Abstract
Human embryonic stem cells (hESCs) hold great promise for future clinical cell therapies because of their unique potential to differentiate into all human cell types. However, the destruction of normal fertilized embryos and the derivation of hESCs for research has resulted in polarized ethical debates, with most of the controversy centered on embryo destruction. Therefore, due to less ethical controversy surrounding them, abnormal fertilized zygotes that are usually discarded are a potential feasible resource for the derivation of hESCs. Microsurgery on human polyspermic zygotes can contribute to the derivation of hESCs, but the efficiency is much lower. Here, we reported a culture system to enhance the developmental competence of such microsurgical human polyspermic zygotes by EGF–BDNF–IGF-1 combination, which eventually resulted in the increased derivation efficiency of hESCs from them. We found that the developmental efficiency of microsurgical enucleated tripronuclear (3PN) embryos cultured with the EGF–BDNF–IGF-1 combination was significantly increased compared with the control group. More importantly, when the microsurgical enucleated 3PN embryos were cultured in medium supplemented with EGF–BDNF–IGF-1, the frequency ratio of chromosome abnormality was reduced. Our present study will facilitate the development of hESC line derivation in subsequent studies and also provide an additional choice for infertile couples.
Introduction
Human embryonic stem cells (hESCs) hold great promise for future clinical cell therapies because of their unique potential to differentiate into all human cell types [1]. Currently, the vast majority of hESC lines are derived from surplus embryos from in vitro fertilization (IVF) treatments. However, the use of embryos for hESC derivation for research and eventual medical applications has resulted in polarized ethical debates, because the process involves the destruction of viable, developing human embryos [2]. Therefore, due to less ethical controversy surrounding them, abnormal fertilized zygotes that are usually discarded are a potential feasible resource for the derivation of hESCs.
Normal human zygotes consist of two pronuclei (PN) that develop from the maternal and paternal genomes. However, IVF, which is one of the mainstream assisted reproductive technologies (ARTs), often results in abnormal zygotes with three or more PN [3]. These zygotes cannot develop into normal humans, because the chromosome is polyploidy instead of diploid and, therefore, usually discarded after IVF manipulation. Among these abnormal fertilized zygotes, the most commonly found are tripronuclear (3PN) zygotes, which are formed because the oocyte genome failed to separate from the sperms or as two sperms entered into the oocyte. The latter happens more often, resulting in ∼5%–7% polyspermic zygotes being formed during the IVF procedure.
Although polyspermic zygotes are usually disregarded in clinical settings, they have the potential to develop into blastocysts and form trophoblast cells and the inner cell mass (ICM) with normal morphologies [4]. Moreover, hESC lines have been shown to exhibit marker expression and differentiation ability in vitro and in vivo comparable to that of normal hESCs [5]. In 1989, Malter and Cohen obtained one reconstructive blastocyst from seven 3PN human zygotes using microsurgery [6]. This microsurgical repair method has also been used in clinical settings. Kattera and Chen transferred repaired embryos into a 38-year-old woman, resulting in a normal, healthy baby boy [7]. Based on these findings, Fan et al. derived the first hESC line using five blastocysts developed from 3PN zygotes repaired by microsurgery [8]. Subsequently Jiang et al. obtained 2 hESC lines using 11 blastocysts generated by the microsurgical method [9]. Since the efficiency (∼20%) was significantly lower than when normal fertilized blastocysts were used (∼40%–50%), the use of polyspermic zygotes in the field of hESCs has been limited. In previous studies, the lower quality of polyspermic zygotes has been shown to result in lower blastocyst formation efficiency and low blastocyst quality. Therefore, improvements in embryo quality could contribute to an increase in hESC derivation [8].
It has been reported that growth factors play key roles in mammalian development and increase the developmental competence of fertilized embryos in mice, rats, cattle, pigs, and humans [10]. Growth factors participate in oocyte maturation, embryonic development, and blastocyst function in an autocrine/paracrine manner, and different growth factors probably play different roles in these processes. Therefore, it is likely that a combination of growth factors would promote embryo development and improve embryo quality [11]. Lorenzo et al. suggested that the EGF–IGF-1 combination could enhance cumulus expansion and nuclear maturation of bovine oocytes [12]. A similar effect of epidermal growth factor (EGF) plus insulin-like growth factor-1 (IGF-1) treatment was reported in studies in sheep, Merino lamb, and porcine embryo development [13–15]. In addition to IGF-1, EGF has also been suggested to play a beneficial role in oocyte maturation and embryo development when combined with gonadotrophin [16], transforming growth factor-α [17], or EGF-like factors [17]. Although EGF has been shown to have tremendous potential for improving oocyte maturation and embryo development due to its stage-dependent functions [18], its negative effects on oocytes in the presence of low gonadotrophins [19] prompted a search for appropriate growth factors to be used in combination with EGF.
Neurotrophic factors were first identified as promoting the growth, survival, and differentiation of neurons. Subsequently, their positive role in the early stages of ovarian folliculogenesis, oocyte maturation, embryo development, and embryo implantation suggested their potential role in the improvement of blastocyst development [20–22]. Linher-Melville and Li suggested that glial cell-line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF), used either alone or in combination, could be added to the media for in vitro oocyte maturation, thereby potentially increasing the production and/or quality of early embryos [23].
Recently, Kawamura et al. used a combination of seven growth factors, including BDNF, colony-stimulating factor (CSF), EGF, granulocyte macrophage colony-stimulating factor (Gm-CSF), IGF-1, GDNF, and artemin, to improve the developmental competence of normal fertilized embryos, polyspermic zygotes, and cloned embryos [24]. Yu et al. demonstrated that embryo development and embryo quality developed from in vitro matured oocytes could be significantly improved by supplementing the in vitro maturation and embryo culture medium with EGF, BDNF, and IGF-1 [22]. More importantly, it was possible to derive hESCs from embryos cultured in media supplemented with these three growth factors. However, whether the combination of these three growth factors can improve the developmental competence and spindle assembly of reconstructed diploid zygotes from polyspermic zygotes is still unknown.
In this study, we investigated the effects of EGF, BDNF, and IGF-1 on the developmental competence and embryo quality of repaired diploid zygotes reconstructed from 3PN embryos and the feasibility of ES derivation using the resulting blastocysts. Moreover, expression of the receptors for these three growth factors was evaluated in these zygotes to explore the potential mechanism of action of the growth factors.
Materials and Methods
Source of 3PN human zygotes
This study was approved by the ethics committees of The Third Affiliated Hospital of Guangzhou Medical University and Peking University Third Hospital. The patients involved in this study knew about and understood the usage of polyspermic zygotes and voluntarily discarded them after signing the informed consent. A total of 364 3PN human zygotes with a visible second polar body were obtained 18–20 h post-insemination.
PN removal by micromanipulation
The 3PN zygotes were transferred into GMOPS media. The male pronucleus was selected for removal. The extra male pronucleus was identified as previously established criteria [7]. The criteria used to identify the male PN were as follows: (1) presence of pronucleus-associated sperm tails, (2) increased pronucleus size, and (3) a greater distance (relative to the female PN) from the second polar body. For the reconstructed diploid zygotes, one pronucleus situated further away from the polar body I was identified clearly through the eyepiece. A hole was made in the zona pellucida using an injector needle of 12–15 μm diameter with the help of a Piezo apparatus (Prime Tech), and the pronucleus was aspired out of the oocyte using the injector needle. The repaired diploid zygotes (r2PN) were transferred into and cultured in Gm media (LGGG-050; LifeGlobal) until the blastocyst stage. To study the effects of growth factors on the development of repaired diploid embryos, some Gm media were supplemented with 10 ng/mL EGF (E-9644), 10 ng/mL BDNF (B-3795), and 10 ng/mL IGF-1 (I-3769). All embryos were maintained in a humidified incubator at 37°C, 5% CO2, and 5% oxygen.
Embryo grading
The repaired diploid embryos were graded in our ART center following previously established eight-cell [25] and blastocyst evaluation [26] criteria. Eight-cell grading was performed as follows: If there were no fragments and uniform blastomeres on day 3, the embryos were classified as 8G1; if the ratio of fragments was 5% and uniform blastomeres were present on day 3, the embryos were classified as 8G2; if the ratio of fragments ranged from 5% to 20% and the blastomere morphology was not uniform, the embryos were classified as 8G3; and if the ratio of fragments was 50% and the blastomeres did not have normal distinct morphology, the embryos were classified as 8G4. Blastocyst grading was as follows: If the blastocyst had a large blastocoel and ICM with a large number of tightly packed cells on day 5, the embryos were classified as BG1; if the blastocyst with the blastocoel expanded to 50%–80% of the volume and the ICM had a few loosely packed cells on day 5, the embryos were classified as BG2; and if the blastocyst with the blastocoel occupied <50% of the volume and the ICM had almost no cells on day 5, the embryos were classified as BG3.
Apoptosis testing and cell number counting
The apoptotic cells were analyzed by TUNEL staining method as in our previous study [27]. The blastocysts were fixed in 4% paraformaldehyde solution for 1 h at room temperature after being rinsed in 1 mg/mL PVP-PBS solution. Then, the plasma membrane of blastomeres was permeabilized in 0.5% Triton X-100 (Sigma-Aldrich) for 1 h. After being treated with DNase, TUNEL staining was performed on the samples as instructed by the TUNEL assay kit manufacturer (Roche). Finally, the samples were cultured in propidium iodide (PI) at a concentration of 0.05 mg/mL plus RNase A (50 mg/mL of each) for 1 h at room temperature in the dark to stain all the nuclei, and then, the samples were mounted on a slide and examined under a NIKON fluorescent microscope.
Blastocyst biopsy
On day 5 or 6, the expanding blastocysts were used for trophectoderm (TE) cell biopsy. Some TE cells (normally 5–10 cells) were aspirated into the biopsy pipette and then cut using the ZILOS-tk™ laser system (Hamilton Thorn Bioscience, Inc.). The biopsied blastocysts were then transferred back into the culture medium. The biopsied TE cells were rinsed in buffer, placed into tubes containing cell lysis buffer, and stored at −20°C till CGH testing.
Array comparative genome hybridization
Genomic DNA from TE cells was amplified using a whole-genome amplification kit. CGH manipulation using a Bluegnome platform was performed according to the instruction book and as previously demonstrated [28]. The amplified DNA samples were labeled with Cy3 or Cy5 using a fluorescent labeling system and mixed with control human DNA. The samples were then dried, resuspended, and loaded onto the BlueGnome 24Sure V3 arrays. The data were analyzed using Bluefuse software (BlueGnome). A similar protocol was used for hESC analysis.
Immunostaining
To determine the expression of the EGF receptor (EGFR), BDNF receptor (TrkB), and IGF-1 receptor (IGF-1R), a total of 30 zygotes from the r2PN group were fixed in 4% paraformaldehyde (w/v) and then permeabilized in PBS containing 0.2% Triton X-100 for 20 min at room temperature. After subsequent washes in PBS, the embryos were blocked for 30 min at room temperature. Slides were incubated separately overnight with the following antibodies at 4°C: rabbit polyclonal antibody to EGFR (1:100, sc374607; Santa Cruz Biotechnology), rabbit polyclonal antibody to TrkB (1:100, ab51190; Abcam, Inc.), and rabbit polyclonal antibody to IGF-1R (1:100, ab90657; Abcam, Inc.). Slides were incubated with FITC-conjugated anti-rabbit or anti-mouse secondary antibodies (Jackson Labs) for 1 h at 37°C before washing again in PBS. Nuclei were stained with propidiumiodide at a final concentration of 0.01 mg/mL for 10 min. HESC identification was performed as described in our previous study. The ESCs were also fixed in 4% PFA (w/v) and incubated overnight with primary antibodies (1:100) at 48°C. The primary antibodies included antibodies against TRA-1-60 (1:200, ab16288; Abcam, Inc.), OCT4 (1:200, ab27985; Abcam, Inc.), and Nanog (1:200, ab80892; Abcam, Inc.). The slides were incubated with FITC-conjugated goat anti-mouse or anti-rabbit secondary antibodies (1:200) for 1 h at 37°C and then washed again in PBS. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) at a final concentration of 0.01 mg/mL for 5 min. The immunostained embryos were mounted on glass slides and examined under a confocal laser scanning microscope (A1-R; NIKON).
hESC derivation
The blastocysts were used for ES derivation at day 6. ICM was isolated by the mechanical method as described in our previous study [8]. After 5–7 days, the human ICM formed a small colony, and the culture medium was changed every 2 days. The small colony was allowed to grow for 7 more days and then mechanically dissociated into three to four small clumps using a micropipette. After five passages, the hESC colonies were propagated with the addition of 1 mg/mL collagenase type IV every 4–7 days.
Karyotype analysis
For chromosome analysis, hESCs obtained after every 10 passage were incubated in culture medium with 0.25 μg/mL colcemid (Gibco, Invitrogen) for 4 h, harvested, and incubated in 0.4% sodium citrate and 0.4% chloratum Kaliumat (1:1, v/v) at 37°C for 5 min. The cells were then fixed thrice in a solution of methanol: acetic acid (3:1, v/v). Subsequently, after Giemsa staining, at least 20 cells were examined in each group for chromosome analysis.
Assessment of the differentiation capacity in vitro and in vivo
Specific gene expression in the embryoid body (EB) was used to assess the differentiation ability of hESCs in vitro. The hESCs were cultured in suspension, and EB growth was observed after 2 weeks. After collection, the EB was subjected to reverse transcription-polymerase chain reaction (RT-PCR) to analyze specific gene expression for three embryonic germ layers: Afp (endoderm), Neurod1 (ectoderm), and Hbz (mesoderm). After being cultured in suspension for 7 days, the EBs were transferred onto a gelatin-coated plate and cultured in the same medium for another 7 days. The differentiated EBs were immunostained with AFP (1:100, Human Germ Layer Marker Kit; Chemicon), α-SMA (1:200, Human Germ Layer Marker Kit; Chemicon), and TUJ1 (1:200, Human Germ Layer Marker Kit; Chemicon). The secondary antibodies used were Alexa 488-conjugated goat anti-mouse IgG (1:500; Invitrogen) and Alexa 488-conjugated goat anti-rabbit IgG (1:500; Invitrogen). Cell nuclei were stained with DAPI.
The differentiation ability of hESCs in vivo was evaluated by teratoma production. The hEScs from passage 10 or beyond were treated with 1 mg/mL collagenase type IV for 10–15 min and then dispensed into 300–400 small hESC colony suspensions. The colonies were collected and subcutaneously injected into the inguinal grooves of 6-week-old male severe combined immunodeficiency (SCID) mice. Eight weeks later, the resultant tumors were removed, fixed for 4–8 h in 4% paraformaldehyde, and embedded in paraffin. After staining with hematoxylin and eosin, the sections were examined under a light microscope for the presence of derivatives from the three germ layers.
Statistic analysis
The results were compared using SPSS software. An independent sample T test was performed when comparing the data from two groups. A one-way ANOVA test was used when comparing the data from more than three groups and followed by least significant differences post hoc analysis. P value<0.05 was considered statistically different.
Results
Effects of PN removal on the developmental competence and quality of repaired embryos
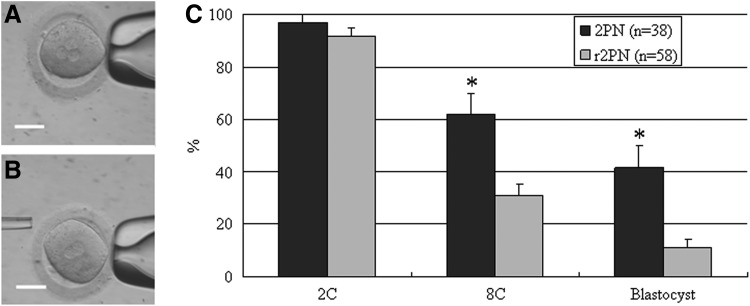
The r2PN embryos were derived from 3PN embryos by removing one pronucleus (Fig. 1A, B). The evaluation of developmental competence showed that the cleavage efficiency was not different between normal 2PN and r2PN groups (P>0.05), but the eight-cell and blastocyst formation efficiency was significantly different between the two groups (P<0.05, Fig. 1C).
FIG. 1.
Human r2PN zygote derived from tripronuclear (3PN) zygote and their subsequent developmental competence. (A) 3PN zygote. (B) r2PN zygote. (C) Developmental efficiency at the eight-cell and blastocyst stages was significantly decreased. Bar=50 μm. * Indicates P<0.05.
EGFR, TrkB, and IGF-1R in human r2PN embryos
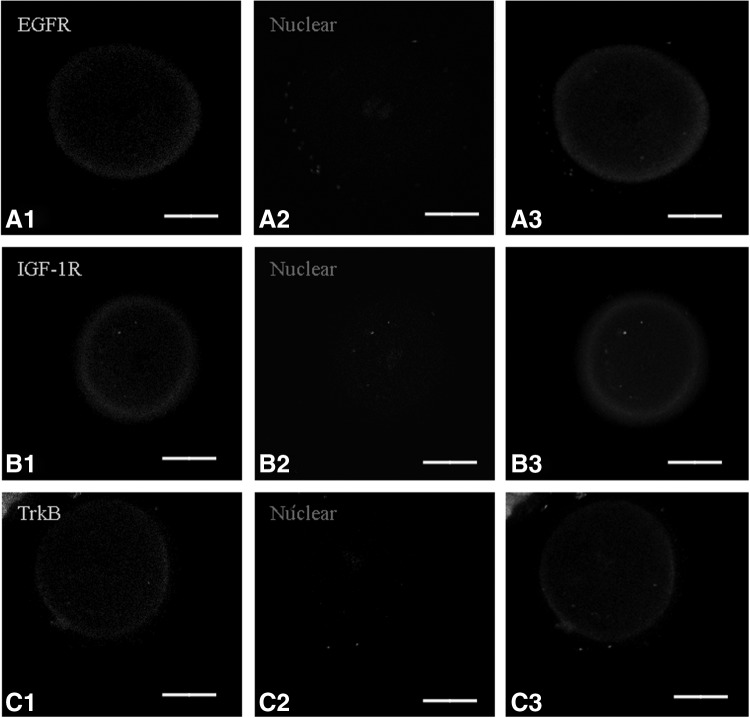
To identify putative molecular mechanisms of the action of the three growth factors on human r2PN embryos, we analyzed the presence of the three growth factor receptors, EGFR, TrkB, and IGF-1R, using immunofluorescence in human r2PN embryos (n=30). The results showed that the growth factor receptors were present in the membranes in human r2PN embryos (Fig. 2).
FIG. 2.
The expression profiles of receptors for epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), and insulin-like growth factor-1 (IGF-1) in human r2PN zygotes (n=30). EGF, BDNF, and IGF-1 receptors (IGF-1Rs) were detected in the membranes of human r2PN zygotes. (A) EGF receptor (EGFR). (B) IGF-1 receptor (IGF-1R). (C) BDNF receptor (TrkB). (A1–A3) Show immunofluorescence for the three different receptors, (B1–B3) show propidium iodide nuclear staining and those in (C1–C3) show the merged images. Scale bar=25 μm.
Effects of different combinations of growth factors on the development of r2PN zygotes
To test the effects of growth factors on the developmental competence of r2PN zygotes, different combinations of EGF, BDNF, and IGF-1 were used and the developmental competence of the groups was compared. The results showed that the developmental competence was identical if only one kind of growth factor was supplemented in culture medium compared with growth in medium without growth factors (P>0.05). When two kinds of growth factors were added to the culture medium (EGF–BDNF, EGF–IGF-1, and BDNF–IGF-1 groups), blastocyst development efficiency of the repaired embryos in the BDNF–IGF-1 group was lower than that in the EGF–BDNF and EGF–IGF-1 groups (P>0.05) but was similar to that of groups treated with only one kind of growth factor supplement. When all three growth factors were added in the culture medium, the developmental efficiency was significantly higher than that of the EGF–BDNF, EGF–IGF-1, and BDNF–IGF-1 groups (P<0.05) (Table 1).
Table 1.
The Effects of Growth Factors Supplement on the Development of Human r2PN Zygotes
| Growth factors | Develop to | |||||
|---|---|---|---|---|---|---|
| EGF | BDNF | IGF-1 | No. of r2PN zygotes | 2C | 8C | B |
| − | − | − | 58 | 53 (91.4)a | 19 (32.8)a | 6 (10.3)a |
| + | − | − | 33 | 31 (93.9)a | 12 (36.4)a | 3 (9.7)a |
| − | + | − | 32 | 29 (90.6)a | 11 (34.4)a | 2 (6.3)a |
| − | − | + | 30 | 27 (90.0)a | 11 (36.7)a | 2 (6.8)a |
| + | + | − | 35 | 33 (94.3)a | 15 (42.9)a, | 6 (17.1)a |
| + | − | + | 34 | 30 (88.2)a | 14 (41.2)a, | 5 (14.7)a |
| − | + | + | 30 | 28 (93.3)a | 13 (46.7)a | 2 (7.1)a |
| + | + | + | 48 | 45 (93.8)a | 29 (60.4)b | 13 (27.1)b |
Values with different superscripts in the same column differ significantly (P<0.05).
EGF, epidermal growth factor; BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth factor-1.
Effects of growth factors on the quality of human r2PN embryos at the eight-cell and blastocyst stage
To verify the effects of growth factors on the quality of r2PN embryos, the embryos at the eight-cell stage on day 3 and at the blastocyst stage on day 5 were evaluated at the ART centers using established clinical criteria. The total cell number and the degree of apoptosis in blastocysts was also evaluated. For eight-cell embryos, the addition of a single growth factor did not significantly improve the embryo grading, but the ratio of 8G2 embryos increased in the EGF–BDNF, EGF–IGF-1, BDNF–IGF-1, and EGF–BDNF–IGF-1 groups. Moreover, the ratio of 8G1 embryos was also increased in the EGF–BDNF–IGF-1 group (Table 2) (P>0.05). The grading of blastocysts could not be improved by the addition of either one or two growth factors, but the number of high grading blastocysts (BG1 and BG2) increased in the EGF–BDNF–IGF-1 group (Table 3).
Table 2.
The Effects of Growth Factors Supplement on the Quality of Human Eight-Cell Embryos Resulting from r2PN Zygotes
| Growth factors | Eight-cell grading | ||||||
|---|---|---|---|---|---|---|---|
| EGF | BDNF | IGF-1 | No. of eight-cell embryos | 8G1 | 8G2 | 8G3 | 8G4 |
| − | − | − | 19 | 2 (10.5)a | 6 (31.6)a | 3 (15.8)a | 8 (42.1)a |
| + | − | − | 12 | 1 (8.3)a | 3 (25.0)a | 3 (25.0)a | 5 (41.7)a |
| − | + | − | 11 | 0 (0)a | 4 (36.4)a | 2 (18.2)a | 5 (45.5)a |
| − | − | + | 11 | 2 (18.2)a | 3 (27.3)a | 0 (0)a | 6 (54.5)a |
| + | + | − | 15 | 2 (13.3)a | 6 (40.0)a | 2 (13.3)a | 5 (33.3)a |
| + | − | + | 14 | 1 (7.1)a | 7 (50.0)a | 2 (14.3)a | 4 (28.6)a |
| − | + | + | 13 | 1 (7.7)a | 6 (46.2)a | 1 (7.7)a | 5 (38.5)a |
| + | + | + | 29 | 7 (24.1)a | 14 (48.3)a | 3 (10.3)a | 5 (17.3)a |
Denote that values do not differ significantly within that column (P>0.05).
Table 3.
Effects of EGF, BDNF, and IGF-1 Supplements on the Quality Grading of Human Blastocysts Resulting from r2PN Zygotes
| Growth factors | Blastocyst grading | |||||
|---|---|---|---|---|---|---|
| EGF | BDNF | IGF-1 | No. of blastocyst | BG1 | BG2 | BG3 |
| − | − | − | 6 | 1 | 1 | 4 |
| + | − | − | 3 | 0 | 1 | 2 |
| − | + | − | 2 | 0 | 1 | 1 |
| − | − | + | 2 | 1 | 0 | 1 |
| + | + | − | 6 | 2 | 1 | 3 |
| + | − | + | 5 | 1 | 2 | 2 |
| − | + | + | 2 | 1 | 0 | 1 |
| + | + | + | 13 | 6 | 4 | 3 |
Sign of subtraction means that this kind of growth factor was not present in culture medium.
Sign of addition means that this kind of growth factor was supplemented in culture medium.
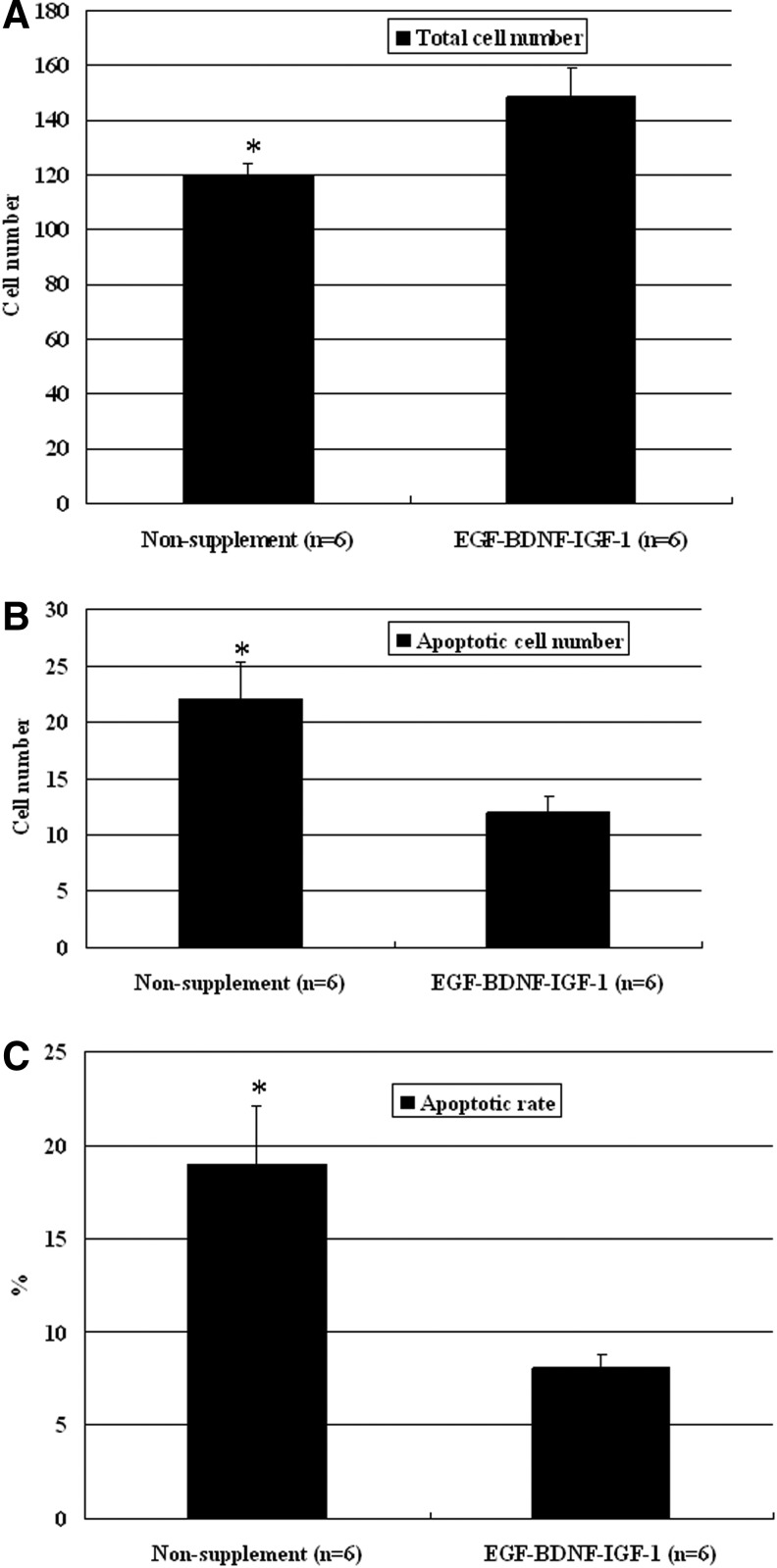
Blastocyst cell number and apoptosis were studied in non-supplemented and EGF–BDNF–IGF-1 groups at day 6 after fertilization. The results showed that the total cell number was significantly increased but that the apoptotic cell number was significantly decreased in the EGF–BDNF–IGF-1 group compared with the non-supplemented group (P<0.05), and, thus, the apoptosis rate of the blastocysts in the EGF–BDNF–IGF-1 group was significantly decreased (Fig. 3) (P<0.05).
FIG. 3.
The comparison of total cell number and apoptotic cell number in r2PN blastocysts cultured with or without EGF–BDNF and IGF-1 combination. A significant increase in total cell number (A) and a significant decrease in apoptotic cell number (B) were observed in blastocysts when they were cultured with the EGF–BDNF and IGF-1 supplement. (C) The apoptosis rate of blastocysts in the EGF–BDNF–IGF-1 group was significantly decreased. Six blastocysts were tested in each group. * Indicates P<0.05.
Effects of growth factor supplement on chromosome aneuploidy in human r2PN embryos
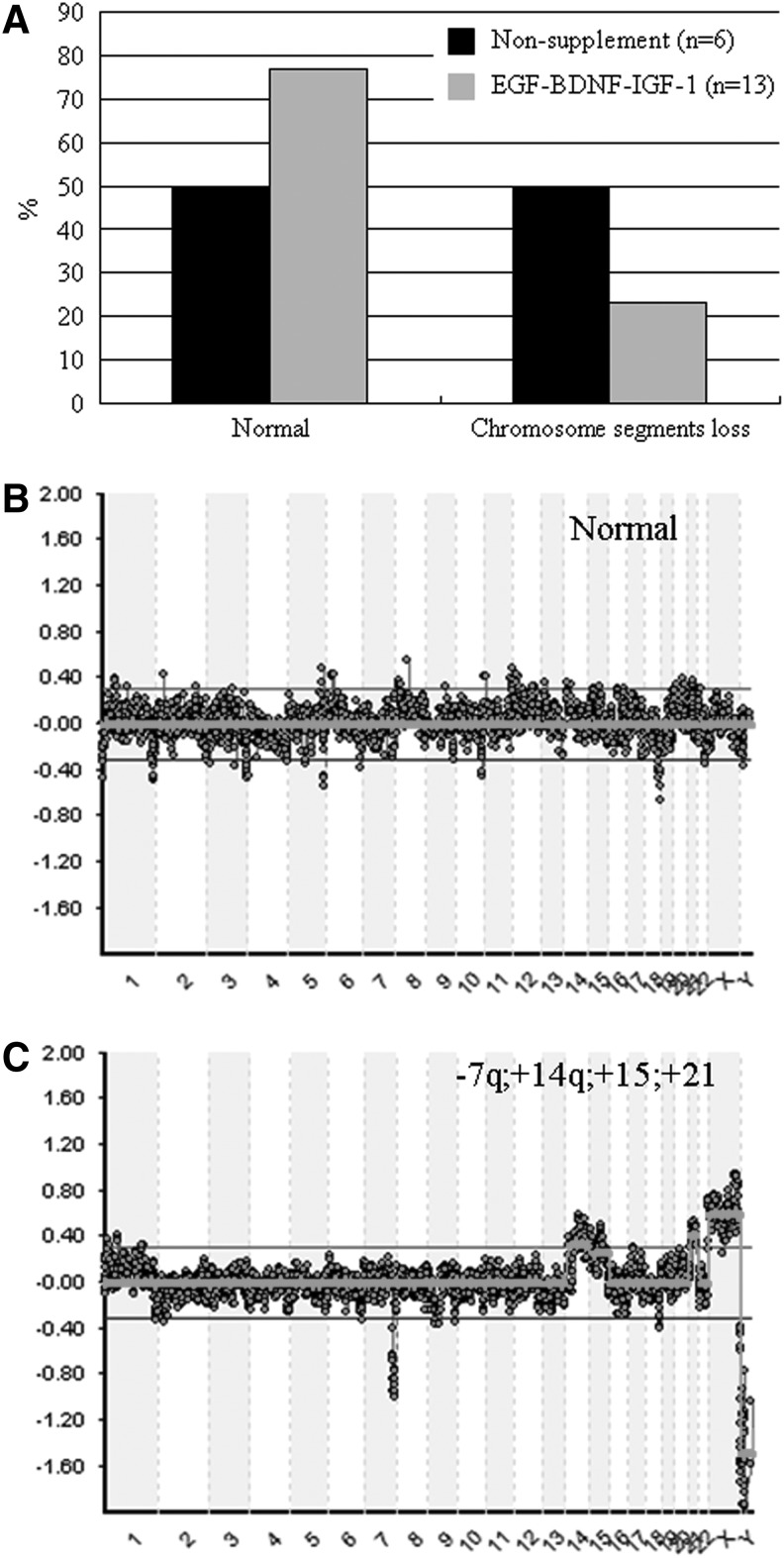
To identify chromosome euploidy, blastocysts in non-supplemented and EGF–BDNF–IGF-1 groups were biopsied, and a group of five to eight trophoblast cells was tested by the CGH method. The results showed that ∼50% blastocysts (3/6) showed chromosome segment loss in non-supplemented groups. In contrast, only 23% blastocysts (3/13) showed similar chromosome segment loss in the EGF–BDNF–IGF-1 group, suggesting that the chromosome euploidy ratio was improved by the addition of growth factors in combination (Fig. 4A). The representative aCGH images for normal aneuploidy and abnormal chromosomes are shown in Figure 4B and C. The results of chromosome euploidy analysis in total human r2PN are summarized in Table 4.
FIG. 4.
Effect of EGF–BDNF–IGF-1 supplement on the chromosome ploidy by array CGH method. (A) Half of the blastocysts in the non-supplement group showed chromosome abnormality, but no more than a quarter of the blastocysts in the EGF–BDNF and IGF-1 supplement group had abnormal chromosomes. (B) A representative image for normal chromosomes in trophectoderm (TE) cells by array CGH. (C) A representative image for abnormal chromosomes in TE cells by array CGH. * Indicates P<0.05.
Table 4.
Chromosome Euploidy Testing by aCGH Method in Human r2PN Embryos Cultured with EGF, BDNF, and IGF-1 Supplement
| Growth factors | CGH testing | ||||
|---|---|---|---|---|---|
| EGF | BDNF | IGF-1 | No. of blastocyst | Normal | Abnormal |
| − | − | − | 6 | 3 | 3 |
| + | − | − | 4 | 1 | 3 |
| − | + | − | 2 | 1 | 1 |
| − | − | + | 2 | 1 | 1 |
| + | + | − | 6 | 4 | 2 |
| + | − | + | 5 | 2 | 3 |
| − | + | + | 2 | 0 | 2 |
| + | + | + | 13 | 10 | 3 |
Sign of subtraction means that this kind of growth factor was not present in culture medium.
Sign of addition means that this kind of growth factor was supplemented in culture medium.
hESC line derivation from the blastocysts that were derived from the r2PN blastocysts
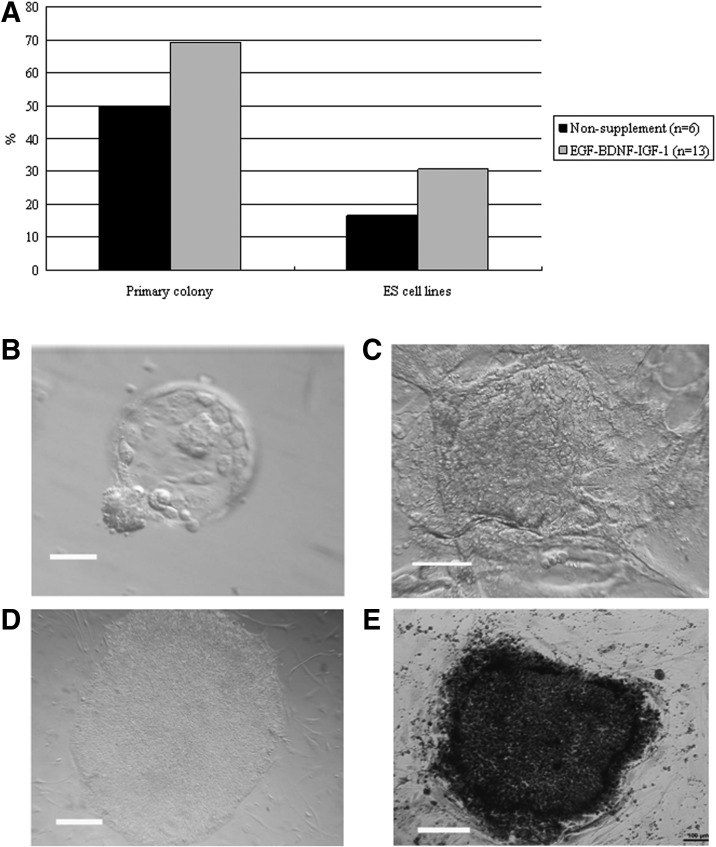
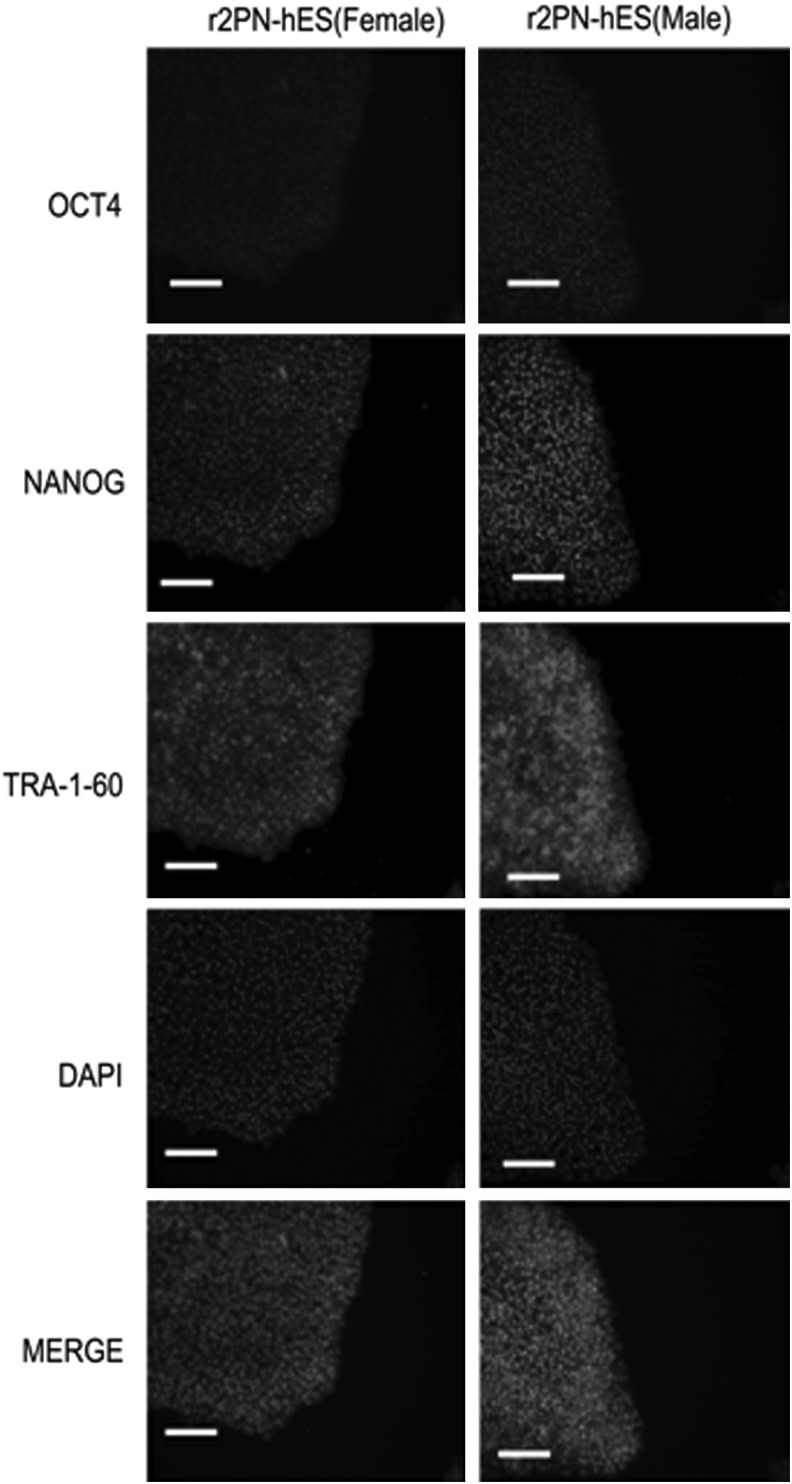
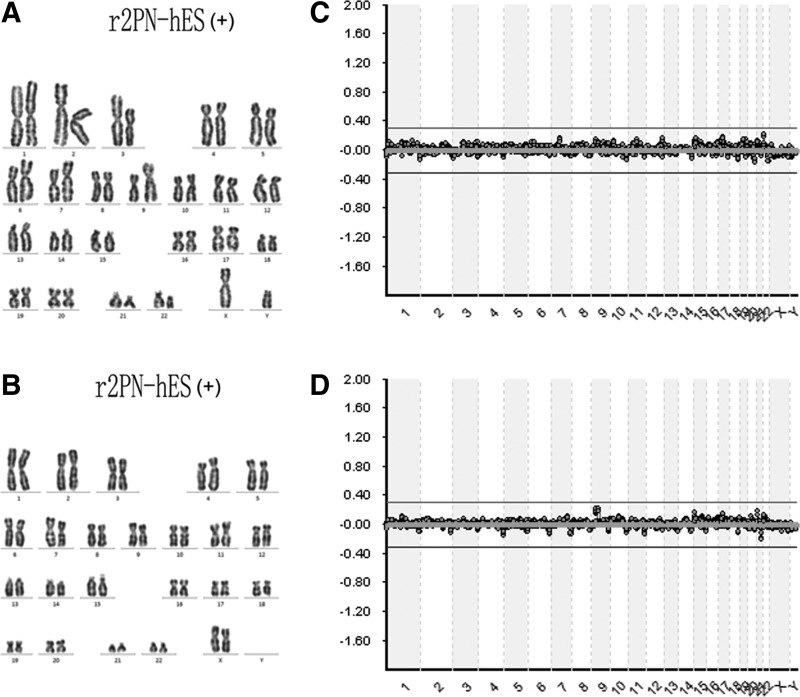
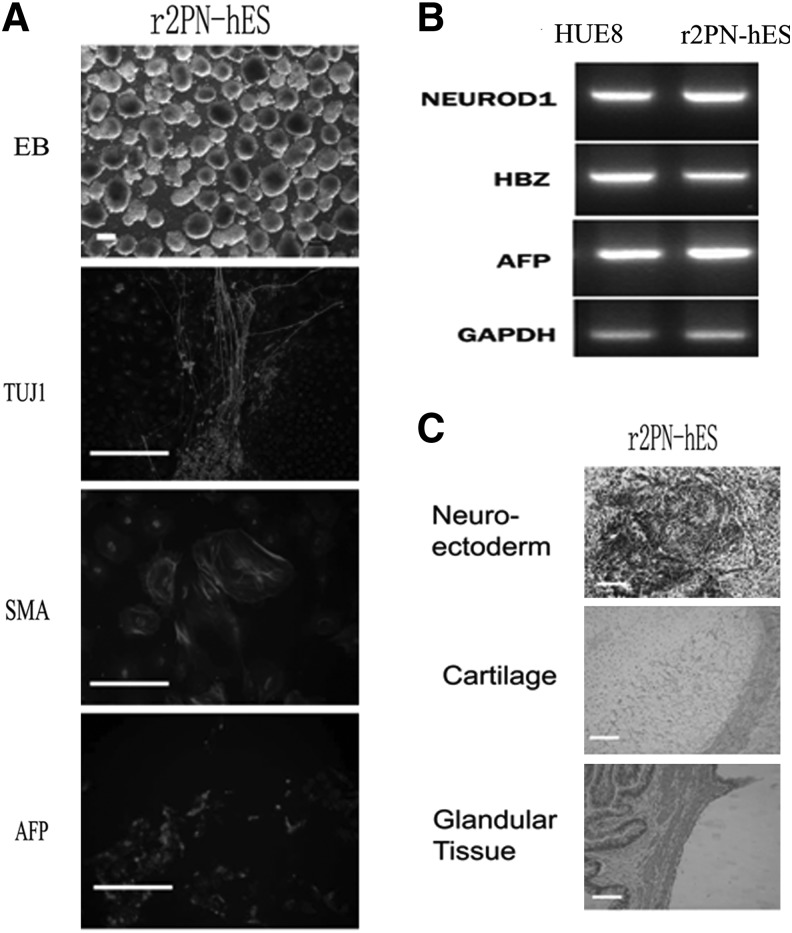
Blastocysts from each group were used to derive hESCs, and the hESCs derivation efficiency was compared between non-supplemented and EGF–BDNF–IGF-1 groups. In the non-supplemented group, a total of six blastocysts were put onto the feeder layers, and three blastocysts attached and formed small cell colonies by the fifth day. After 1 week, the growing cell colonies were divided into four or five clumps, and one hESC line was successfully derived. In the EGF–BDNF–IGF-1 group, 13 blastocysts were put on feeder layers, and nine small colonies were formed after 5 days, resulting in four hESC lines that were successfully derived, indicating significantly improved efficiency (Fig. 5A). The data for ESC derivation in other groups are shown in Table 5. The established hESC lines displayed normal morphology, including distinct cell colony boundaries, high nuclear/cytoplasm ratio, tightly packed colonies, and positive AP staining (Fig. 5B, C). The pluripotent marker (OCT4, NANOG) and cell surface marker (TRA-1-60) expression was detected in the five hESC lines (Fig. 6). Karyotyping analysis showed that all the r2PN-hESC lines from human r2PN blastocysts were diploid (three for 46XX and four for 46XY) (Fig. 7A, B), and they also showed the normal chromosome euploidy after long-term propagation tested by aCGH (passage 15 and passage 30, Fig. 7C, D). These hESCs differentiated to EBs in vitro and expressed SMA (mesoderm), TUJ1 (ectoderm), and AFP (endoderm) markers indicative of the three germ layers (Fig. 8A). In addition, RT-PCR results confirmed the expression of marker genes of the three germ layers (Afp-endoderm, Hbz-mesoderm, and Neurod1-ectoderm) (Fig. 8B). Typical tissues from the three germ layers, neuroectoderm (ectoderm), cartilage (mesoderm), and glandular tissue (endoderm), were identified using HE staining when the hESCs were injected into SCID mice for 2 months (Fig. 8C).
FIG. 5.
Derivation of human embryonic stem cell (hESC) lines from r2PN blastocysts cultured with or without the EGF–BDNF–IGF-1 combination. (A) Derivation efficiency, including primary colony formation and ESC line establishment, was significantly increased when the culture medium was supplemented with EGF–BDNF–IGF-1. (B) Expanded blastocysts in r2PN cultured with EGF–BDNF–IGF-1. (C) Primary colony at day 7 after inner cell mass was implanted onto the feeder layers. (D) Typical hESC colony with distinct boundary, high nuclear/cytoplasm ratio, and tightly packed together. (E) Positive AP activity for hESC colony. Scale bar=50 μm.
Table 5.
Summary of ES Derivation from Human r2PN Blastocysts Cultured with EGF, BDNF, and IGF-1 Supplement
| Growth factors | ES derivation | ||||
|---|---|---|---|---|---|
| EGF | BDNF | IGF-1 | No. of blastocyst | Primary colony | ESC lines |
| − | − | − | 6 | 3 | 1 |
| + | − | − | 3 | 1 | 0 |
| − | + | − | 2 | 1 | 0 |
| − | − | + | 2 | 2 | 1 |
| + | + | − | 6 | 2 | 1 |
| + | − | + | 5 | 3 | 1 |
| − | + | + | 2 | 1 | 0 |
| + | + | + | 13 | 9 | 4 |
Sign of subtraction means that this kind of growth factor was not present in culture medium.
Sign of addition means that this kind of growth factor was supplemented in culture medium.
ESC, embryonic stem cell.
FIG. 6.
The expression of specific markers of hESC lines. The hESCs express the pluripotent markers (OCT4 and NANOG) and the hESCs surface marker (TRA-1-60). Scale bar=50 μm.
FIG. 7.
Chromosome euploidy identification by cell karyotyping and aCGH methods. (A) Karyotyping for male r2PN-hESCs (46, XY); (B) Karyotyping for female r2PN-hESCs (46, XX); was observed in r2PN-hESC. (C) aCGH chart for female r2PN-hESC identification at passage 15; (D) aCGH chart for female r2PN-hESC identification at passage 30. No chromosome abnormalities were detected.
FIG. 8.
Evaluation of in vitro and in vivo differentiation in hESC by embryoid body (EB) and teratoma formation. (A) Typical EBs with round and smooth morphology were formed when the r2PN hESCs were cultured in suspension. The EBs expressed TUJ1 (ectoderm), SMA (mesoderm), and AFP (endoderm). (B) Genes from the endoderm (AFP), mesoderm (HBZ), and ectoderm (NEUROD) were identified in differentiated EB clumps. (C) Tissues from the three embryonic germ layers were identified in teratomas, including neuroectoderm (ectoderm), cartilage (mesoderm), and glandular tissue (endoderm). Scale bar=100 μm.
Discussion
hESC lines are typically derived from the ICM of embryos at the blastocyst stage. hESCs are self-renewing and have the ability to differentiate into all cell types. These characteristics provide HESCs with great potential for use in modern regenerative medicine and cell-based drug discovery. However, the destruction of normal fertilized embryos and the derivation of hESCs for research has resulted in polarized ethical debates, with most of the controversy centered on embryo destruction [2]. The results from this study suggest that modified diploid fertilized embryos from polyspermic zygotes can be used to derive hESC lines, and the derivation efficiency was comparable with that of normal fertilized embryos when cultured with the EGF–BDNF–EGF-1 growth factor combination. Therefore, we have demonstrated that these abnormal fertilized zygotes, which are destined to be discarded in IVF cycles, are alternative resources for hESC production. This option could be of particular interest in countries where the use of normal embryos or the creation of human embryos for stem cell research is prohibited.
With the development of knowledge and technology, more and more resources have been used to derive hESC lines. Zhang et al. were the first to report that hESC lines could be successfully derived using arrested development embryos at the four- and eight-cell stages. In their study, the embryos without any morphological changes within 48 h after fertilization were designated as arrested development embryos and co-cultured with hESCs to yield normal hESC lines [29]. Chung et al. collected single blastomeres from fertilized mouse embryos at the eight-cell stage using a biopsy method that was frequently applied in pre-implantation genetic diagnosis (PGD) and co-cultured this single blastomere along with mouse ESC. The blastomere eventually grew and propagated to form the mouse ESC line [30]. Moreover, the feasibility of this method using human embryos was demonstrated [31,32]. However, since all hESC lines have chromosomal abnormalities, the risks involved in this method need to be evaluated [33]. In our previous study, we also indicated that the diploid hESC lines can be derived from 3PN zygotes when one pronuclear is removed [8], and Jiang et al. subsequently proved our results [9]; however, the lower efficiency limited the development of this method. Here, we have developed a new culture system to promote the application of 3PN zygotes.
In the process of IVF, which is the most popular ART, amount of sperms are added into the fertilization medium containing the cumulus-oocyte complexes. Very often (5%–7% incidence rate), two sperms enter the oocyte, forming polyspermic zygotes (also named 3PN zygotes, because three pronuclear ones are observed in these zygotes). In a previous study, Fan et al. demonstrated that the cytoplasm of 3PN zygotes can reprogram human somatic cells by nuclear transfer technology, indicating that the cytoplasm of 3PN zygotes is comparable to that of normal zygotes [8], and these results were further corroborated by Kawamura et al. [24]. Therefore, the concern was whether diploid zygotes could be obtained after the removal of a redundant pronucleus. A study by Kattera and Chen showed for the first time that 3PN embryos repaired by microsurgery could develop into a viable offspring [7]. However, the lower developmental efficiency of this process limited its application in the clinical setting, and only about 10% of the repaired 3PN zygotes could develop into the blastocyst stage [8,34]. Although Jiang et al. showed that 25 r2PN embryos could develop into blastocysts using this method, the fact that only 2 hESC lines were derived from 11 blastocysts indicated the lower quality of the blastocysts obtained [9].
In a previous study, Roblero and Garavagno proved that the phenomenon of embryo loss would be reversed when the steroid hormone-depleted mice were treated with progesterone and estrogen, which suggested that additional paracrine factors were also key to the full complement of preimplantation embryo development [35], though embryos can develop to the blastocyst stage without these factors. Paria and Dey proved that specific growth factors which were secreted in an autocrine or paracrine manner regulated the embryo development and were responsible for the retarded development of preimplantation embryos in vitro [11]. Recently, Pacella et al. indicated that follicular cell metabolism and follicle fluid metabolites levels were altered in the female patients with either reduced ovarian reserve or advanced maternal age. More importantly, they suggested that oocyte development competence would be impaired in such a perturbed environment and resulted in a lower embryo development outcome [36]. Varnagy also demonstrated that the concentration of resistin factor was beneficial with regard to the outcome of IVF [37]. Moreover, Petro et al. proved that the fertilization rate was decreased and, consequently, with a lower chance of an oocyte to develop into a high-quality embryo if there are higher concentration endocrine disruptors in a follicle micro-environment [38]. Thus, these studies indicated that these autocined or paracined factors are responsible for the oocytes and the resultant embryo development in mammals, and the dilution of these factors which are released by the embryos in the culture medium would probably result in the reduction of developmental competence.
Growth factors, one kind of important autocined or paracined factors, have been shown to exhibit tremendous potential in the improvement of embryo development and quality, and, more importantly, 3PN embryo development, which is regarded as abnormal fertilization in the IVF procedure, can also be significantly improved by supplementing seven growth factors [24]. Of these seven growth factors, EGF, BDNF, and IGF-1 have been suggested to play a significant role in improving human embryo development and are the focus of this study. EGF, when used in combination with BDNF or EGF-1, significantly improved developmental efficiency, but the combination of BDNF and IGF-1 had no effect, suggesting that EGF plays a key role in the process. It is well known that EGF promotes oocyte maturation in vivo and in vitro, and sufficient maturation has been closely correlated with embryo development and quality [19]. In addition, EGF has been suggested to directly promote post-fertilization embryonic development [39]. However, in this study, the addition of EGF alone was unable to improve developmental efficiency, indicating that BDNF and IGF-1 also play important roles. Similar to EGF, IGF-I has traditionally been used as a growth factor in development, and its positive effects on pre-implantation development by autocrine stimulation of cumulus and/or granulosa cells has been reported in pigs [40], cattle [41], and mice [42]. In these earlier studies, IGF-1 was typically used in combination with EGF to improve maturation and development. A few recent studies investigating the role of BDNF in embryo development have indicated that it improves development at the eight-cell and blastocyst stages, while a few other studies support its role in the cleavage or four-cell stages [20,43].
Although the combination of EGF and BDNF or IGF-1 promotes the developmental efficiency of r2PN embryos, the quality of eight-cell embryos and blastocysts significantly increases only in the EGF–BDNF–IGF-1 combination group. This result indicates that embryos cultured with growth factor supplement may promote the developmental efficiency and embryo quality, and infertile couples can obtain better quality embryos for transfer. Embryo quality was assessed based on embryo grading criteria, cell number, and apoptotic cell number. The embryo grading criteria are used in ART centers worldwide to evaluate embryo development competence by non-invasive observation using an optic microscope. Typically, embryos are graded at four stages, including oocyte, zygote, eight-cell, and blastocyst stages [22,44], although the latter two grading stages are the most popular. The other criterion that reflected quality was the cell number of blastocysts. It is well established that successful embryo implantation is determined by blastocoel expansion-induced blastocyst hatching. Blastocoel formation is a normal developmental period in which a sufficient number of cells is generated by mitosis. In this study, decreased blastocyst cell numbers and increased apoptotic cell numbers were observed in embryos cultured without growth factor supplement, suggesting that they would probably fail to form blastocoels, thereby arresting further embryo development. Furthermore, the enzymes secreted by the cells would most likely be insufficient to digest the zona pellucida, leading to failure in hatching. Kawamura et al. observed that several blastocysts which developed from 3PN zygotes failed to expand and hatch, which can be explained by the fewer cells in those blastocysts [24].
Chromosome euploidy has been closely correlated with embryo quality [45]. In our previous study, we found that aneuploidy was higher in r2PN embryos compared with normal 2PN embryos and could potentially contribute to the lower quality of the r2PN embryos [8]. Reverte et al. observed that abnormal spindle positioning and movements might also interfere with cytokinesis and lead to the accumulation of tetraploid cells [46]. In our previous study, fluorescence in situ hybridization (FISH)-based identification of chromosome euploidy and aneuploidy was specifically performed for chromosomes 18, X and Y only, and not the entire set [8]. In this study, an array-based CGH analysis was performed for all 23 chromosomes together. Lestou et al. applied this method to screen chromosomal aneuploidy in the clinical setting and confirmed that CGH is a molecular cytogenetic method which enables easier analysis of both embryonic (amnion) and extraembryonic (chorion) cell lineages compared with FISH [47]. Subsequently, in the PGD procedure, the chromosomes of blastomeres at the eight-cell or blastocyst stages were analyzed by CGH instead of traditional PCR or FISH [22,48]. The application of CGH analysis in blastocyst biopsy is regarded as one of best methods of single embryo transfer in IVF. Moreover, Sher et al. demonstrated that the transfer of previously vitrified blastocysts derived from CGH-normal embryos significantly improves implantation and birth rates per embryo transferred and reduces the rate of miscarriage [49]. In this study, the EGF–BDNF–IGF-1 group had fewer embryos with small chromosome section loss, strongly suggesting the higher quality of blastocysts and increased hESC derivation efficiency.
To explore the potential mechanism of action of the growth factors, the expression of growth factor receptors in r2PN embryos was investigated. The embryos were found to express receptors for EGF, BDNF, and IGF-1 on their membranes, indicating that EGF, BDNF, and IGF-1 are able to enter into the cytoplasm via their corresponding receptors and carry out their functions in the oocytes. In the ooplasm, EGF, IGF-1, and BDNF have been shown to bind to their respective receptor tyrosine kinases to activate downstream phophotidyinositol-3-kinase-Akt (PI3-K) signaling [24,50]. PI3-K is a regulator of several physiological responses, including cellular proliferation, growth, survival, and glucose metabolism, and is vital for preimplantation embryo survival and development [51,52]. Consistent with our results, some reports have shown that inhibition of PI3-K induces apoptosis in blastocysts [53,54]. The other role of the PI3-K pathway is to regulate glucose uptake mainly via the translocation of glucose transporter proteins to the plasma membrane. A study by Riley demonstrated that iodoacetate, a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibitor, induces apoptosis in blastocysts and TE cells, suggesting that the maintenance of glycolysis is important for embryo survival because the inhibition of this pathway induces cell death [54].
In our previous study, we derived one hESC line using five blastocysts developed from 2PN zygotes repaired by microsurgery [8]. Jiang et al. also indicated the lower efficiency of hESC lines derivation using similar r2PN embryos (2 cell lines from 11 blastocysts) [9]. In another study, Lerou et al. derived hESC lines using poor-quality embryos such as early arrested or highly fragmented embryos and suggested a statistical correlation between the developmental stage of such poor-quality embryos and the yield of hESC lines [55]. Improvements in hESC derivation efficiency have also been reported in previous studies. Using murine embryos, Cortes et al. developed a new strategy based on the combination of whole blastocyst culture followed by laser drilling destruction of the trophoectoderm for improving the efficiency of ICM isolation and ESC derivation [56]. They went on to demonstrate that the ROCK inhibitor Y23672 increases post-thaw embryo survival and that hESCs may also facilitate the efficiency of hESC derivation from frozen, poor-quality embryos [57]. Whichever method was applied, an improvement in embryo quality was essential for enhancing hESC line derivation efficiency. In this study, only one hESC line was established from six blastocysts that were developed from the repaired 2PN zygotes, which is consistent with the efficiency observed in the previous study. However, this number was significantly increased when the repaired 2PN zygotes were cultured with EGF–BDNF–IGF-1, resulting in 5 hESC lines that were successfully derived from 13 blastocysts. The derived hESC lines shared features with normal hESCs [1], including similar morphology, normal karyotype, expression of alkaline phosphatase and pluripotency genes such as OCT4 and NANOG, expression of the cell surface marker TRA-1-61, and the ability to form teratomas in SCID mice and to differentiate in vitro into cells with three embryonic germ layers.
In summary, our results show that r2PN zygotes from polyspermic zygotes have the potential to be an alternative resource for deriving hESC lines. The quality of r2PN zygotes was significantly improved when they were cultured with the EGF–BDNF–IGF-1 cocktail and resulted in more efficient hESC derivation. Moreover, the expression of EGF, BDNF, and IGF-1Rs on the membrane of r2PN zygotes is essential for improving embryo development and quality. Our data will facilitate the development of hESC line derivation in subsequent studies and will also provide an additional choice for infertile couples.
Acknowledgments
This work was supported in part by grants from the Ministry of Science and Technology of China (973 program; 2011CB944504 and 2014CB943203), the Specialized Research Fund for the Doctoral Program of Higher Education (20110001120008), the National Natural Science Foundation of China (31371521, 81100473, 81370766, and U1132005), Guangdong Natural Science Funds (S2013010014781), and the Zhujiang Science and Technology Star Project of Guangzhou (2012J2200006).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS. and Jones JM. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 2.Cohen CB. (2009). Ethical and policy issues surrounding the donation of cryopreserved and fresh embryos for human embryonic stem cell research. Stem Cell Rev 5:116–122 [DOI] [PubMed] [Google Scholar]
- 3.Englert Y, Puissant F, Camus M, Degueldre M. and Leroy F. (1986). Factors leading to tripronucleate eggs during human in-vitro fertilization. Hum Reprod 1:117–119 [DOI] [PubMed] [Google Scholar]
- 4.Balakier H. (1993). Tripronuclear human zygotes: the first cell cycle and subsequent development. Hum Reprod 8:1892–1897 [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Luo Y, Fan Y, Yue L, Wu X, Chen Y. and Sun X. (2012). Triploid and diploid embryonic stem cell lines derived from tripronuclear human zygotes. J Assist Reprod Genet 29:713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malter HE. and Cohen J. (1989). Embryonic development after microsurgical repair of polyspermic human zygotes. Fertil Steril 52:373–380 [DOI] [PubMed] [Google Scholar]
- 7.Kattera S. and Chen C. (2003). Normal birth after microsurgical enucleation of tripronuclear human zygotes: case report. Hum Reprod 18:1319–1322 [DOI] [PubMed] [Google Scholar]
- 8.Fan Y, Li R, Huang J, Yu Y. and Qiao J. (2013). Diploid, but not haploid, human embryonic stem cells can be derived from microsurgically repaired tripronuclear human zygotes. Cell Cycle 12:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang C, Cai L, Huang B, Dong J, Chen A, Ning S, Cui Y, Qin L. and Liu J. (2013). Normal human embryonic stem cell lines were derived from microsurgical enucleated tripronuclear zygotes. J Cell Biochem 114:2016–2023 [DOI] [PubMed] [Google Scholar]
- 10.Hegde A. and Behr B. (2012). Media composition: growth factors. Methods Mol Biol 912:177–198 [DOI] [PubMed] [Google Scholar]
- 11.Paria BC. and Dey SK. (1990). Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A 87:4756–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo PL, Illera MJ, Illera JC. and Illera M. (1994). Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin-like growth factor I. J Reprod Fertil 101:697–701 [DOI] [PubMed] [Google Scholar]
- 13.Grupen CG, Nagashima H. and Nottle MB. (1997). Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro. Reprod Fertil Dev 9:571–575 [DOI] [PubMed] [Google Scholar]
- 14.Kelly JM, Kleemann DO, Maxwell WM. and Walker SK. (2008). Effects of insulin-like growth factor-I, epidermal growth factor and cysteamine on the in vitro maturation and development of oocytes collected from 6- to 8-week-old Merino lambs. Reprod Fertil Dev 20:570–578 [DOI] [PubMed] [Google Scholar]
- 15.Shabankareh HK. and Zandi M. (2010). Developmental potential of sheep oocytes cultured in different maturation media: effects of epidermal growth factor, insulin-like growth factor I, and cysteamine. Fertil Steril 94:335–340 [DOI] [PubMed] [Google Scholar]
- 16.Merriman JA, Whittingham DG. and Carroll J. (1998). The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod 13:690–695 [DOI] [PubMed] [Google Scholar]
- 17.Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI. and Conti M. (2010). Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod 25:2569–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JL, Hammar K, Smith PJ. and Gross DJ. (1999). Stage-dependent effects of epidermal growth factor on Ca2+ efflux in mouse oocytes. Mol Reprod Dev 53:244–253 [DOI] [PubMed] [Google Scholar]
- 19.Tkachenko OY, Delimitreva S, Isachenko E, Valle RR, Michelmann HW, Berenson A. and Nayudu PL. (2010). Epidermal growth factor effects on marmoset monkey (Callithrix jacchus) oocyte in vitro maturation, IVF and embryo development are altered by gonadotrophin concentration during oocyte maturation. Hum Reprod 25:2047–2058 [DOI] [PubMed] [Google Scholar]
- 20.Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD. and Hsueh AJ. (2005). Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci U S A 102:9206–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J. and Tanaka T. (2009). Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 150:3774–3782 [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Yan J, Li M, Yan L, Zhao Y, Lian Y, Li R, Liu P. and Qiao J. (2012). Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Hum Reprod 27:2146–2159 [DOI] [PubMed] [Google Scholar]
- 23.Linher-Melville K. and Li J. (2013). The roles of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor and nerve growth factor during the final stage of folliculogenesis: a focus on oocyte maturation. Reproduction 145:R43–R54 [DOI] [PubMed] [Google Scholar]
- 24.Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao J, Behr B, Pera RA. and Hsueh AJ. (2012). Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One 7:e49328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veeck L. (1990). The morphological assessment of human oocytes and early conception. In: Handbook of the Laboratory Diagnosis and Treatment of Infertility. Keel BA, Webster BW, eds. CRC Press, Boca Raton, Pp. 353–356 [Google Scholar]
- 26.Dokras A, Sargent IL. and Barlow DH. (1993). Human blastocyst grading: an indicator of developmental potential? Hum Reprod 8:2119–2127 [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Ding C, Wang E, Chen X, Li X, Zhao C, Fan Y, Wang L, Beaujean N, et al. (2007). Piezo-assisted nuclear transfer affects cloning efficiency and may cause apoptosis. Reproduction 133:947–954 [DOI] [PubMed] [Google Scholar]
- 28.Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, Ubaldi FM, Iammarrone E, Gordon A. and Pantos K. (2011). PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod 26:1925–1935 [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M. and Stojkovic M. (2006). Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells 24:2669–2676 [DOI] [PubMed] [Google Scholar]
- 30.Chung Y, Klimanskaya I, Becker S, Marh J, Lu SJ, Johnson J, Meisner L. and Lanza R. (2006). Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature 439:216–219 [DOI] [PubMed] [Google Scholar]
- 31.Klimanskaya I, Chung Y, Becker S, Lu SJ. and Lanza R. (2006). Human embryonic stem cell lines derived from single blastomeres. Nature 444:481–485 [DOI] [PubMed] [Google Scholar]
- 32.Geens M, Mateizel I, Sermon K, De Rycke M, Spits C, Cauffman G, Devroey P, Tournaye H, Liebaers I. and Van de Velde H. (2009). Human embryonic stem cell lines derived from single blastomeres of two 4-cell stage embryos. Hum Reprod 24:2709–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feki A, Hovatta O. and Jaconi M. (2008). Derivation of human embryonic stem cell lines from single cells of 4-cell stage embryos: be aware of the risks. Hum Reprod 23:2874. [DOI] [PubMed] [Google Scholar]
- 34.Gu YF, Lin G, Lu CF. and Lu GX. (2009). Analysis of the first mitotic spindles in human in vitro fertilized tripronuclear zygotes after pronuclear removal. Reprod Biomed Online 19:745–754 [DOI] [PubMed] [Google Scholar]
- 35.Roblero LS. and Garavagno AC. (1979). Effect of oestradiol-17 beta and progesterone on oviductal transport and early development of mouse embryos. J Reprod Fertil 57:91–95 [DOI] [PubMed] [Google Scholar]
- 36.Pacella L, Zander-Fox DL, Armstrong DT. and Lane M. (2012). Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertil Steril 98:986–994.e1–e2. [DOI] [PubMed] [Google Scholar]
- 37.Varnagy A, Bodis J, Kovacs GL, Sulyok E, Rauh M. and Rascher W. (2013). Metabolic hormones in follicular fluid in women undergoing in vitro fertilization. J Reprod Med 58:305–311 [PubMed] [Google Scholar]
- 38.Petro EM, Leroy JL, Covaci A, Fransen E, De Neubourg D, Dirtu AC, De Pauw I. and Bols PE. (2012). Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod 27:1025–1033 [DOI] [PubMed] [Google Scholar]
- 39.Hardy K. and Spanos S. (2002). Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol 172:221–236 [DOI] [PubMed] [Google Scholar]
- 40.Xia P. (1997). Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod 12:1750–1755 [DOI] [PubMed] [Google Scholar]
- 41.Makarevich AV. and Markkula M. (2002). Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor I during in vitro maturation and culture. Biol Reprod 66:386–392 [DOI] [PubMed] [Google Scholar]
- 42.Demeestere I, Gervy C, Centner J, Devreker F, Englert Y. and Delbaere A. (2004). Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice. Biol Reprod 70:1664–1669 [DOI] [PubMed] [Google Scholar]
- 43.Martins da Silva SJ, Gardner JO, Taylor JE, Springbett A, De Sousa PA. and Anderson RA. (2005). Brain-derived neurotrophic factor promotes bovine oocyte cytoplasmic competence for embryo development. Reproduction 129:423–434 [DOI] [PubMed] [Google Scholar]
- 44.Scott L, Alvero R, Leondires M. and Miller B. (2000). The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod 15:2394–2403 [DOI] [PubMed] [Google Scholar]
- 45.Hardarson T, Caisander G, Sjogren A, Hanson C, Hamberger L. and Lundin K. (2003). A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human pre-embryos compared with control blastocysts. Hum Reprod 18:399–407 [DOI] [PubMed] [Google Scholar]
- 46.Reverte CG, Benware A, Jones CW. and LaFlamme SE. (2006). Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell Biol 174:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lestou VS, Desilets V, Lomax BL, Barrett IJ, Wilson RD, Langlois S. and Kalousek DK. (2000). Comparative genomic hybridization: a new approach to screening for intrauterine complete or mosaic aneuploidy. Am J Med Genet 92:281–284 [DOI] [PubMed] [Google Scholar]
- 48.Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG. and Wells D. (2010). Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril 94:1700–1706 [DOI] [PubMed] [Google Scholar]
- 49.Sher G, Keskintepe L, Keskintepe M, Maassarani G, Tortoriello D. and Brody S. (2009). Genetic analysis of human embryos by metaphase comparative genomic hybridization (mCGH) improves efficiency of IVF by increasing embryo implantation rate and reducing multiple pregnancies and spontaneous miscarriages. Fertil Steril 92:1886–1894 [DOI] [PubMed] [Google Scholar]
- 50.Ben-Shlomo I, Yu Hsu S, Rauch R, Kowalski HW. and Hsueh AJ. (2003). Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Sci STKE 2003:RE9. [DOI] [PubMed] [Google Scholar]
- 51.Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Ratajczak CK. and Moley KH. (2005). The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev Biol 284:377–386 [DOI] [PubMed] [Google Scholar]
- 52.Halet G, Viard P. and Carroll J. (2008). Constitutive PtdIns(3,4,5)P3 synthesis promotes the development and survival of early mammalian embryos. Development 135:425–429 [DOI] [PubMed] [Google Scholar]
- 53.Purcell SH. and Moley KH. (2011). The impact of obesity on egg quality. J Assist Reprod Genet 28:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riley JK, Carayannopoulos MO, Wyman AH, Chi M. and Moley KH. (2006). Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem 281:6010–6019 [DOI] [PubMed] [Google Scholar]
- 55.Lerou PH, Yabuuchi A, Huo H, Takeuchi A, Shea J, Cimini T, Ince TA, Ginsburg E, Racowsky C. and Daley GQ. (2008). Human embryonic stem cell derivation from poor-quality embryos. Nat Biotechnol 26:212–214 [DOI] [PubMed] [Google Scholar]
- 56.Cortes JL, Sanchez L, Catalina P, Cobo F, Bueno C, Martinez-Ramirez A, Barroso A, Cabrera C, Ligero G, et al. (2008). Whole-blastocyst culture followed by laser drilling technology enhances the efficiency of inner cell mass isolation and embryonic stem cell derivation from good- and poor-quality mouse embryos: new insights for derivation of human embryonic stem cell lines. Stem Cells Dev 17:255–267 [DOI] [PubMed] [Google Scholar]
- 57.Cortes JL, Sanchez L, Ligero G, Gutierrez-Aranda I, Catalina P, Elosua C, Leone PE, Montes R, Bueno C, et al. (2009). Mesenchymal stem cells facilitate the derivation of human embryonic stem cells from cryopreserved poor-quality embryos. Hum Reprod 24:1844–1851 [DOI] [PubMed] [Google Scholar]