Abstract
Modeling of cellular environments with nanofabricated biomaterial scaffolds has the potential to improve the growth and functional development of cultured cellular models, as well as assist in tissue engineering efforts. An understanding of how such substrates may alter cellular function is critical. Highly plastic central nervous system hippocampal cells and non-network forming peripheral nervous system dorsal root ganglion (DRG) cells from embryonic rats were cultured upon laminin-coated degradable polycaprolactone (PCL) and nondegradable polystyrene (PS) electrospun nanofibrous scaffolds with fiber diameters similar to those of neuronal processes. The two cell types displayed intrinsically different growth patterns on the nanofibrous scaffolds. Hippocampal neurites grew both parallel and perpendicular to the nanofibers, a property that would increase neurite-to-neurite contacts and maximize potential synapse development, essential for extensive network formation in a highly plastic cell type. In contrast, non-network-forming DRG neurons grew neurites exclusively along fibers, recapitulating the simple direct unbranching pathway between sensory ending and synapse in the spinal cord that occurs in vivo. In addition, the two primary neuronal types showed different functional capacities under patch clamp testing. The substrate composition did not alter the neuronal functional development, supporting electrospun PCL and PS as candidate materials for controlled cellular environments in culture and electrospun PCL for directed neurite outgrowth in tissue engineering applications.
Introduction
Biomaterial substrates have long been investigated for use as structural support and both mechanical and chemical control of cellular growth in culture and tissue regeneration. Neuronal growth is highly dependent upon the chemical and physical properties of the environment, so cell growth and functional development should be optimal for conditions that most closely match the natural cellular environment.1–3 Nanofabricated electrospun polymer substrates are a promising means by which to mimic that natural neural cellular environment both in culture and later for implantation in tissue regeneration applications.
Cellular electrophysiological activity is an important function that is especially critical for neurons, since their function is characterized by the cell's ability to adequately control transmembrane ion flux to enable generation of action potentials (APs), the unit of neuronal intercellular communication.
Electrophysiological studies of cultured primary neurons have shown that the cellular function was not altered when neurite outgrowth directionality was controlled by guidance cues presented by patterned protein deposition on 2D surfaces,4–6 or when cells were plated with patterned inkjet cell printing techniques.7 Nor has there been reported changes in the ability of cells to form synapses on patterned or textured 2D surfaces as measured by synapsin I staining8,9 and synaptic currents.10 Neurons cultured on two-dimensional cast polymer surfaces also display normal function.11 However, many polymer-based scaffolds are nanofabricated, increasing the ratio of material surface area to volume which, in turn, increases the contact area between the cells and substrate,12 potentially magnifying effects the material may have on cell function. Increasing the surface area also increases degradation rates of degradable polymers13 which, along with any resultant by-products, has the potential to alter the electrophysiological function with time in extended culture. Polymer substrates have previously been treated to attach peptides to surfaces, particularly for electrospun polycaprolactone (PCL), where ethylenediamine treatment has been used to partially degrade and aminolyse the surface to facilitate peptide attachment.14,15 This PCL aminolysation treatment has not been tested for effects on cell function.
The electrophysiological function and network formation appear normal in primary neurons cultured in a three-dimensional Matrigel16 and collagen matrices.17,18 Changes in the resting membrane potential and altered duration of the after hyperpolarization following an AP have been reported in hippocampal cells during early culture in collagen gel,19 however, the capacity of cells to fire APs was not altered. However, gene expression and protein transcription of various proteins, not necessarily related to the electrophysiological function, can be regulated within certain cell types by cellular interactions with the nanostructure,20 by changes in cell morphology arising from surface chemistry,21 and by topographical guidance cues22 presented by the substrates upon which cells are grown. If substrate nanoarchitecture can influence expression of proteins related to electrophysiological function, such as Na+, K+, Ca2+, and Cl− ion channels, then it is also likely that nanoarchitecture may alter the cell's ability to fire APs, a critical requirement of functional neurons.
Shahbazi et al. found that when differentiating stem cells into neurons rather than using primary neuronal cultures, polyamide nanofibers regulated gene expression and transcription of ion channel proteins, including various voltage-gated K+, Na+, and Ca2+ channels, resulting in increased duration and amplitude of inward currents as well as increased K+ currents.23 Acute application of nanoparticles can also alter cellular functions, with carbon nanotubes able to block transmission of ions through ion channels involved in electrophysiological function, decreasing K+ current densities in PC12 cells24 and in transfected CHO cells.25 Incorporation of carbon nanotubes into biomimetic scaffolds may, however, improve the neuronal network formation,26 possibly by the generation of electrical shortcuts between cells.27 This dichotomy of effects of nanoparticles illustrates the need to thoroughly test their effects on cell function.
Sensory dorsal root ganglion (DRG) neurons have been well studied electrophysiologically.28–32 During development, DRG neurons send processes from the spinal cord to the body's extremities to form the sensory peripheral nervous system. It is not surprising then that, in culture, DRG neurons have the propensity to extend long neurites, as they do in vivo, which are strongly guided by structural cues in the underlying substrate.33–37 In contrast, hippocampal neurons, often studied for their electrophysiological activity and network formation in culture,38–41 form highly plastic networks with GABAergic and glutamatergic synapses involved in memory formation. Unlike DRG neurons, Nagata et al. showed that central nervous system neuroblasts, including those from the hippocampus, extended neurites primarily perpendicular, but also parallel, to the existing aligned neurite bundles.42 Later studies extended this to the artificial topographic cues,43,44 however, parallel hippocampal neurite outgrowth has been reported upon electrospun fibers.45 DRG and hippocampal neurons provide biomaterial research with two very different neuronal models, representing the two extremes of the neuronal spectrum: a low plasticity peripheral nervous system model that typically does not form networks in culture, and a highly plastic network-forming central nervous system model.
Functional assessment of neurons with time in culture out to maturity in response to biomaterial substrates has not been reported, nor has the effect of biomaterial degradation on neuronal function been investigated. Direct comparison of functional activity of central and peripheral primary neurons has also not been made within the biomaterial discourse. This study investigated the growth patterns and functional development of rat embryonic day 18 (E18) DRG and hippocampal neurons cultured on electrospun polymer substrates of nondegradable polystyrene (PS) and degradable PCL with and without prior ethylenediamine treatment. Neuronal function was assessed by investigating AP firing patterns during current injection using whole cell patch clamp techniques. Cells were studied for cellular functional maturity in extended cultures.
Materials and Methods
Electrospinning
PCL (Sigma Aldrich; Mn 70,000–90,000) was dissolved 9% (w/v) in chloroform and methanol at a ratio of 3:1, whereas PS (Sigma-Aldrich; Mw ∼280,000) was dissolved in chloroform and dimethylformamide at a ratio of 1:1 at 10% (w/v) or 12.5% (w/v) for small and large diameter fibers, respectively (all solvents from Merck). Solutions were transferred to a 10-mL glass syringe and electrospun through an 18G drawing needle with an acceleration voltage of 18 kV for PCL and 20 kV for PS across a working distance of 6.5 cm. Aligned fibers were collected on a rotating conductive mandrel with a diameter of 4.8 cm. PS film strips (Goodfellow; 0.05 mm thick) 2 cm wide were attached lengthwise along the mandrel and aligned polymer fibers collected on the PS film.
The PS film with aligned fibers was collected and heated to 45°C for 10 min to securely attach fibers to the PS film and to remove the residual solvent. Substrates were stored in an evacuated dehumidified chamber until use.
Preparation of substrates
Glass coverslips were acid washed in 37% HCl, rinsed, and then stored in 100% analytic ethanol until use. Half of the PCL substrates were treated for 60 min in 0.05 M ethylenediamine (Sigma-Aldrich) in 2-propanol (Merck) to partially degrade and aminolyse the PCL surface, then rinsed in ice-cold MilliQ water. Fibrous substrates and PS film without fibers were sterilized in 80% (v/v) analytic ethanol for 1 h, then rinsed six times over 1 h to remove residual ethanol. Glass coverslips (Menzel Glasser) were incubated at 37°C in 0.01% poly-l-ornithine (Sigma Aldrich) for 4 h. All substrates, including glass coverslips, were incubated in 10 μg/mL natural mouse laminin (Invitrogen) in Dulbecco's modified Eagle's medium with Ham's F12 nutrient mixture (DMEM/f12, Invitrogen; 11330-032) for 4 h at 37°C, rinsed, and returned to DMEM/f12 until culture.
DRG explants and culture
Pregnant Sprague Dawley rats were anesthetized with isoflurane and decapitated. E18 pups were removed, decapitated, and DRG removed and placed in an ice-cold sterile Hank's buffered saline solution (HBSS, Invitrogen; 14025-092). Cells were mechanically dissociated by trituration in HBSS using 200 μL pipette tips, followed by addition of the same volume of growth medium. The growth medium contained DMEM/f12, 0.5 ng/mL nerve growth factor (Alomone, Israel; N-240-A 2.5s), 2 ng/mL glial cell-derived neurotrophic factor (Invitrogen; PHC 7044), 1:100 N2 (Invitrogen), 5% (v/v) heat inactivated fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (pen/strep; Invitrogen).
Cell suspensions were plated on substrates at 30,000 cells/cm2. Cells were incubated for 1 h to allow for attachment, after which 1 mL of culture medium was added to each well in 12-well culture plates. Seventy percent of medium was replaced every 3 days.
Hippocampal dissection and culture
Brains were isolated from the E18 pups into sterile HBSS, the meninges removed, and hippocampi carefully dissected. Cells were mechanically dissociated by trituration in HBSS using 200 μL pipette tips, and the cell suspension centrifuged at 100 g for 4 min. HBSS was aspirated and replaced with a plating medium and cells were dispersed by mechanical trituration. The plating medium consisted of DMEM/f12, 10% FBS, and pen/strep. Cell suspensions were plated on substrates at 30,000 cells/cm2. Cells were incubated in the plating medium for 5 h to allow cell attachment, after which 1 mL of serum-free growth medium was added to each well in 12-well culture plates. The serum-free growth medium contained the Neurobasal medium (Invitrogen; 21103-049), 1:100 N2, 1:50 B27 (Invitrogen; 17504-044), and pen/strep. Seventy percent of medium was replaced every 3 days.
Immunostaining
Cells were washed in PBS, and fixed in ice-cold 4% paraformaldehyde for 20 min. Samples were rinsed 3×5 min in PBS, then blocked with 5% normal goat serum (NGS; Invitrogen) and 0.25% Triton X-100 in PBS for 30 min at room temperature. Samples were incubated in the 1:2000 mouse anti-TuJ1 primary antibody (Covance; MMS-435P) in 2.5% NGS and 0.25% Triton X-100 for 18 h at 4°C. Samples were rinsed 3×5 min in PBS, then incubated in 1:500 goat anti-mouse Alexa Fluor 488 (Invitrogen; A11029) in 2.5% NGS and 0.25% Triton X-100 in PBS for 1 h at room temperature. Samples were mounted on glass slides with the fluorescent mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Invitrogen), coverslipped, and imaged on a confocal Nikon C1 inverted microscope. Images were processed in ImageJ (version 1.47q, National Institute of Health), utilizing Fourier analysis through the Directionality plugin (version 2.0, June 8, 2010, created by Jean-Yves Tinevez) to generate neurite directional histograms for cells on coverslips and thin PS fiber substrates.
Electrophysiology
Every 5th day in culture, cells were transferred to a perfusion bath with an artificial extracellular solution containing (in mM) 137 NaCl, 1.3 CaCl2, 5.4 KCl, 0.44 KH2PO4, 0.5 MgCl2, 0.4 MgSO4, 0.3 Na2HPO4, 4 NaHCO3, 5.6 D-glucose, 10 HEPES, and 0.02 EDTA, at pH 7.4. Glass pipette electrodes (Harvard Apparatus; PG150T-15) of resistance 2–5 MΩ were pulled (Flaming-Brown Puller; Sutter Instruments), fire polished (Narishige), and filled with a pseudo-intracellular solution containing (in mM) 115 K-L-glutamate, 15 KCl, 1.2 MgCl2, 10 HEPES, 1 ATP, 0.3 EGTA, pH 7.2.
Cells were patched using patch clamp in whole cell mode, where current was injected for 800 ms using Axopatch 200A series amplifier hardware controlled by Axoclamp version 10 software. Transmembrane voltage signals were recorded and stored. APs were induced and recorded in 200 DRG neurons across 4 independent cultures out to 20 days in vitro (DIV), and in 129 hippocampal cells across 5 independent cultures out to 25 DIV, with 4 more hippocampal cells firing APs upon current injection at 50 DIV.
Fiber diameter measurements
Substrate samples were collected, mounted on aluminium stubs, gold coated, and imaged on a Hitachi S570 scanning electron microscope. Images were processed in ImageJ and fiber diameters of each substrate measured. Diameters are presented as mean+/− standard deviation.
Results and Discussion
Scaffold characterization
Diameters of PCL and small PS fibers were designed to be comparable with the range of diameters of neuronal outgrowths within the body46 to maximize the likelihood that cellular outgrowths would interact with the protein covered fibers as they would with surrounding cellular processes in vivo. Fibers were aligned, and diameters were 458+/−209 nm (n=500), 402+/−99 nm (n=50), and 1697+/−445 nm (n=48) for PCL, small diameter PS, and large diameter PS, respectively.
Neurite outgrowth characteristics
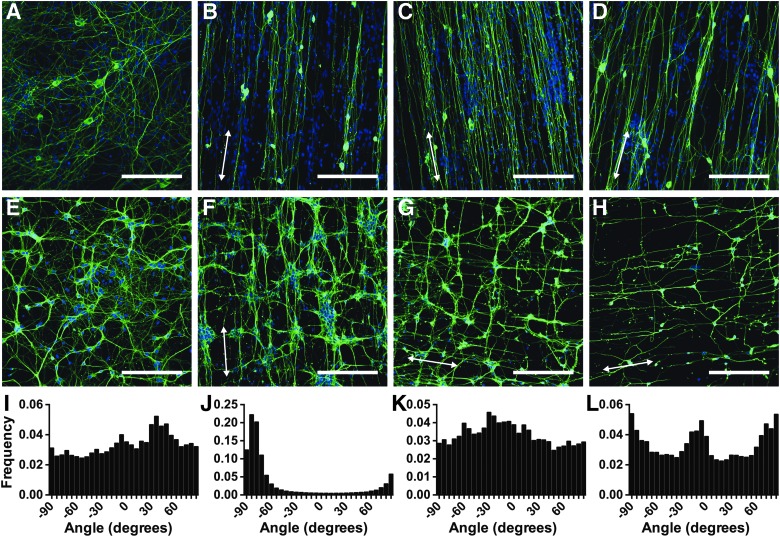
Immunostaining of hippocampal neurons showed that neurites extended both perpendicular and parallel to fibers of all three types and diameters (Fig. 1F–H). Clear dual peaks in alignment occurred at −90o and −6o (Fig. 1L) corresponding to hippocampal neurite growth perpendicular and parallel to PS fibers. Light micrographs of hippocampal neurons studied electrophysiologically show clear extension of neurites perpendicular to PCL fibers on day 10 (Fig. 3H) and day 50 (Fig. 3J) in culture. The nanofibers, being laminin coated and of a diameter similar to natural neurites, were likely regarded by the cells as a similar topographic guidance cue to natural hippocampal neurites. Growth of hippocampal neurites parallel and perpendicular to existing neurites would increase the number of neurite-to-neurite contacts and so increase the number of potential synapses. This growth pattern would maximize intercellular communication and network formation, expected of a cell type making up highly plastic networks of the hippocampus that are involved in memory formation.
FIG. 1.
TuJ1, a neuron-specific β-3 Tubulin antibody (green) and Dulbecco's modified Eagle's medium (nuclear, blue) immunostaining of dorsal root ganglion (DRG) (A–D) and hippocampal cells (E–H) at 5 days in vitro (DIV) on substrates of glass coverslips (A, E), aligned polycaprolactone (PCL) (B, F), aligned small diameter polystyrene (PS) scaffolds (C, G), and aligned large diameter PS scaffolds (D, H) (scale bar 200 μm). In each case, cells on glass coverslips sent out neurites in random orientation. While DRG cells sent out neurites along fibers, hippocampal cells sent out neurites both parallel and perpendicular to fibers. Direction histograms for DRG on coverslips (I) and thin PS fibers (J), and hippocampal cells on coverslips (K) and thin PS fibers (L) have been included. Arrows show the direction of fiber alignment.
FIG. 3.
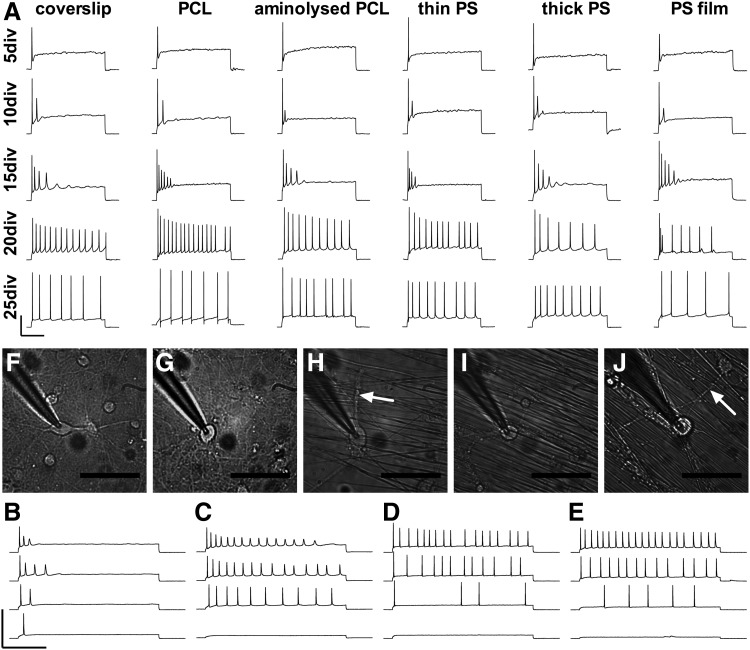
(A) E18 rat hippocampal cells were cultured on substrates and patch clamped every 5 days out to 25 days in culture (scale 250 ms horizontal, 50 mV vertical). APs induced by current injection improved in function over time in culture, with no difference in functional development between substrates. Application of increasing current steps early in culture resulted in significant loss of amplitude of repeated APs (B: 15 DIV, C: 20 DIV), a trend that persisted until cell maturity at 25 days in culture (D). Function at 50 DIV (E) was similar to 25 DIV, suggesting cellular functional maturity from 25 days in culture (scale 250 ms horizontal, 200 mV vertical). Optical micrographs (scale 50 μm) of patched hippocampal cells on glass coverslips at 10 DIV (F) and 25 DIV (G) and PCL substrates at 10 DIV (H) and 25 DIV (I) show clean and consistent extended cultures, remaining clean out to 50 days in culture (J). Arrows point to hippocampal neurites clearly growing perpendicular to fibers. Electrophysiology on hippocampal neurons was from 5 independent cultures, with APs induced in 129 neurons up to 25 DIV and another 4 neurons at 50 DIV.
DRG neurons in vivo extend axons side-by-side from the spinal column out to the extremities without synapsing with other DRG neurons. Similarly, in the present study, processes from DRG neurons in culture were guided along the nanofibers, with similar growth patterns on PCL fibers (Fig. 1B) and small and large diameter PS fibers (Fig. 1C, D). A strong single peak in alignment occurred at −84o (Fig. 1J) corresponding to the direction of the fibers.
In contrast, random outgrowth of both DRG and hippocampal neurites occurred on glass coverslips, where no topographic growth cues were supplied (Fig. 1A, E), with no clear peak in alignment observed in directionality histograms (Fig. 1I, K).
DRG cellular function
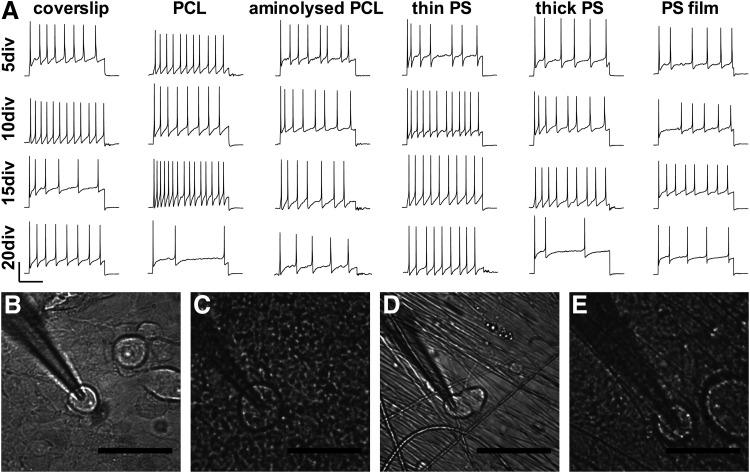
DRG neurons on all substrates fired repeated APs upon current injection from 5 to 20 DIV (Fig. 2A). There was a large range in the amplitude (66.4–136.1 mV, from AP peak to the peak after hyperpolarization potential) and number of induced APs (from 3 to 15 per 800 ms) between recordings presented within this study (Fig. 2A), which likely reflects the electrophysiologically heterogeneous neuronal population known to exist within the DRG.28–32 Neither substrate composition nor time in culture affected the ability of the cells to fire APs upon current injection. Figures 2B and D show clean light micrographs of DRG neurons that had been grown on glass coverslips and PCL fibers, respectively, and studied electrophysiologically. At 20 DIV, DRG neurons were difficult to patch due to a buildup of protein and lipid droplets on the surface of the cells (Fig. 2C, E), later time points could not be studied electrophysiologically, and this occurred equally on all substrates.
FIG. 2.
(A) Embryonic day 18 (E18) rat DRG cells cultured on substrates and patch clamped at 5, 10, 15, and 20 DIV (scale 250 ms horizontal, 50 mV vertical). Cells on all substrates were capable of firing action potentials (APs) throughout the culture period in response to current injection. Optical micrographs of patched cells in the perfusion bath (scale bar 50 μm) on glass coverslips at 10 DIV (B) and 20 DIV (C), and PCL substrates at 10 DIV (D) and 20 DIV (E). Cultures at 20 days show a buildup of protein and lipid over the culture making patching of cells difficult. Electrophysiology on DRG neurons was from 4 independent cultures, with APs induced in 200 neurons up to 20 DIV.
Hippocampal cellular function
The ability of hippocampal neurons to fire repeated APs upon current injection developed over time in culture, with only a single AP at 5 DIV (Fig. 3A). Repeated APs with rapidly decreasing amplitude were observed at 10 and 15 DIV. At 20 DIV, current injection induced APs throughout the 800 ms period of stimulation, but still with some decline in amplitude.
Increasing the magnitude of injected current at 15 and 20 DIV (Fig. 3B, C) resulted in a greater decline in amplitude of repeated APs, suggesting that cells were not functionally mature. The process of functional development of hippocampal cells appeared to plateau by 25 DIV, where the amplitude of repeated APs remained constant (Fig. 3A) and increasing current injections resulted in minimal decline in AP amplitude (Fig. 3D). This sustained amplitude of repeated APs, even with injection of increased current, remained a consistent characteristic out to 50 DIV (Fig. 3E), suggesting functional maturity of hippocampal cells by 25 days in culture.
Previous modeling of APs based on ion channel densities by Arhem et al. predicts a decline in the amplitude of repeated APs in cases where there is an inadequate ratio of Na+ to K+ current densities. The model suggests that once an adequate ratio of Na+ to K+ current densities is reached, repeated APs will not decline in amplitude and cells will function as mature neurons.47,48 This decline in repeated AP amplitude was observed in hippocampal cells within this study (Fig. 3A), with improvement over time in culture to reach minimal repeated AP amplitude decline by 25 DIV. This model would suggest that the observed functional improvement in hippocampal cells over time in culture was primarily due to the development of Na+ and K+ channel densities toward physiological levels, and that hippocampal functional maturity was reached by 25 days in culture.
The functional development of hippocampal cells occurred at a similar rate on all substrates, including glass coverslips, aminolysed and nonaminolysed PCL fibers, small and large diameter PS fibers, and PS film.
Conclusions
Electrospun fibers of a diameter comparable to that of neuronal processes have differential effects on neurite outgrowth for central versus peripheral neurons. DRG neurites were guided exclusively along fibers, a property that might be expected of a cell type whose processes travel long distances within the body. Conversely, hippocampal neurons sent out processes both parallel and perpendicular to substrate fibers, a property that would facilitate neurite-to-neurite contacts, and so maximize the number of synapses, a characteristic required of a highly plastic neuronal cell type to enable extensive network formation. However, the observed perpendicular contact guidance of hippocampal neurons may have a significant impact upon guiding neurites to their target in engineering of tracts within the central nervous system.
Longitudinal electrophysiological studies of electrically active cells cultured upon and within biomaterial substrates are critical in ensuring that substrates, particularly those that degrade with time, do not interfere with cellular function over time in culture. Furthermore, such longitudinal electrophysiological studies are important with biomaterial substrates before implantation for tissue engineering applications to ensure that substrates are not likely to interfere with endogenous tissue function. The intrinsic functional activity of DRG and hippocampal neurons was not the same, however, neither the functional capacity of DRG neurons nor the functional development of hippocampal neurons was altered by the composition of the substrates upon which they were grown. Nondegradable PS and degradable PCL nanofibrous substrates, with or without prior partial degradation through ethylenediamine treatment, all resulted in a similar time course of development of hippocampal cell functional, thus PCL degradation did not negatively impact upon cellular function and development. Having shown that these substrates do not impact upon the rate of functional development of primary neurons supports electrospun PCL and PS substrates as candidates for culture substrates with electrophysiologically active cells and also supports the case for implantation of degradable nanofibrous PCL substrates in tissue engineering applications.
In culture, as in the developing body, growth patterns and rates of functional development of neurons from the central and peripheral nervous systems are remarkably different. The results of this study highlight the importance of studying cells of more than one origin when developing biomaterials for use with neuronal tissue. The central and peripheral models used in this study encapsulate the extremes of the neuronal spectrum, providing powerful tools for understanding the control of neuronal cell growth and functional activity for biomaterial product development leading to clinical applications.
Acknowledgments
We thank Monash Micro Imaging for provision of instrumentation and training in optical microscopy techniques, and the National Health and Medical Research Council for the Dora Lush Biomedical Postgraduate Scholarship to J.B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hutmacher D.W.Biomaterials offer cancer research the third dimension. Nat Mater 9,90, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Lutton C., and Goss B.Caring about microenvironments. Nat Biotechnol 26,613, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Edelman D.B., and Keefer E.W.A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp Neurol 192,1, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kleinfeld D., Kahler K.H., and Hockberger P.E.Controlled outgrowth of dissociated neurons on patterned substrates. J Neurosci 8,4098, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnusson A.K., Linderholm P., Vieider C., Ulfendahl M., and Erlandsson A.Surface protein patterns govern morphology, proliferation, and expression of cellular markers but have no effect on physiological properties of cortical precursor cells. J Neurosci Res 86,2363, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Heller D.A., Garga V., Kelleher K.J., Lee T.-C., Mahbubani S., Sigworth L.A., et al. . Patterned networks of mouse hippocampal neurons on peptide-coated gold surfaces. Biomaterials 26,883, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Xu T., Gregory C.A., Molnar P., Cui X., Jalota S., Bhaduri S.B., et al. . Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27,3580, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ma W., Liu Q.-Y., Jung D., Manos P., Pancrazio J.J., Schaffner A.E., et al. . Central neuronal synapse formation on micropatterned surfaces. Brain Res Dev Brain Res 111,231, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Liu Q.-Y., Coulombe M., Dumm J., Shaffer K.M., Schaffner A.E., Barker J.L., et al. . Synaptic connectivity in hippocampal neuronal networks cultured on micropatterned surfaces. Brain Res Dev Brain Res 120,223, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Thalhammer A., Edgington R.J., Cingolani L.A., Schoepfer R., and Jackman R.B.The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials 31,2097, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Ramos C., Lainez S., Sancho F., García Esparza M.A., Planells-Cases R., García Verdugo J.M., et al. . Differentiation of postnatal neural stem cells into glia and functional neurons on laminin-coated polymeric substrates. Tissue Eng Part A 14,1365, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Stevens M.M., and George J.H.Exploring and engineering the cell surface interface. Science 310,1135, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Bölgen N., Menceloğlu Y.Z., Acatay K., Vargel I., and Pişkin E.In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polym Ed 16,1537, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Horne M.K., Nisbet D.R., Forsythe J.S., and Parish C.L.Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev 19,843, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Nisbet D.R., Yu L.M.Y., Zahir T., Forsythe J.S., and Shoichet M.S.Characterization of neural stem cells on electrospun poly(epsilon-caprolactone) submicron scaffolds: evaluating their potential in neural tissue engineering. J Biomater Sci Polym Ed 19,623, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Irons H.R., Cullen D.K., Shapiro N.P., Lambert N.A., Lee R.H., and Laplaca M.C.Three-dimensional neural constructs: a novel platform for neurophysiological investigation. J Neural Eng 5,333, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ma W., Fitzgerald W., Liu Q.-Y., O'Shaughnessy T.J., Maric D., Lin H.J., et al. . CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol 190,276, 2004 [DOI] [PubMed] [Google Scholar]
- 18.O'Shaughnessy T.J., Lin H.J., and Ma W.Functional synapse formation among rat cortical neurons grown on three-dimensional collagen gels. Neurosci Lett 340,169, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Xu T., Molnar P., Gregory C., Das M., Boland T., and Hickman J.J.Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3D collagen hydrogel. Biomaterials 30,4377, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Mendonça G., Mendonça D.B.S., Simões L.G.P., Araújo A.L., Leite E.R., Duarte W.R., et al. . The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials 30,4053, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Shao H.-J., Lee Y.-T., Chen C.-S., Wang J.-H., and Young T.-H.Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials 31,4695, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Chew S.Y., Mi R., Hoke A., and Leong K.W.The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 29,653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahbazi E., Kiani S., Gourabi H., and Baharvand H.Electrospun nanofibrillar surfaces promote neuronal differentiation and function from human embryonic stem cells. Tissue Eng Part A 17,3021, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Xu H., Bai J., Meng J., Hao W., Xu H., and Cao J.-M.Multi-walled carbon nanotubes suppress potassium channel activities in PC12 cells. Nanotechnology 20,285102, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Park K.H., Chhowalla M., Iqbal Z., and Sesti F.Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem 278,50212, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lovat V., Pantarotto D., Lagostena L., Cacciari B., Grandolfo M., Righi M., et al. . Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett 5,1107, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Cellot G., Cilia E., Cipollone S., Rancic V., Sucapane A., Giordani S., et al. . Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol 4,126, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Ho C., and O'Leary M.E.Single-cell analysis of sodium channel expression in dorsal root ganglion neurons. Mol Cell Neurosci 46,159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scroggs R.S., and Fox A.P.Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol (Lond) 445,639, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scroggs R.S., Todorovic S.M., Anderson E.G., and Fox A.P.Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J Neurophysiol 71,271, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Villière V., and McLachlan E.M.Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol 76,1924, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Caffrey J.M., Eng D.L., Black J.A., Waxman S.G., and Kocsis J.D.Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res 592,283, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Xie J., MacEwan M.R., Li X., Sakiyama-Elbert S.E., and Xia Y.Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano 3,1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnell E., Klinkhammer K., Balzer S., Brook G., Klee D., Dalton P., et al. . Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28,3012, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Daud M.F.B., Pawar K.C., Claeyssens F., Ryan A.J., and Haycock J.W.An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 33,5901, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Corey J.M., Lin D.Y., Mycek K.B., Chen Q., Samuel S., Feldman E.L., et al. . Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A 83,636, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Wang H.B., Mullins M.E., Cregg J.M., McCarthy C.W., and Gilbert R.J.Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater 6,2970, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Eubanks J.H., Perez-Velazquez J.L., Kerr R.G., Carlen P.L., Mills L.R., and Jones O.T.Three distinct neuronal phenotypes exist in embryonic rat hippocampal neurons cultured in basic fibroblast growth factor. Neurosci Lett 204,5, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Bi G.Q., and Poo M.M.Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18,10464, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao H., Zhang L.I., Bi G., and Poo M.Selective presynaptic propagation of long-term potentiation in defined neural networks. J Neurosci 20,3233, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H.-X., Gerkin R.C., Nauen D.W., and Bi G.-Q.Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat Neurosci 8,187, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Nagata I., and Nakatsuji N.Rodent CNS neuroblasts exhibit both perpendicular and parallel contact guidance on the aligned parallel neurite bundle. Development 112,581, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Nagata I., Kawana A., and Nakatsuji N.Perpendicular contact guidance of CNS neuroblasts on artificial microstructures. Development 117,401, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Fozdar D.Y., Lee J.Y., Schmidt C.E., and Chen S.Hippocampal neurons respond uniquely to topographies of various sizes and shapes. Biofabrication 2,035005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J.Y., Bashur C.A., Gomez N., Goldstein A.S., and Schmidt C.E.Enhanced polarization of embryonic hippocampal neurons on micron scale electrospun fibers. J Biomed Mater Res A 92,1398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson K., Brisby H., Johansson B.R., Runesson E., and Rydevik B.Electron microscopy analysis of neurites extending from dorsal root ganglia in vitro following exposure to intervertebral disc cells. Cells Tissues Organs (Print) 196,82, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Arhem P., Klement G., and Blomberg C.Channel density regulation of firing patterns in a cortical neuron model. Biophys J 90,4392, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arhem P., and Blomberg C.Ion channel density and threshold dynamics of repetitive firing in a cortical neuron model. BioSystems 89,117, 2007 [DOI] [PubMed] [Google Scholar]