS) assay
For the determination of CD4+ and CD8+ T cell responses, an eight-color ICS assay was performed on fresh PBMCs, which had been rested overnight following the purification procedure. PBMCs (0.5×106) were incubated in 96-well round-bottom plates with costimulatory anti-CD28 (1 μg/ml) and anti-CD49d (1 μg/ml) monoclonal antibodies (Becton Dickinson, BD Pharmingen, San Diego, CA) in either medium only (negative control) or in medium containing a mixture of staphylococcal enterotoxin A and B (SEAB, 1 μg/ml, Sigma-Aldrich Logistic GmbH, Germany), CEF peptide pool (1 μg/ml, NMI Technologie Transfer, Germany), CMV peptide pool (PepMix, 0.125 μg/ml, JPT, Innovative Peptide solutions, Germany), HIV-1 Gag-specific and Env-specific peptide pools (Table 1), and Brefeldin A (10 μg/ml, Sigma-Aldrich, Germany). Samples were incubated for 6 h at 37°C in a 7.5% CO2 incubator and were stored at 4°C overnight.
The next day, 20 μl (20 mM, pH 8) of EDTA was added to each well and incubated for 15 min. Thereafter, the samples were transferred to a V-bottom plate and centrifuged for 5 min. Cells were washed twice with phosphate-buffered saline (PBS), stained with anti-CD4-PerCP-Cy5.5, anti-CD8-AmCyan (BD), and the viability marker VIVID-Pacific blue (Life Technologies), and incubated for 30 min at 4°C (in the dark). Cells were washed twice in wash buffer [PBS+0.1% fetal calf serum (FCS)], permeabilized in BD Cytofix/Cytoperm buffer for 20 min at 4°C (in the dark), and washed once in BD Perm/Wash buffer. Cells were then stained intracellularly for 30 min at 4°C with an antibody cocktail containing anti-CD3–APC-Cy7, anti-MIP-1β-PE, anti-TNF-α-PE-Cy7, anti-IFN-γ-FITC, and anti-IL-2-APC (Becton Dickinson, San Jose, CA). At the end of the incubation, cells were washed twice in Perm/Wash buffer, fixed in BD CellFix solution, and stored at 4°C in the dark until acquisition. Acquisition of samples was performed using a FACSCanto II flow cytometer (BD Biosciences) where a volume of 80% (200 μl) of each sample was acquired using a threshold of 200 on FSC. A minimum of 50,000 CD3+ lymphocytes per well was required for a sample to be included in the analysis. The samples were analyzed using FlowJo software, version 8.7.1 (Tree Star, Ashland, OR) and distributions were presented using PESTLE and SPICE, version 5.1 (kindly provided by Mario Roederer, Vaccine Research Center, NIH at http://exon.niaid.nih.gov/spice).
Background levels for Gag and Env were established using Swedish blood donor samples (n=15). ICS responses to Gag were considered positive if they were at least 2.5-fold higher than the mean of background (medium control) and above 0.05% gated positive CD4+ T lymphocytes and above 0.1% CD8+ T cells. Using these criteria, one of 15 blood donors had a CD4+ T cell reactivity to Gag I expressing IFN-γ (0.061%) and MIP1-β (0.1%). Another blood donor had a borderline CD4+ T cell reactivity to Gag WR expressing IFN-γ (0.052%). All blood donors' CD8+ T cells were negative for IFN-γ, interleukin (IL)-2, tumor necrosis factor (TNF)-α, and macrophage inflammatory protein (MIP)-1β expression. ICS responses to Env were considered positive if they were >2.5-fold higher than the mean of medium control and >0.05% gated positive CD4+ or CD8+ T lymphocytes. Using these criteria, one of 15 blood donors had CD4+ T cell reactivity to Env I expressing IL-2 (0.270%), to Env II expressing IFN-γ (0.067%), to Env WR expressing IFN-γ (0.150%), and to Env WR expressing MIP-1β (0.096%). Another blood donor had CD8+ T cell reactivity to Env II expressing IL-2 (0.140%). The analysis of reactivity to Env III in the CD4+ T cell population and to Env WR among CD8+ T cells was excluded due to high background levels in blood donor samples.
Lymphoproliferation assay
A tritiated [3H]thymidine uptake lymphoproliferation assay was used as described previously.13 Here, Aldrithiol-2 (AT-2)-treated HIV-1MN (subtype B isolate), HIV-1KNH (subtype A isolate), HIV-1TZA (subtype C isolate), HIV-1CM235 (subtype CRF01_AE isolate), and SUPT1 microvesicles or Jurkat (control antigens) were applied. The antigens were kindly provided by Dr. J. Lifson, SAIC Frederick, Inc., Frederick, MD. T cell proliferation was reported as stimulation index (SI). An SI ≥8 was considered positive based on the mean background reactivity in 30 healthy Swedish blood donors. The SI mean+3 SD was 7.57 for HIV-1MN, 5.51 for HIV-1KNH, 4.53 for HIV-1TZA, 3.34 for HIV-1CM, 7.79 for SUPT1 microvesicles, and 7.82 for Jurkat.
Humoral immunogenicity assessment
Assessment of binding antibodies
Antibodies to Gag (recombinant p17/24 protein kindly provided by Programme EVA, Centre for AIDS Reagents, NIBSC, Potters Bar, UK),17 native gp160 subtype B (HIV-1IIIB, Advanced Biotechnologies Inc., Columbia, MD), and recombinant gp140 subtype C (HIV-196ZM651, kindly provided by Programme EVA, Centre for AIDS Reagents, NIBSC, Potters Bar, UK) were determined by use of standard validated ELISAs. Briefly, ELISA plates (Nunc, Maxisorp, Odense, Denmark) were coated with Env proteins at a concentration of 0.5 μg/ml and incubated overnight at +4–+8°C. Plates were washed and blocked with 10% FCS in PBS. Serum dilutions in duplicate, titrated beginning at 1:100 using 2-fold dilutions, were added and incubated overnight at +4–+8°C. Antigen–antibody complexes were detected using rabbit antihuman immunoglobulin G antibodies conjugated to horseradish peroxidase (Dako Cytomation, Aarhus, Denmark). Plates were developed for 15 min by adding O-phenylenediamine buffer (Dako). The color reaction was stopped using 2 M H2SO4 and the optical density was read using dual wavelengths, 490 nm and 630 nm. The cutoff was based on each volunteers' baseline reactivity. The mean of the duplicate optical density values was calculated for both preimmunization and postimmunization samples. A sample was positive in a dilution of 1:100 or 1:200 if the absorbance value was more than twice that of the preimmunization sample at a 1:100 or a 1:200 dilution, respectively. A sample was positive in a dilution >1:200 if the absorbance value was more than twice the mean of the preimmunization sample run at a 1:200 dilution. The results were reported as reciprocal end-point titers.
Anti-Env IgG subclasses were assessed in an in-house ELISA using native HIV-1IIIB gp160 (Advanced Biotechnologies Inc., Columbia, MD). Testing was performed as described above using horseradish peroxidase-conjugated sheep antihuman IgG1, IgG2, IgG3, or IgG4 (The Binding Site, Birmingham, UK) as conjugate.
Measurement of envelope glycoprotein-specific antibody avidity was determined using an in-house ELISA employing recombinant HIV subtype B gp160 (Protein Sciences Corporation, Meridian, CT) essentially performed as described previously17 with and without a urea wash. Briefly, serum dilutions were added to plates in duplicate and one-half of the samples were incubated in 8 M urea (Sigma-Aldrich, Germany) and the other half were incubated in saline for 5 min following incubation of serum dilutions. The avidity index was calculated as the ratio of the absorbance value obtained with urea treatment to that observed with saline multiplied by 100. Avidity index values <30% were designated low avidity, those with index values between 30% and 50% were designated intermediate avidity, and those >50% was designated high avidity.18
Neutralization assays
Neutralizing antibody (NAb) was measured in both the TZM-bl and PBMC neutralization assay platforms using pseudovirus and Renilla luciferase expressing infectious molecular clones (IMC), respectively, as reported previously.19,20 Sera were screened at 1:20 dilution in the TZM-bl assay; 1×104 cells were incubated with equal volumes of virus and sera and then cultured for 48 h in the presence of 40 μg/ml DEAE-dextran. Cell-expressed luciferase relative luminescence units (RLU) were detected using Britelite substrate and a Victor Light luminometer (Perkin Elmer, Waltham, MA). Neutralization was calculated as the reduction in RLU in wells containing postvaccination sera compared to wells without sera. A result ≥50% was considered a positive response.
Sera were serially titered in the PBMC assay; 1.5×105 cells were incubated with equal volumes of virus and sera and then cultured for 96 h in the presence of IL-2 and phytohemagglutinin (PHA). IMC-expressed luciferase RLU were detected using Renilla luciferase substrate and an Envision luminometer (Perkin Elmer, Waltham, MA). Neutralization was calculated as the reduction in RLU in wells containing postvaccination sera compared to wells containing prevaccination sera. Reported ID50 values are the average of two independent experiments for which the results were within a 2.5-fold range.
Detection of neutralizing plasma IgA1 in samples collected 4 weeks after the HIV-MVA vaccination was performed on IgA1 samples that had been purified from plasma. The neutralization assay was performed using 20–30 TCID50 of HIV-1BZ167 virus (Clade B, NIH AIDS Reagent and Reference Program) as reported previously.21,22 Neutralization capacity was defined as more than 67% reduction of p24 antigen in the supernatant compared with p24 content in baseline samples.
Analysis of antivaccinia neutralizing antibody titers was performed using vaccinia virus strain Elstree (Bernbiotech, Bern, Switzerland), as described earlier.16
Data analysis
Clinical and safety laboratory data were entered in the computerized hospital patient registry system under the national identification code and full name. Study data were entered under study code and initials on clinical report forms. Specimens for immunological and virological studies were sent under study code to SMI, which remained blinded to the randomization. Clinical and safety laboratory data were entered in Access and immunological laboratory data in Excel. Volunteer data and immunological data were analyzed in SPSS 15.0 under study code. Most data are presented without statistical analysis since this is a descriptive, hypothesis generating study. The immune responses were compared using the Mann–Whitney U-test. Correlations between results obtained by the PBMC-based neutralizing antibody assay, the gp140 ELISA, and gp160 IgG3 subclass assay were estimated by the nonparametric Spearman rank correlation test. A p value of <0.05 was considered statistically significant.
Ethical approval
The protocols and products were approved by the Karolinska Institutet and Regional Ethics Committees and the Swedish Medical Products Agency. Informed consent was obtained from all participants.
Results
Safety
The HIV-1 MVA boosting vaccination was well tolerated. There was no influence of vaccinations on hemoglobin, white blood cell, neutrophil, lymphocyte, or platelet count, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, creatinine, or fasting blood glucose levels. There were no changes in ECG.
Vaccine-induced T cell responses
IFN-γ ELISpot
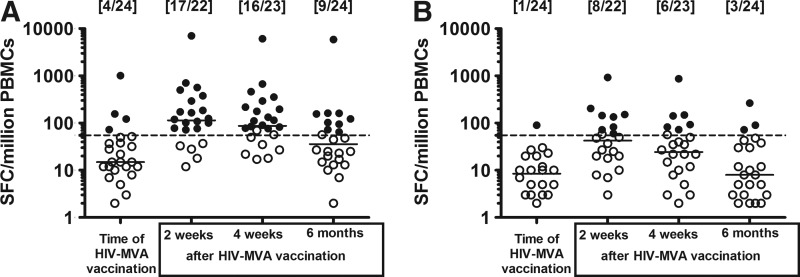
Table 2 summarizes the IFN-γ ELISpot response rates to the various HIV peptide pools in vaccinees on the day of the second HIV-MVA vaccination (delivered approximately 3 years after the previous HIV-MVA vaccination) and 2 and 4 weeks as well as 6 months after the second HIV-MVA vaccination. On the day of vaccination, four (17%) of 24 vaccinees had IFN-γ responses to Gag, while one (4%) of the 24 vaccinees had a response to Env. The Env responder responded to both Env I (gp120 V1 and V2) and Env III (gp41) peptide stimulation. Two weeks after the second HIV-MVA vaccination, 18 (82%) of 22 evaluable vaccinees had IFN-γ responses to Gag, while 10 (45%) of the 22 vaccinees had a response to Env. Similarly, 4 weeks after the second HIV-MVA vaccination, 16 (70%) of 23 evaluable vaccinees had IFN-γ responses to Gag and seven (30%) of the 23 vaccinees had a response to Env. Six months after the second HIV-MVA vaccination, nine (37%) of 24 vaccinees had IFN-γ responses to Gag and three (12%) of the 24 vaccinees had a response to Env. None of the vaccinees responded to the Pol WR peptide pool at any of the four time points.
Table 2.
Summary of Interferon-γ ELISpot Response Rates to Various Peptide Pools in Vaccinees
| At the time of HIV-MVA boost, n=24 | Two weeks after HIV-MVA boost, n=22 | Four weeks after HIV-MVA boost, n=23 | Six months after HIV-MVA boost, n=24 | |||||
|---|---|---|---|---|---|---|---|---|
| Peptide pool | No. (%)a | Median (range)b | No. (%)a | Median (range)b | No. (%)a | Median (range)b | No. (%)a | Median (range)b |
| Gag I | 1 (4) | 62 | 5 (23) | 80 (77–180) | 4 (17) | 100 (78–133) | 2 (8) | 93 (82–102) |
| Gag II | 1 (4) | 780 | 8 (36) | 107 (60–6,825) | 10 (43) | 90 (67–6,500) | 2 (8) | (193–6,560) |
| Gag WR | 4 (17) | 139 (73–1,010) | 17 (77) | 165 (72–7,060) | 16 (70) | 155 (77–6,120) | 9 (37) | 123 (65–5,905) |
| Env I | 1 (4) | 90 | 8 (36) | 140 (60–930) | 6 (26) | 117 (73–867) | 3 (13) | 90 (72–267) |
| Env II | 0 (0) | 1 (4) | 80 | 1 (4) | 60 | 0 (0) | ||
| Env III | 1 (4) | 60 | 3 (13) | 98 (65–273) | 1 (4) | 195 | 0 (0) | |
| Any Gag | 4 (17) | 18 (82) | 16 (70) | 9 (37) | ||||
| Any Env | 1 (4) | 10 (45) | 7 (30) | 3 (12) | ||||
| Gag or Env | 4 (17) | 18 (82) | 18 (78) | 10 (42) | ||||
Number of responders given as percentage of total number of vaccinees.
Median and range given for responders only.
MVA, modified vaccinia virus Ankara.
Medians and ranges in IFN-γ ELISpot responders for the peptide pools are shown in Table 2 and Fig. 1. Two weeks after the second HIV-MVA vaccination the median responses in the responders were modest; the median Gag WR response was 165 SFC/million PBMCs (range 72–7,060) and the median Env I response was 140 SFC/million PBMCs (range 60–930). There was no significant difference between the magnitude of Gag WR responses in responding vaccinees at 2 and 4 weeks after the second HIV-MVA vaccination, median 165 vs. 155 SFC/million PBMCs, respectively, p=0.0906 (Wilcoxon signed matched pair test). Six months after the second HIV-MVA vaccination the level of reactivity to Gag WR in the nine responders was 123 SFC/million PBMCs (range 65–5,905). T cell epitope mapping using IFN-γ ELISpot was performed in 10 vaccinees on cryopreserved samples collected 2 or 4 weeks after the second HIV-MVA vaccination. Samples with >200 SFC/million PBMCs to either a Gag or an Env peptide pool were selected. Deconvolution of the peptide matrices revealed minimum median epitope counts of 2.5 per responder subject against Gag (range 0–4), 2 per responder subject against Env (range 0–4), and 4 per responder subject against either Gag or Env (range 1–8) (data not shown).
FIG. 1.
The magnitude of interferon (IFN)-γ enzyme-linked immunospot (ELISpot) responses in the vaccinees at four time points after stimulation with (A) the Gag WR peptide pool and (B) the Env I peptide pool. The number of responders per number of evaluable vaccinees is given in brackets. Median values are given by the bars. ELISpot responses were considered positive if the number of spot-forming cells (SFC) was >55 spots/million peripheral blood mononuclear cells (PBMCs) and four times the background value. The dashed line is at 55 SFC/million PBMCs. Responders are indicated by filled circles and nonresponders are given by open circles.
Intracellular cytokine staining
HIV-specific CD4+ and CD8+ T cell responses to three Gag and four Env peptide pools (Table 1) were determined by eight-color ICS assay 4 weeks after the second HIV-MVA boosting vaccination (Table 3). Of the 24 vaccinees, 23 (95%) had CD4+ and/or CD8+ T cell responses expressing at least one cytokine in response to Gag or Env. A balanced CD4+ and CD8+ T cell response was observed, where 18 (75%) vaccinees exhibited CD4+ T cell reactivity, 13 (54%) to Gag and 16 (67%) to Env, and 18 (75%) vaccinees had CD8+ T cell responses, 15 (62%) to Gag and six (25%) to Env.
Table 3.
CD4+ and CD8+ T Cell Responses Determined by Intracellular Cytokine Staining 2 Weeks After the Second HIV-Modified Vaccinia Virus Ankara Boost
| IFN-γ | IL-2 | TNF-α | MIP-1ß | Any cytokine | |||||
|---|---|---|---|---|---|---|---|---|---|
| Peptide pool | No. (%)a | Median (range)b | No. (%)a | Median (range)b | No. (%)a | Median (range)b | No. (%)a | Median (range)b | No. (%)a |
| Overall CD4+ and /or CD8+ T cell responses (n=24) | |||||||||
| Gag or Env | 16 (67) | 15 (62) | 13 (54) | 13 (54) | 23 (95) | ||||
| Any Gag | 12 (50) | 12 (50) | 11 (46) | 10 (42) | 19 (79) | ||||
| Any Env | 11 (46) | 12 (50) | 8 (33) | 8 (33) | 19 (79) | ||||
| CD4+ T cell responses | |||||||||
| Gag I | 2 (8) | 0.162 (0.006–0.256) | 1 (4) | 0.305 | 0 | 1 (4) | 0.145 | 2 (8) | |
| Gag II | 5 (21) | 0.100 (0.070–0.150) | 7 (29) | 0.098 (0.061–0.240) | 2 (8) | 0.225 (0.170–0.280) | 3 (12) | 0.100 (0.061–0.120) | 8 (33) |
| Gag WRc | 8 (35) | 0.106 (0.056–0.265) | 9 (39) | 0.125 (0.072–0.250) | 8 (35) | 0.135 (0.085–0.305) | 3 (13) | 0.0905 (0.072–0.094) | 12 (50) |
| Any Gag | 9 (38) | 10 (42) | 8 (33) | 4 (17) | 13 (54) | ||||
| Env I | 5 (21) | 0.100 (0.055–0.260) | 4 (17) | 0.155 (0.110–0.360) | 2 (8) | 0.259 (0.088–0.430) | 3 (12) | 0.077 (0.072–0.160) | 7 (29) |
| Env II | 2 (8) | 0.099 (0.089–0.110) | 2 (8) | 0.195 (0.170–0.220) | 0 | 1 (4) | 0.180 | 4 (17) | |
| Env WRc | 9 (39) | 0.190 (0.055–0.390) | 12 (52) | 0.175 (0.072–0.530) | 7 (30) | 0.190 (0.087–0.610) | 7 (30) | 0.120 (0.097–0.190) | 15 (62) |
| Any Env | 9 (37) | 12 (50) | 7 (29) | 6 (25) | 16 (67) | ||||
| Gag or Env | 12 (50) | 14 (58) | 10 (42) | 7 (29) | 18 (75) | ||||
| CD8+ T cell responses | |||||||||
| Gag I | 3 (12) | 0.12 (0.100–0.375) | 3 (12) | 0.160 (0.120–0.375) | 2 (8) | 0.435 (0.110–0.760) | 1 (4) | 0.470 | 6 (25) |
| Gag II | 4 (17) | 0.250 (0.150–15.20) | 3 (12) | 0.150 (0.110–5.700) | 4 (17) | 0.170 (0.100–15.70) | 7 (29) | 0.540 (0.140–16.20) | 11 (46) |
| Gag WRc | 5 (22) | 0.270 (0.120–14.95) | 3 (13) | 0.275 (0.13–4.195) | 4 (17) | 0.215 (0.115–16.05) | 4 (17) | 0.400 (0.345–17.00) | 8 (33) |
| Any Gag | 7 (29) | 6 (25) | 7 (29) | 8 (33) | 15 (62) | ||||
| Env I | 0 | 0 | 0 | 1 (4) | 0.330 | 1 (4) | |||
| Env II | 2 (8) | 0.215 (0.210–0.220) | 0 | 1 (4) | 0.660 | 3 (12) | 0.310 (0.300–2.090) | 5 (21) | |
| Env IIIc | 3 (13) | 0.350 (0.180–0.350) | 0 | 1 (4) | 0.520 | 2 (9) | 1.240 (0.340–2.140) | 4 (17) | |
| Any Env | 3 (12) | 0 | 1 (4) | 3 (12) | 6 (25) | ||||
| Gag or Env | 8 (35) | 6 (25) | 8 (35) | 10 (42) | 18 (75) | ||||
Number of responders given as percentage of total number of vaccinees.
Median and range are given for responders only.
Data missing for one vaccinee (n=23).
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; MIP, macrophage inflammatory protein.
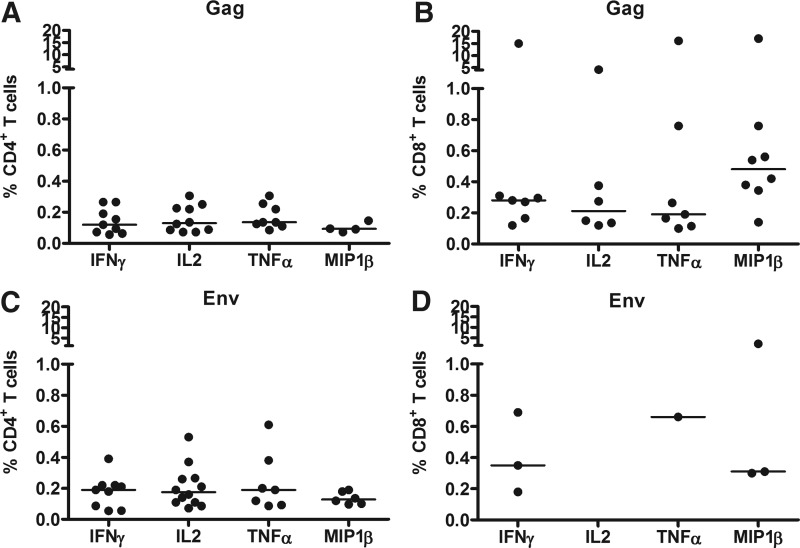
The magnitudes of the CD4+ and CD8+ T cell responses against Gag and Env are shown in Fig. 2 and Table 3. The median CD4+ T cell responses to any Gag peptide was similar for all cytokines (Fig. 2A). CD8+ T cells showed stronger IFN-γ and MIP-1β responses to Gag compared to CD4+ T cells (p=0.011 and 0.0081, respectively, Mann–Whitney test). No differences in magnitude were noted for the other two cytokines. Analysis of single cytokines revealed CD4+ and /or CD8+ T cell production of three or more cytokines in 12 (52) of 23 ICS responders, 11 (48%) to Gag and six (25%) to Env, and production of two cytokines in 18 (78%) of 23 responders, 13 of whom had production of both IFN-γ and IL-2.
FIG. 2.
The magnitude of HIV-specific T cell responses as assessed by eight-color intracellular cytokine staining (ICS) 4 weeks after the second HIV-modified vaccinia virus Ankara (MVA) vaccination. Reactivities in responders to any Gag peptide pool by (A) CD4+ T cells and (B) CD8+ T cells are shown. Reactivities in responders to any Env peptide pool by (C) CD4+ T cells and by (D) CD8+ T cells are also shown. Median values are given by the bars.
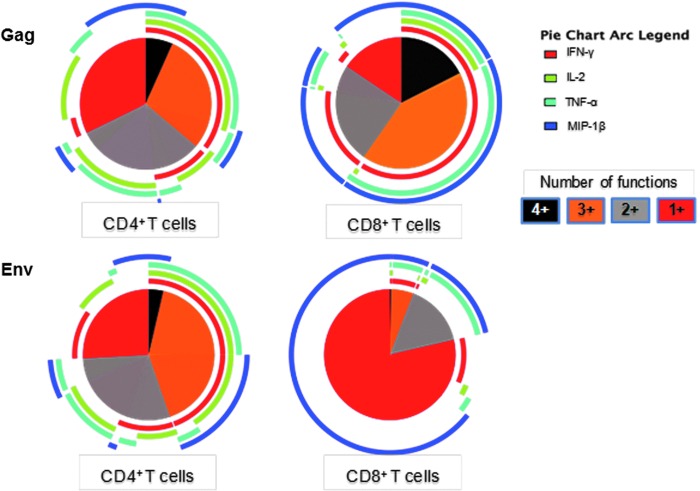
Polyfunctional analysis based on Boolean gating to create combinational events of IFN-γ, IL-2, TNF–α, and/or MIP-1β revealed that approximately 25% of the Gag WR-responding CD4+ T cells were dual functional, with a predominance of IFN-γ/IL-2 and IL-2/TNF-α, and 40% were polyfunctional, predominantly expressing IFN-γ/IL-2/TNF-α. Among the Gag responding CD8+ T cells, approximately 25% were dual functional, mainly producing IFN-γ/MIP-1β, and 60% were polyfunctional, predominantly expressing IFN-γ/IL-2/TNF-α/MIP-1β and IFN-γ/TNF-α/MIP-1β (Fig. 3). Among Env-specific responding CD4+ T cells, approximately 25% were dual functional, with a predominance of IFN-γ/IL-2 and IL-2/TNF-α expression, and 40% were polyfunctional, producing IFN-γ/IL-2 coexpressed with either TNF-α or MIP-1β. Most of the Env-specific responding CD8+ T cells (75%) were monofunctional, mainly expressing MIP1-β. The dual-functional and trifunctional cells (25%) expressed TNF-α/MIP-1β with or without IFN-γ.
FIG. 3.
Polyfunctional analysis of Gag-WR and Env-specific responses in CD4+ and CD8+ T cells from all 24 vaccinees. The pie charts show the fraction of the responses based on the number of functions from one (red) to four (black) functions whereas the pie arcs show the relative contribution of each individual cytokine.
Lymphoproliferative responses
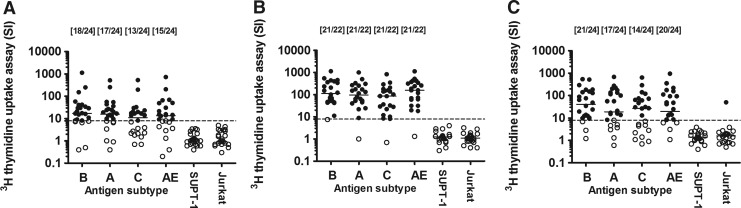
HIV-specific T cell responses were also measured using LPA on the day of the second HIV-MVA vaccination, 2 weeks and 6 months after vaccination (Fig. 4). Notably, on the day of the second HIV-MVA boosting vaccination, 3 years after the first HIV-MVA vaccination, a high frequency of the 24 volunteers exhibited a positive LPA (SI >8) in response to AT-2-treated HIV-1 antigens of four different subtypes: 18 (75%) to HIV-1MN (subtype B), 17 (71%) to HIV-1KNH (subtype A), 13 (54%) to HIV-1TZA (subtype C), and 15 (62%) to HIV-1CM235 (subtype CRF01_AE). The overall response rate to any of the antigens tested was 21 (87%) of 24. The median (range) proliferative response in responders to HIV-1MN was 29.3 SI (8.2–1,126), to HIV-1KNH 24.0 SI (9.6–511), to HIV-1TZA 19.9 SI (8.2–529), and to HIV-1CM235 21.4 SI (9.2–725). Two weeks after the second HIV-MVA boost the response rate had increased and 21 (95%) of 22 evaluable vaccinees were responders to all four antigens used. The median (range) proliferative response in responders to HIV-1MN was 123 SI (11.2–1,139), to HIV-1KNH 115 SI (8.9–1,007), to HIV-1TZA 86 SI (8.1–827), and to HIV-1CM235 157 SI (18.2–1,130). A high response rate was also detected 6 months after the second HIV-MVA boosting vaccination with 21 (87%) of 24 vaccinees responding to HIV-1MN, 17 (71%) of 24 to HIV-1KNH, 14 (58%) of 24 to HIV-1TZA, and 20 (83%) of 24 to HIV-1CM235. The overall response rate to any of the antigens tested was 23 (96%) of 24, and 21 (87%) of 24 exhibited responses to two or more of the antigens. The median (range) proliferative response in responders to HIV-1MN was 64 SI (9.3–538), to HIV-1KNH 100 SI (8.3–678), to HIV-1TZA 62 SI (13–639), and to HIV-1CM235 45 SI (8.3–949). One vaccinee was consistently LPA negative at all time points tested.
FIG. 4.
T cell proliferative responses to Aldrithiol-2 (AT-2)-treated HIV antigens (HIV-1MN subtype B, HIV-1KNH subtype A, HIV-1TZA subtype C, and HIV-1CM235 subtype CRF01_AE) and control antigen (SUPT-1 and Jurkat) as measured by [3H]thymidine uptake assay (A) at the time of, (B) 2 weeks after, and (C) 6 months after the second HIV-MVA vaccination. The number of lymphoproliferative assay (LPA)-reactive vaccinees per number of evaluable vaccinees is given in brackets. Median values are given by the bars. T cell proliferative responses were considered positive if the stimulation index (SI) >8. The dashed line is at 8 SI. LPA reactive vaccinees are given by filled circles and nonreactive vaccinees are given by open circles.
Vaccine-induced antibody responses
Binding antibodies
Antibody testing was performed on serum or plasma samples collected 4 weeks and 6 months after the second HIV-MVA vaccination. Four weeks after the second HIV-MVA vaccination all (100%) of 24 volunteers were reactive in the IMxHIV-1/HIV-2 III Plus (Abbott) ELISA, while 13 (54%) of 24 volunteers were reactive in the Enzygnost HIV Integral II ELISA. Thirteen (54%) of 24 volunteers were also positive in Western blot using the Centers for Disease Control and Prevention criteria, which requires a reactivity to at least two of the following antigens for a positive classification: p24, gp4, and gp120/160. At 6 months, 23 (96%) of 24 volunteers were still reactive in IMxHIV-1/HIV-2 III Plus (Abbott) ELISA and 10 (42%) of 24 were reactive in Enzygnost HIV Integral II ELISA and Western blot, respectively (data not shown).
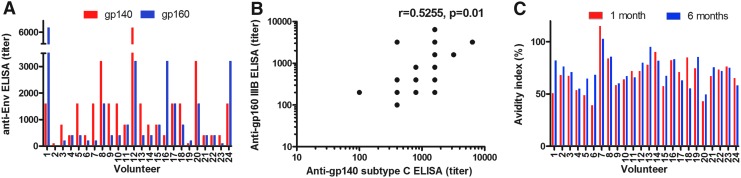
Binding antibody titers to recombinant Gag (p17/24 protein), native HIV-1IIIB subtype B gp160, and recombinant HIV-196ZM651 subtype C gp140 were determined by ELISA. One month after the second HIV-MVA vaccination all (100%) of 24 volunteers exhibited anti-Gag antibodies (median titer 1,600, range 100–6,400). Furthermore, 23 (96%) of 24 volunteers exhibited antibodies to native HIV-1IIIB subtype B gp160 (median titer 400, range 100–6,400) and 24 (100%) of 24 had antibodies to recombinant HIV-1 subtype C gp140 (median titer 1600, range 100–6,400) (Fig. 5A). Six months after the HIV-boosting vaccination 20 (83%) of 24 were reactive to native HIV-1IIIB gp160 (median 200, range 100–6,400). The HIV-1IIIB gp160 antibody titers 1 month after the second HIV-MVA vaccination correlated with HIV-1 subtype C recombinant gp140 antibody titers, r=0.5255, p=0.0100 (Fig. 5B).
FIG. 5.
HIV-specific antibody responses as determined by enzyme immunoassay (EIA). (A) Antibody endpoint titers to recombinant HIV-196ZM651 gp140 (red bars) and native HIV-1IIIB gp160 (blue bars) 1 month after the second HIV-MVA vaccination. (B) Correlation between anti-HIV-1IIIB gp160 (subtype B) and anti-HIV-196ZM651 gp140 (subtype C) enzyme-linked immunosorbent assay (ELISA) titers 1 month after the second HIV-MVA vaccination. (C) Anti-gp160 avidity indices 1 and 6 months after the second HIV-MVA vaccination.
To further define the Env-specific antibody response, IgG subclasses were determined using native HIV-1IIIB gp160 antigen. One month after the second HIV-MVA vaccination 19 (79%) of 24 vaccinees displayed IgG1-specific anti-gp160 antibodies (median titer 200, range 100–800) and five (21%) of 24 had IgG3-specific anti-gp160 antibodies (median titer 200, range 200–800). None had IgG2 or IgG4 subclass-specific responses to gp160 (data not shown).
The avidity of antibodies to gp160 was determined by measuring the resistance of serum antibody-envelope gp160 immune complexes to disruption by treatment of 8 M urea in gp160 ELISA. One month after the second HIV-MVA boosting vaccination, antibodies, with avidity indices greater than 50%, were detected in 21 (87%) of 24 vaccinees. Furthermore, at 6 months, 23 (96%) of 24 had high-avidity envelope-specific antibodies while one vaccinee had an avidity index of 49% (Fig. 5C).
Neutralizing antibodies
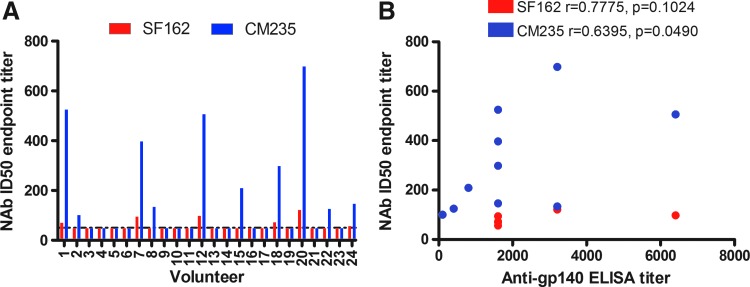
Neutralizing antibody testing was performed in samples collected from vaccinees 1 month after the HIV-MVA boosting vaccination. There was no demonstrable NAb activity in the TZM-bl pseudovirus assay using SF162 subtype B or CM235 subtype CRF01_AE pseudoviruses in samples tested at a 1:20 dilution (data not shown). In contrast, NAbs were demonstrated when the PBMC assay was applied using samples diluted 1:50 against SF162 (6/24, 25%) and CM235 (10/24, 42%). Vaccinee sera that screened positive were further titrated to calculate ID50 values; on average responder ID50 values were higher for CM235 (median titer 254, range 101–698) than for SF162 (median titer 84, range 57–121) (Fig. 6A). Furthermore, neutralizing antibody titers to CM235 correlated with HIV-1 subtype C gp140 ELISA antibody titers (Fig. 6B).
FIG. 6.
Neutralizing antibody titers as determined by peripheral blood mononuclear cell/infectious molecular clone neutralizing antibody (PBMC/IMC Nab) assay. (A) NAb titers to SF162 subtype B (red bars) or CM235 subtype CRF01_AE isolates (blue bars) are shown. (B) Correlation between NAb titers to SF162 or CM235 and anti-subtype C gp140 ELISA titers.
Purified plasma IgA1-mediated neutralization was assessed in samples collected from vaccinees 1 month after the second HIV-MVA vaccination. None of the 24 IgA1 samples exhibited HIV neutralizing activity.
The first HIV-MVA vaccination influenced the vaccine-induced antibody-mediated immune response after the second HIV-MVA
Of the 24 volunteers rerecruited to receive a late second HIV-MVA vaccination, 13 volunteers had previously received 108 pfu HIV-MVA im and 11 volunteers had received 107 pfu HIV-MVA id as a boost after three HIV-DNA immunizations. There was no statistical difference in ELISpot response rate to Gag WR stimulation between the two groups at any of the time points tested. Nor was there any difference in lymphoproliferative responses at any of the time points tested (data not shown). Anti-gp160 IIIB antibody reactivity and avidity indices were also similar between the two groups, p=0.2969 and p=0.2970, respectively. In contrast, gp140 ELISA antibody titers were higher after the second HIV-MVA vaccination in vaccinees who had received 108 pfu HIV-MVA im (median 1,600, range 100–6,400) compared to vaccinees who had received 107 pfu id (median 400, range 100–1,600) at the first HIV-MVA vaccination, p=0.0136. Anti-gp160 IIIB IgG3 subclass antibodies were detected in only a proportion of the vaccinees who had received two vaccinations of 108 pfu HIV-MVA im following the HIV-DNA priming immunizations (Table 4). Furthermore, neutralizing antibodies detected by a PBMC-based assay using subtype B (HIV-1SF162) and CRF01_AE (HIV-1CM235) viral isolates were frequent in vaccinees who had received 108 pfu of HIV-MVA im, while they were rare in vaccinees who had received 107 pfu id before the late second HIV-MVA vaccination (Table 4).
Table 4.
Antibody Responses After the Late Second HIV-Modified Vaccinia Virus Ankara Vaccination in Vaccinees According to the Dose and Route of Immunizations at the Time of the First HIV-Modified Vaccinia Virus Ankara Vaccination
| First HIV-MVA vaccination | |||
|---|---|---|---|
| Antibody response | 108 pfu im | 107 pfu id | p-value |
| Anti-HIV-1IIIB gp160 IgG3 antibodies | 5/13 (38%) | 0/11 (0%) | 0.0411 |
| SF162 neutralizing antibodies | 6/13 (46%) | 0/11 (0%) | 0.0162 |
| CM235 neutralizing antibodies | 8/13 (62%) | 2/11 (18%) | 0.0414 |
Antivaccinia neutralizing antibodies
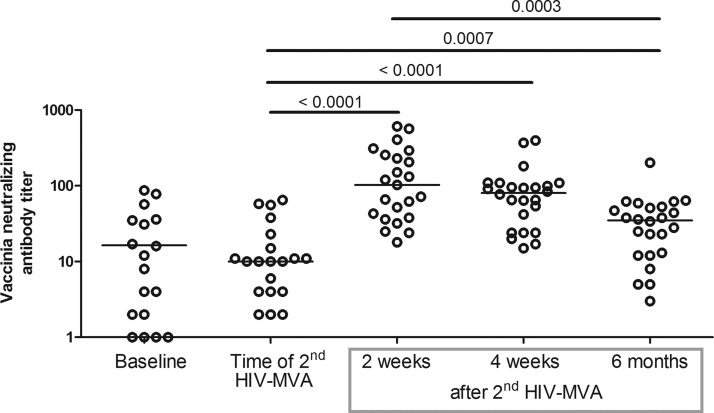
Figure 7 shows the antivaccinia neutralizing antibody responses at baseline (prior to the first HIV-DNA vaccination), the time of the second HIV-MVA, 2 weeks, 4 weeks and 6 months after the vaccination. At the time of the second HIV-MVA vaccination the magnitude of antivaccinia Nab was low and similar to the level seen at baseline. NAb responses against vaccinia peaked 2 weeks after the second HIV-MVA vaccination with a mean NAb titer of 167, and declined over time to a mean NAb titer of 39, 6 months after the second HIV-MVA vaccination (p=0.0003).
FIG. 7.
Antivaccinia neutralizing antibody titers in vaccinees. Median values are given by the bars. p-values are given for comparisons in median titers between the time of the second HIV-MVA and 2 weeks, 4 weeks, and 6 months after the second HIV-MVA, respectively. A p-value is also given for the comparison in titers between 2 weeks and 6 months after the second HIV-MVA.
Discussion
In the present trial we explored the safety and immunogenicity of a second HIV-MVA boosting vaccination delivered 3 years after receipt of three HIV-DNA immunizations and an HIV-MVA boost. Twenty-four vaccinees received the second late HIV-MVA vaccination, which was well tolerated.
Interestingly, on the day of the second HIV-MVA vaccination, a high proportion (87%) of the volunteers exhibited strong T cell proliferative responses in response to AT-2-treated HIV-1 antigens of four different HIV subtypes (A, B, C, and CRF01_AE). Furthermore, a proportion (17%) of the vaccinees had IFN-γ ELISpot responses to Gag peptide pool stimulation. Thus, the initial immunization scheme applied in the HIVIS01/02 trial12 consisting of three HIV-DNA immunizations given at 0, 1, and 3 months followed by an HIV-MVA vaccination at 9 months induced a durable and robust cell-mediated immune response lasting more than 3 years. To our knowledge this is the first report describing a long-term 3-year follow-up of a prophylactic HIV vaccine candidate using HIV-DNA as prime and HIV-MVA as boost.
Two weeks after the second HIV-MVA immunization of vaccinees previously given three HIV-DNA immunizations and one HIV-MVA vaccination a high proportion (82%) of the volunteers exhibited IFN-γ ELISpot responses, 82% to Gag and 45% to Env. We have previously reported that after delivery of three HIV-DNA immunizations and a single HIV-MVA vaccination in the HIVIS01/02 trial, the response rate was 86% to Gag and 65% to Env (HIVIS01/0212). Using the same vaccines for vaccination of Tanzanian volunteers and priming with three injections of HIV-DNA (1 mg id or 3.8 mg im) and boosting twice with 108 pfu HIV-MVA, a higher proportion of IFN-γ ELISpot responders was found: 93% to Gag and 79% to Env (HIVIS03).15 The difference in IFN-γ ELISpot response rates between the Swedish and the Tanzanian trials can be attributed to several factors. The Tanzanian trial was guided by the results from the HIVIS01/02 trial in Sweden and the two most promising HIV-DNA priming modes were selected for use. In the Tanzanian trial all vaccinees received two doses of 108 pfu of HIV-MVA vaccination. The Swedish vaccinees received either 107 pfu id or 108 pfu im of HIV-MVA at the time of the first HIV-MVA vaccination depending on randomizations. At the time of the second HIV-MVA all volunteers received the 108 pfu HIV-MVA dose. Additionally, the volunteers in Tanzania were younger (18–40 years) compared to those in Sweden (18–60 years).
The same HIV-MVA construct used in Sweden has also been evaluated in a phase I safety and immunogenicity trial in healthy volunteers in the United States and Thailand. After three immunizations of 108 pfu HIV-MVA, 90% of the volunteers exhibited IFN-γ ELISpot responses, predominantly to Env,22 but the magnitude of responses was lower than in the present trial and in the Tanzanian HIVIS03 trial, which both included priming with HIV-DNA. The Eurovacc02 trial of a vaccine regimen including two primes with HIV-DNA and one boost with NYVAC poxvirus vector, both expressing HIV-1 Env and Gag/Pol/Nef, showed a high IFN-γ ELISpot response rate, 90%, predominantly to Env and polyfunctional CD4+ and CD8+ T cell responses.23 The use of HIV-1 DNA prime and MVA boost vaccines based on T cell epitopes in phase I/II clinical trials resulted in a low frequency of IFN-γ ELISpot responses.24,25 Using a multiclade HIV-1 DNA and rAd5 boost, Churchyard et al. reported IFN-γ ELISpot responses in 70.8% of vaccinees overall, 54.7% to Gag and 54.2.% to Env.26 Other clinical trials using HIV-DNA prime and recombinant Ad5 boost regimens have reported IFN-γ ELISpot response rates ranging from 35.5% to 100%, primarily targeting Env.27–30
In the present trial, eight-color ICS performed 2 weeks after the second HIV-MVA vaccination showed equally high frequencies (75%) of HIV-specific CD4+ and CD8+ T cell responses. However, CD8+ T cell cytokine responses were more frequent to Gag (69%) than to Env (28%), whereas the CD4+ T cell response rates to Gag and Env were more balanced (54% and 67%, respectively). After two Geovax pGA2/JS7 DNA and two MVA/HIV62 vaccinations in the HVTN065 trial the CD4+ T cell response rate of 77% (77% to Gag and 62% to Env) was similar to that in the present study, whereas the CD8+ T cell response was somewhat lower at 42% (35% to Gag and 23% to Env).31 In a phase IIA clinical trial (HVTN204) of a multiclade HIV DNA prime and rAd5 boost a balanced CD4+and CD8+ T cell ICS response rate was demonstrated (41.8% and 47.2%, respectively).26
For the present trial, T cell epitope mapping was performed after the second HIV-MVA vaccination using samples from a subset of 10 volunteers having >200 SFC/million PBMCs in IFN-γ ELISpot to either Gag or Env. A balanced response was observed with a mean minimal epitope count of 2.5 to Gag, 2.1 to Env, and 4.6 to either Gag or Env. The overall mean number of epitopes recognized was comparable to that reported for the EuroVacc02 phase I study (4.2 to Env),23 the RV144 phase III trial (3.6 to Env),11 a multiclade DNA prime recombinant adenovirus 5 boost regimen trial, VRC009 (3.3 to Env or Gag),27 but greater than that reported for the Step trial (1 to Gag).32
A broad multisubtype HIV-specific T cell response was also elicited as measured by LPA. Two weeks after the second HIV-MVA vaccination 21 (95%) of 22 evaluable vaccinees were responders to all four antigens of different subtypes (AT-2-treated HIV-1MN, HIV-1KNH, HIV-1TZA, and HIV-1CM235) used for testing. The highest stimulation indices were recorded for the HIV-MVA homologous subtype CRF01_AE HIV-1CM235 (157 SI) followed by subtype B HIV-1MN (123 SI), subtype A HIV-1KNH (115 SI), and subtype C HIV-1TZA (86 SI). These results extend our previous findings from the HIVIS03 trial in which all (100%) of the vaccinees had LPA responses to HIV-1CM235 antigen 2 weeks after the second HIV-MVA vaccination.15
Notably, following the second HIV-MVA vaccination HIV-specific ELISA antibody responses were detected in all vaccinees by routine diagnostic serological tests, and 96% had antibodies to subtype B Env gp160 and 100% had antibodies to subtype C Env gp140. Antibodies with HIV-neutralizing activity in a PBMC/IMC assay were more common among recipients of two 108 pfu HIV-MVA im vaccinations than among vaccinees who had received a 107 pfu HIV-MVA id vaccination and a 108 pfu HIV-MVA im vaccination after the three HIV-DNA priming immunizations. Thus, the vaccine dose and/or mode of delivery used at the first HIV-MVA vaccination given approximately 3 years before the second HIV-MVA vaccination influenced the neutralizing antibody response rates after the second (late) HIV-MVA vaccination. Using the same HIV-MVA vaccine, Currier et al. previously reported that antibodies exhibiting antibody-dependent cellular cytotoxicity (ADCC) activity were more frequent in vaccinees who had received three doses of 108 pfu im compared to three doses of 107 pfu id.22 Goepfert et al. reported higher antibody response rates after two immunizations with 3 mg of Geovax pGA2/JS7 DNA followed by two vaccinations using 108 TCID50 MVA/HIV62 compared to two immunizations with 0.3 mg pGA2/JS7DNA followed by two vaccinations using 107 TCID50 MVA/HIV62.31
The antibody findings in the present trial support and extend our previous findings from the HIVIS03 trial in Tanzania in which all vaccinees became HIV antibody positive and a high proportion of the vaccinees exhibited NAbs to CM235 clade CRF01_AE using the PBMC/IMC assay after a second 108 pfu HIV-MVA vaccination delivered 1 year after receipt of three HIV-DNA and a 108 pfu HIV-MVA boosting vaccination.15 Recently, high neutralizing antibody response rates were reported using the TZMbl assay in vaccinees who had received two ADVAX DNA vaccinations followed by two vaccinations of recombinant MVA encoding HIV genes (TBC-M4).34
In the present study, the majority of vaccinees (87%) had high-avidity anti-Env antibodies 4 weeks after the second HIV-MVA vaccination. The number of vaccinees having high avidity antibodies increased to 23/24 (96%) 6 months after the second HIV-MVA boost. In a preclinical evaluation of a DNA prime MVA boost vaccine candidate Zhao et al.33 reported an inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia in the absence of detectable levels of neutralizing Ab suggesting Fc-mediated mechanisms of viral control. Here, NAbs were detected when using the PBMC/IMC assay but not when using the TZMbl/pseudovirus assay. In the HIVIS03 trial, 83% of the vaccinees neutralized CM235 subtype CRF01_AE when the PBMC/IMC assay was used, while no neutralizing activity was detected using the TZMbl/pseudovirus assay.15 In a standard PBMC-based assay with a p24-read out antibodies and virus inocula are washed out, while when using the PBMC/IMC assay Renilla reneformis luciferase activity is detected and the cells are not washed. Brown et al.35 recently reported a substantial influence of natural killer cells when the PBMC/IMC assay was applied. Thus, the virus neutralizing activity seen after the second HIV-MVA may be Fc mediated and further assessment of the vaccine-induced antibody-mediated antiviral activity is merited.
Antivaccinia vector responses were determined at four time points. Importantly, at the time of the second HIV-MVA vaccination, 3 years after the first HIV-MVA vaccination, antivaccinia levels were at pre-MVA vaccination levels. Peak antivaccinia levels were detected 2 weeks after the second HIV-MVA vaccination. The levels of antivaccinia NAb decreased considerably after 6 months. Preexisting immunity to vaccinia virus did not reduce the proportion of responders to HIV, but lowered the magnitude of HIV responses after a single HIV-MVA vaccination.16 Here, the second HIV-MVA vaccination induced a broad and potent immune response to HIV-1 antigens confirming that vaccinia-based vectors can be used for repeated boosting despite previous exposure to vaccinia virus.
In summary, a second HIV-MVA boost delivered approximately 3 years after the initial HIV-DNA/HIV-MVA vaccinations induced strong and broad cellular immune responses to both Gag and Env. Furthermore, a potent antibody-mediated response was induced. The data support further exploration of this vaccine concept.
Supplementary Material
Acknowledgments
The study was funded by the European Union (INCO-DEV A4 ICFP501A4PR03 to E.S. and AVIP 503487 and Europrise to B.W. and E.S.), the Swedish International Development Cooperation Agency (Sida) (SWE-2004-120 to C.N. and HIV2004-000809 and 2004:813 to G.B.), the Swedish Research Council (Vetenskapsrådet, K2004-16x-07743-19 to B.W.), and Läkare mot AIDS Forskningsfond (04-050301 and 01-051101 to E.S.). None of these institutions had any influence on the study or its analysis. Documentation and production of the HIV-DNA vaccines were supported by Vecura, Karolinska University Hospital. Construction of the HIV-1 MVA was supported by the Division of Intramural Research, NIAID, NIH and the U.S. Military HIV Research Program. The production costs were funded by the U.S. Military HIV Research Program, WRAIR.
We extend special thanks to the study volunteers. We also thank Ulrika. Edbäck, Ida Ericsson, Linda Jernberg, Katarina. Karlén, and Teghesti Tecleab for excellent technical assistance, Inger Petz, Ronnie Ask, and Karin Reinhard, for their devoted clinical work, and Gunnel Engström for vaccine documentation. We are grateful to Dr. Jeffrey Lifson, SAIC Frederick, Inc., Frederick, Maryland for providing AT-treated antigens. We thank the Programme Eva Centre for AIDS Reagents, NIBSC, Potters Bar for providing recombinant gp140 subtype C (HIV-196ZM651) and recombinant p17/24 protein.
Presented in part at the 2010 AIDS Vaccine conference in Atlanta, GA. Abstracts OA03.08 and P14.03.
The views and opinions expressed herein do not necessarily reflect those of the U.S. Army or the Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS: 2012 UNAIDS report on the global AIDS epidemic. www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf
- 2.Girard MP, Osmanov S, Assossou OM, and Kieny M-P: Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: A review. Vaccine 2011;29:6191–6218 [DOI] [PubMed] [Google Scholar]
- 3.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, et al. : Prevention of infection by granulocyte macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 2011;204:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letvin NL, Rao SS, Montefiori D, Seaman MS, Sun Y, Lim S-Y, et al. : Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 2011;3:81ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. : Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012;482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freel SA, Saunders KO, and Tamaras GD: CD8+ T-cell-mediated control of HIV and SIV infection. Immunol Res 2011;49:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soghoidan DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, et al. : HIV-specific cytolytic CD4 T cell responses during acute infection predict disease outcome. Sci Trans Med 2012;4:123ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Rerks-Ngarm S, Excler J-L, and Michael NL: HIV vaccines: Lessons learned and the way forward. Curr Opin HIV AIDS 2010;5:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al.: Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Eng J Med 2009;362:2209–2220 [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Gilbert PB, McElrath JM, Zolla-Pazner S, Tomaras GD, Alam M, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Eng J Med 2012;366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, et al. : The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012;188:5166–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandström E, Nilsson C, Hejdeman B, Bråve A, Bratt G, Robb M, et al.: Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis 2008;198:1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboud S, Nilsson C, Karlén K, Marovich M, Wahren B, Sandström E, et al.: Strong HIV-specific CD4+ and CD8+ T-lymphocytes proliferative responses in healthy individuals immunized with an HIV-1 DNA vaccine and boosted with recombinant modified vaccinia virus Ankara expressing HIV-1 genes. Clin Vaccine Immunol 2010;17:1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl PL, Cotter C, Moss B, VanCott T, Currier J, Eller LA, et al. : Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 2009;27:5885–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakari M, Aboud S, Nilsson C, Francis J, Buma D, Moshiro C, et al.: Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011;28:8417–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudmundsdotter L, Nilsson C, Bråve A, Hejdeman B, Earl P, Moss B, et al.: Recombinant modified vaccinia Ankara (MVA) efficiently boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009;27:4468–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bråve A, Boberg A, Gudmundsdotter L, Rollman E, Hallermalm K, Ljungberg K, et al.: A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1 specific CD8+ T cells and humoral responses in mice. Mol Ther 2007;15:1724–1733 [DOI] [PubMed] [Google Scholar]
- 18.Cole KS, Rowles JL, Jagerski BA, Murphey-Corb M, Unangst T, Clements JE, et al. : Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol 1997;71:5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montefiori DC: Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. Curr Protocall Immunol 2004;Chapter 12, unit 11(12.11.1–12.11.17). [DOI] [PubMed] [Google Scholar]
- 20.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, et al. : Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 2010;408:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasselrot K, Bratt G, Hirbod T, Säberg P, Ehnlund M, Lopalco L, et al.: Orally exposed uninfected individuals have systemic anti-HIV responses associating with partners' viral load. AIDS 2010;24:35–43 [DOI] [PubMed] [Google Scholar]
- 22.Currier JR, Ngauy V, de Souza MS, Ratto-Kim S, Cox JH, Polonis VR, et al. : Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia–Ankara-HIV-1 vaccine candidate. PLoS One 2010:5:e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harari A, Bart P-A, Stöhr W, Tapia G, Garcia M, Medjitna-Rais E, et al.: An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med 2008;205:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke T, Goonetilleke N, McMichael AJ, and Dorrell L: Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol 2007;88:1–12 [DOI] [PubMed] [Google Scholar]
- 25.Gorse GJ, Newman MJ, deCamp A, Hay CM, De Rosa SC, Noonan E, et al. : DNA and modified vaccinia virus Ankara vaccines encoding multiple cytotoxic and helper-T lymphocyte epitopes of human immunodeficiency virus type 1 (HIV-1) are safe but weakly immunogenic in HIV-1-uninfected, vaccinia virus-naïve adults. Clin Vaccine Immunol 2012;5:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, et al. : A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011;8:e2122521857901 [Google Scholar]
- 27.Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, et al. : Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One 2010;5:e9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, et al. : A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-uninfected east Africans. J Infect Dis 2010;201:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, et al.: Safety and immunogenicity study of multiclade HIV-1 adenovirus vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine alone. PLoS One 2010;5:e12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koblin BA, Casapia M, Morgan C, Qin L, Wang ZM, Defawe OD, et al. : Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: A randomized clinical trial. PLoS One 2011;6:e24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, et al. : Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2010;203:610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elrath MJ: Immune responses to HIV vaccines and potential impact on control of acute HIV-1 infection. J Infect Dis 2010;202(Suppl2):S323–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, et al.: Preclinical studies of human immunodeficiency virus/AIDS vaccines: Inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J Virol 2009;83:4102–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehendale S, Thankar M, Sahay S, Kumar M, Shete A, Sathyamurthi P, et al.: Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: A phase I randomized trial in HIV-uninfected Indian volunteers. PLoS One 2013;8:e55831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown BK, Wieczorek L, Jijak G, Lombardi K, Currier J, Wesberry M, et al. : The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS One 2012;7:e29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.