Abstract
A prospective molecular epidemiology study of HIV-1 infection was conducted in newly diagnosed and antiretroviral-naive patients in Northern Greece between 2009 and 2010 using a predefined enrolling strategy. Phylogenetic trees of the pol sequences obtained in this study with reference sequences indicated that subtypes B and A1 were the most common subtypes present and accounted for 44.9% and 42.9%, respectively, followed by subtype C (3.1%), CRF02_AG (4.1%), CRF04_cpx (2.0%), and subtypes CRF01_01, F1, and G (1.0%). A high rate of clustered transmission of subtype A1-resistant strains to reverse transcriptase (RT) inhibitors was observed among men having sex with men. Indeed, 15 out of 17 study subjects (88.2%) infected with transmitted drug resistance (TDR) strains were implicated in transmission clusters, 10 of whom (66.7%) were men who have sex with men (MSM), and were also infected with subsubtype A1 strains. The main cluster within subtype A1 (I) included eight men reporting having sex with men from Thessaloniki infected with dual-class RT-resistant strains carrying both T215C and Y181C mutations.
The human immunodeficiency virus type 1 (HIV-1) genome exhibits high genetic heterogeneity, leading to three genetic groups (M, N, and O) and numerous closely related subtypes. HIV-1 group M is further classified in a proposed consensus of nine distinct subtypes (A–D, F–H, J, O, and P) and an increasing number of intersubtype circulating recombinant forms (CRFs).1,2 In a recent molecular epidemiology study of HIV-1 infection in Europe, the most prevalent subtypes/CRFs were subtype B (66.1%), followed by subsubtype A1 (6.9%), subtype C (6.8%), and CRF02_AG (4.7%) with substantial differences in subtype distribution among European countries, immigrant populations, and patient risk groups.3
In this study, we analyzed pol sequences of HIV-1 isolates from a prospective molecular epidemiology study, in which we recruited individuals newly diagnosed with HIV-1 infection from 2009 to 2010 in Northern Greece in an effort to characterize the epidemiological and genetic diversity of HIV-1 infection in the region, to determine the prevalence of transmitted drug resistance (TDR), and to gain further insight in the potential risk factors of TDR in relation to the reported risk behavior. The predefined enrollment strategy was for the most part based on the European prospective program (SPREAD) guidelines. Adult, newly diagnosed HIV-1-infected study subjects who had never been exposed to antiretroviral drugs were prospectively recruited. The blood sample was obtained within 3 months of HIV-1 seropositive diagnosis and isolated plasma was used for genotypic resistance analysis. Epidemiological, demographic, and clinical data were collected from each study subject using a standardized questionnaire.
The study subjects were consenting newly diagnosed HIV-1-seropositive patients attending the Division of Infectious Diseases of the AHEPA University Hospital in Thessaloniki, Greece between 2009 and 2010 (Table 1). A table summarizing the detailed analyses of the characteristics of the study subjects is presented in a concurrent study utilizing the same study cohort.4 The study was approved by the Bioethics Committee of the Medical School of the Aristotle University of Thessaloniki. The HIV-1 serostatus of each subject was previously established by commercial enzyme-linked immunoassay and confirmed by Western blotting and blood was drawn within 3 months of HIV-1 diagnosis. An informed consent form was signed by each subject, a questionnaire was filled in with an interviewer, and blood samples were taken by qualified personnel. All samples and questionnaires were coded with a laboratory identifier number so as to ensure patient anonymity. Ninety-eight individuals were included in this study, representing 63.7% of antiretroviral-naive newly diagnosed HIV-1-seropositive patients registered at the database of the AIDS National Reference Laboratory of Northern Greece for the period 2009–2010.
Table 1.
Clinical and Epidemiological Information for Study Patients
| Patienta | Sexb | Age (years) | Collection datec | Weeks of infectiond | Country of origine | Transmission risk groupf | CD4 (cells/mm3) | Plasma HIV-1 RNA (copies×103/ml) | Epidemiological informationf |
|---|---|---|---|---|---|---|---|---|---|
| GR013 | M | 29 | June, 2009 | 1 | Greece | MSM | 844 | 40.5 | Diagnosed with lymphadenopathy |
| GR015 | M | 30 | June, 2009 | 4 | Greece | MSM | 583 | 120.2 | Diagnosed with lymphadenopathy |
| GR020 | M | 43 | June, 2009 | 1 | Greece | MSM | 305 | 1,340.7 | ASM |
| GR024 | M | 38 | July, 2009 | 12 | Greece | MSM | 300 | 100.1 | Diagnosed with lymphadenopathy |
| GR027 | M | 22 | Aug., 2009 | 1 | Greece | MSM | 625 | 90.5 | Diagnosed with Infectious Mononucleosis |
| GR028 | M | 27 | Aug., 2009 | 1 | Greece | Unknown | 946 | 160.1 | ASM |
| GR029 | M | 48 | Aug., 2009 | 12 | Albania | MSM | 160 | 90.7 | ASM |
| GR031 | M | 24 | Sept., 2009 | 8 | Greece | MSM | 265 | 30.1 | ASM |
| GR033 | M | 29 | Sept., 2009 | 12 | Greece | MSM | 378 | 160.2 | ASM |
| GR035 | M | 35 | Sept., 2009 | 1 | Greece | HSX | 805 | 10.6 | ASM |
| GR036 | M | 53 | Sept., 2009 | 1 | Greece | MSM | 134 | 30.6 | ASM |
| GR041 | M | 35 | Oct., 2009 | 1 | Cameroon | HSX | 317 | 150.0 | Diagnosed with lymphadenopathy and VZV |
| GR043 | F | 20 | Oct., 2009 | 2 | Romania | HSX | 241 | 660.0 | Diagnosed with lymphadenopathy |
| GR044 | M | 37 | Oct., 2009 | 1 | Greece | MSM | 656 | 3.0 | ASM |
| GR046 | M | 44 | Oct., 2009 | 1 | Greece | MSM | 234 | 110.8 | Diagnosed with VZV |
| GR048 | M | 24 | Nov., 2009 | 2 | Greece | MSM/IDU | 413 | 10.8 | Diagnosed with lymphadenopathy |
| GR050 | F | 52 | Nov., 2009 | 1 | Greece | HSX | 1,568 | 20.2 | ASM |
| GR051 | M | 28 | Nov., 2009 | 1 | Greece | MSM | 529 | 230.1 | Diagnosed with cervical lymphadenopathy |
| GR052 | F | 35 | Nov., 2009 | 2 | Nigeria | HSX | 593 | 60.8 | ASM |
| GR054 | M | 68 | Nov., 2009 | 1 | Greece | MSM | 817 | 10.8 | ASM |
| GR055 | M | 23 | Nov., 2009 | 1 | Greece | MSM | 405 | 290.7 | ASM |
| GR056 | M | 31 | Nov., 2009 | 2 | Greece | MSM | 436 | 20.6 | ASM |
| GR057 | M | 28 | Nov., 2009 | 2 | Greece | MSM | 684 | 40.0 | Diagnosed with lymphadenopathy, anal warts, and thrombocytopenia |
| GR058 | F | 27 | Nov., 2009 | 2 | Greece | HSX | 309 | 5,280.5 | Diagnosed with lymphadenopathy and high fever |
| GR060 | M | 56 | Dec., 2009 | 2 | Greece | Unknown | 324 | 10.9 | ASM |
| GR061 | M | 28 | Dec., 2009 | 2 | Greece | MSM | 1,086 | 2.0 | ASM |
| GR062 | F | 38 | Dec., 2009 | 2 | South Africa | HSX | 38 | 160.6 | Heterosexual partner of GR063 |
| GR063 | M | 40 | Dec., 2009 | 2 | Greece | HSX | 495 | 3.0 | Heterosexual partner of GR062 |
| GR064 | M | 28 | Dec., 2009 | 2 | Greece | IDU | 675 | 30.7 | Diagnosed with lymphadenopathy |
| GR065 | M | 32 | Dec., 2009 | 12 | Greece | MSM | 1002 | 2.0 | N/A |
| GR066 | M | 44 | Jan., 2010 | 1 | Greece | MSM | 691 | 110.4 | Flu-like symptoms with high fever |
| GR068 | M | 38 | Jan., 2010 | 1 | Greece | MSM | 396 | 8.0 | ASM |
| GR070 | M | 32 | Jan., 2010 | 2 | Greece | HSX | 732 | 10.2 | Married to seropositive individual |
| GR073 | M | 32 | Feb., 2010 | 2 | Greece | MSM | 584 | 5.0 | Diagnosed with lymphadenopathy and syphilis |
| GR074 | M | 32 | Feb., 2010 | 1 | Greece | MSM | 700 | 40.5 | Diagnosed with lymphadenopathy |
| GR075 | M | 38 | Feb., 2010 | 1 | Greece | MSM | 633 | 10.3 | Diagnosed with lymphadenopathy |
| GR076 | M | 22 | Feb., 2010 | 3 | Greece | MSM | 741 | 90.5 | Diagnosed with high fever and diarrhoic syndrome |
| GR079 | M | 30 | Feb., 2010 | 1 | Greece | MSM | 376 | 200.5 | Diagnosed with high fever |
| GR080 | M | 23 | Feb., 2010 | 1 | Greece | MSM | 737 | 4.0 | Diagnosed with lymphadenopathy and VZV |
| GR083 | M | 32 | Mar., 2010 | 3 | Greece | MSM | 228 | 100.0 | Diagnosed with high fever |
| GR086 | M | 33 | Mar., 2010 | 3 | Greece | MSM | 496 | 10.9 | Diagnosed with lymphadenopathy |
| GR087 | M | 22 | Mar., 2010 | 2 | Greece | MSM | 481 | 20.8 | ASM |
| GR088 | M | 47 | Apr., 2010 | 3 | Greece | MSM | 777 | 9.0 | Diagnosed with lymphadenopathy and ocular HSV |
| GR090 | M | 33 | Apr., 2010 | 3 | Greece | MSM | 564 | 500.7 | Partner of GR114; diagnosed with syphilis |
| GR091 | F | 35 | Apr., 2010 | 2 | Ukraine | IDU | 288 | 380.0 | HCV positive |
| GR092 | M | 36 | Apr., 2010 | 3 | Greece | MSM | 477 | 410.5 | HSV-1 |
| GR093 | M | 49 | Apr., 2010 | 2 | Greece | HSX | 389 | 190.0 | ASM |
| GR094 | M | 28 | Apr., 2010 | 4 | Greece | MSM | 737 | 40.2 | Diagnosed with lymphadenopathy |
| GR096 | M | 23 | May, 2010 | 1 | Greece | MSM | 220 | 130.0 | Possible Kaposi sarcoma; seropositive partner |
| GR097 | M | 30 | May, 2010 | 2 | Greece | MSM | 543 | 420.8 | Diagnosed with lymphadenopathy |
| GR098 | M | 30 | May, 2010 | 3 | Greece | MSM | 438 | 100.2 | Diagnosed with lymphadenopathy |
| GR099 | M | 37 | May, 2010 | 3 | Greece | MSM | 260 | 330.4 | Diagnosed with lymphadenopathy and HSV-1 |
| GR100 | M | 36 | May, 2010 | 1 | Greece | MSM | 297 | 290.0 | Relapsing aphthous stomatitis |
| GR101 | M | 21 | May, 2010 | 1 | Russia | MSM | 694 | 9.0 | Diagnosed with lymphadenopathy and fever |
| GR102 | M | 21 | June, 2010 | 2 | Greece | MSM | 613 | 610.0 | ASM |
| GR104 | M | 34 | June, 2010 | 4 | Russia | IDU | 250 | 210.0 | ASM |
| GR105 | M | 35 | June, 2010 | 2 | Albania | MSM | 350 | 330.0 | Diagnosed with high fever |
| GR106 | M | 26 | June, 2010 | 1 | Greece | MSM | 324 | 10.6 | ASM |
| GR107 | M | 25 | June, 2010 | 1 | Greece | HSX | 238 | 10.5 | Diagnosed with lymphadenopathy, HSV-1, and syphilis |
| GR108 | M | 49 | June, 2010 | 1 | Greece | N/A | 153 | 60.9 | Diagnosed with neurosyphilis |
| GR109 | M | 39 | June, 2010 | 2 | Albania | N/A | 320 | 6.0 | Diagnosed with syphilis |
| GR110 | M | 40 | June, 2010 | 1 | Greece | MSM | 460 | 68.0 | Diagnosed with infectious mononucleosis |
| GR111 | M | 36 | June, 2010 | 1 | Greece | MSM | 623 | 10.7 | Diagnosed with lymphadenopathy with fever |
| GR112 | M | 24 | June, 2010 | 1 | Georgia | MSM | 322 | 210.0 | Diagnosed with lymphadenopathy |
| GR113 | M | 32 | July, 2010 | 2 | Greece | MSM | 710 | 250.1 | Diagnosed with syphilis |
| GR114 | M | 23 | July, 2010 | 1 | Greece | MSM | 398 | 90.8 | Partner of GR090, diagnosed with syphilis |
| GR115 | M | 27 | Aug., 2010 | 3 | Greece | MSM | 496 | 17.0 | ASM |
| GR116 | M | 51 | Aug., 2010 | 1 | Greece | MSM | 264 | 240.0 | ASM |
| GR117 | M | 48 | Aug., 2010 | 3 | Greece | HSX | 154 | 230.0 | ASM |
| GR118 | M | 38 | Sept., 2010 | 3 | Greece | MSM | 51 | 6.0 | ASM |
| GR119 | F | 41 | Sept., 2010 | 3 | Greece | HSX | 290 | 120.4 | Oral candidiasis |
| GR120 | M | 40 | Sept., 2010 | 1 | Greece | MSM | 675 | 340.9 | Flu-like symptoms that lasted for 2 months |
| GR121 | M | 31 | Sept., 2010 | 2 | Greece | MSM | 428 | 10.1 | Tonsilitis |
| GR122 | M | 25 | Sept., 2010 | 1 | Greece | MSM | 372 | 250.5 | Diagnosed with cervical lymphadenopathy and rash |
| GR123 | M | 25 | Sept., 2010 | 2 | Greece | MSM | 720 | 90.6 | ASM |
| GR124 | M | 57 | Sept., 2010 | 1 | Greece | MSM | 452 | 10.9 | ASM |
| GR125 | M | 26 | Sept., 2010 | 2 | Greece | MSM | 526 | 390.3 | ASM |
| GR126 | M | 33 | Sept., 2010 | 1 | Greece | HSX | 184 | 30.2 | Diagnosed with cervical lymphadenopathy, fever, and oral candidiasis |
| GR127 | F | 22 | Sept., 2010 | 1 | Nigeria | HSX | 777 | 3.0 | ASM |
| GR128 | M | 47 | Sept., 2010 | 1 | Greece | MSM | 489 | 10.6 | ASM |
| GR129 | M | 22 | Oct., 2010 | 3 | Greece | MSM | 950 | 9.0 | ASM |
| GR130 | M | 30 | Oct., 2010 | 1 | Russia | IDU | 753 | 10.8 | ASM |
| GR131 | M | 29 | Oct., 2010 | 1 | Greece | MSM | 297 | 4.0 | Frequent tonsilitis |
| GR132 | F | 51 | Oct., 2010 | 1 | Greece | Unknown | 963 | <50 | ASM |
| GR133 | M | 30 | Oct,. 2010 | 1 | Greece | MSM | 205 | 110.3 | ASM |
| GR134 | M | 23 | Oct., 2010 | 2 | Greece | MSM | 442 | 50.5 | ASM |
| GR135 | M | 24 | Nov., 2010 | 3 | Greece | MSM | 611 | 210.3 | Fever for over 2 weeks with relapsing aphthous stomatitis |
| GR136 | M | 26 | Nov., 2010 | 4 | Greece | MSM | 899 | 2.0 | High fever that lasted 5 days |
| GR137 | M | 24 | Nov., 2010 | 1 | Greece | MSM | 512 | 10.0 | ASM |
| GR138 | M | 23 | Nov., 2010 | 1 | Greece | MSM | 333 | 70.3 | Tonsilitis |
| GR139 | M | 33 | Nov., 2010 | 1 | Greece | MSM | 340 | 30.4 | Diagnosed with VZV |
| GR140 | M | 25 | Nov., 2010 | 12 | Greece | MSM | 154 | 130.0 | Decimal fever |
| GR141 | M | 37 | Nov., 2010 | 2 | Greece | MSM | 746 | 40.4 | ASM |
| GR142 | M | 28 | Dec., 2010 | 2 | Greece | MSM | 379 | 60.3 | ASM |
| GR143 | F | 31 | Dec., 2010 | 8 | Russia | HSX | 382 | 70 | ASM |
| GR144 | M | 36 | Dec., 2010 | 2 | Greece | MSM | 305 | 1,090.0 | ASM |
| GR145 | M | 41 | Dec., 2010 | 1 | Greece | MSM | 656 | 2.0 | ASM |
| GR146 | M | 50 | Dec., 2010 | 2 | Greece | MSM | 496 | 120.0 | ASM |
Indicates the laboratory code for each study subject.
F, female; M, male.
Indicates the date of the sample collection.
Indicates the duration from the first known positive HIV antibody test.
Country of birth of the study subjects.
Information provided by the study subjects.
MSM, men who have sex with men; HSX, heterosexual contact; IDU, intravenous drug user, N/A, not available; ASM, asymptomatic; VZV, varicella zoster virus; HSV, herpes simplex virus; HCV, hepatitis C virus.
All blood samples were processed for population-based nucleotide sequencing of plasma HIV-1 RNA encoding regions of reverse transcriptase (RT) and protease (PR) genes at the National AIDS Reference Laboratory of Northern Greece of the Aristotle University of Thessaloniki by using commercially available kits.5 The phylogenetic analyses were performed at the Laboratory of Biotechnology and Molecular Virology of the University of Cyprus according to previously published methodologies.6,7 The GenBank accession numbers for the reference sequences used in the phylogenetic analyses of the pol regions are A1-DQ676872, A1-AB253429, A1-AF004885, A1-AB253421; A2-AF286238, A2-AF286237; B-K03455, B-AY331295, B-AY173951, B-AY423387; C-U52953, C-U46016, C-AY772699, C-AF067155; D-AY253311, D-U88824, D-K03454; F1-AF077336, F1-AF075703, F1-AF005494, F1-AJ249238; F2-AJ249236, F2-AJ249237, F2-AY371158, F2-AF377956; G-AF061641, G-AF084936, G-U88826, G-AY612637; H-AF190128, H-AF005496, H-AF190127; J-AF082395, J-AF082394, J-EF614151; K-AJ249235, K-AJ249239; 01AE-U54771, 01AE-AB220944; 02AG-L39106, 02AG-AY271690; 03AB-AF414006, 03AB-AF193276; 04cpx-AF119820, 04cpx-AF049337, 04cpx-AF119819. Transmitted drug resistance mutations (TDRM) were defined according to the mutation list published for surveillance of transmitted drug resistance as recommended by the World Health Organization.8 Potential drug resistance transmission clusters were define as sequences sharing a most recent common ancestor with >85% bootstrap support and a mean genetic distance of <0.015 nucleotide substitutions per site.9
The study group consisted of 98 HIV-1 newly diagnosed individuals. Eighty-one subjects were Greek citizens living permanently in Northern Greece at the time of the study, although a number of them reported traveling or living abroad in the past, whereas 13 subjects were born in Albania (two subjects), Cameroon, Romania, Nigeria (two subjects), South Africa, Ukraine, Russia (four subjects), and Georgia. Eighty-eight study subjects (89.8%) were male and 10 (10.2%) were female with a median age of 34 years (IQR, 27–41). The most common reported risk factor of HIV-1 transmission was homosexual contact (74.5%), followed by heterosexual contact (15.3%), intravenous drug usage (5.1%), and of unknown reason (5.1%). Investigation for other sexually transmitted diseases showed that seven of the patients were found to be positive for syphilis and one for HCV. Most of the patients were diagnosed during stage A of their infection according to CDC guidelines and at the time of diagnosis the median CD4 count and the plasma virus load were 468.5 cells/μl (IQR, 307–679.5) and 4.6 log copies/ml (IQR, 4.15–5.31), respectively. Analyses of the HIV-1 pol sequences indicated that subtypes B and A1 were the most common subtypes present and accounted for 44.9% and 42.9%, respectively, followed by subtype C (3.1%), CRF02_AG (4.1%), CRF04_cpx (2.0%), and subtypes CRF01_01, F1, and G (1.0%). The GenBank accession numbers obtained in this study for HIV-1 pol sequences are KF671758-KF671855 for protease sequences and KF671856-KF671953 for reverse transcriptase sequences.
A summary of the characteristics of patients with transmitted drug resistance mutations is shown in Table 2. The overall prevalence of transmitted drug resistance mutations (TDRM) to current HIV-1 antiretroviral drugs in the studied patients was 17.4%, of whom 29.4% were infected with viruses carrying a single TDRM. Dual-class and multiclass-resistant mutations were observed in 64.7% and 5.9% of the patients, respectively. The prevalence of nucleoside reverse-transcriptase inhibitor (NRTI) resistance was 12.24% (12 of 98 patients), the prevalence of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance was 17.35% (17 of 98 patients), and the prevalence of protease inhibitor (PI) was 1.02% (1 of 98 patients). NRTI TDRM were found in 11 patients infected with HIV-1 subtype A1 (T215C, nine patients; T215S, two patients) and in one patient with subtype C (D67N). The highest prevalent mutation was the revertant mutations at position 215 (C/S, 91.7%) followed by D67N (8.3%). NNRTI TDRM were found in all 14 patients infected with HIV-1 subtype A1 (Y181C, nine patients; K103N, five patients), two patients with subtype C subtype (Y181I), and one patient with B subtype (G190A). PI TDRM was found in a single patient (GR087) infected with subtype A1 (N88S).
Table 2.
Characteristics of Patients with Transmitted Drug Resistance Mutations
| Surveillance drug resistance mutationse | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patienta | Cluster | Sexb | Age (years) | Weeks of infectionc | Country of origind | Transmission risk groupe | CD4 (cells/mm3) | Plasma HIV-1 RNA (copies×104/ml) | Subtype | NRTI | NNRTI | PI |
| GR013 | I | M | 29 | 1 | Greece | MSM | 844 | 4.5 | A1 | T215C | Y181C | — |
| GR015 | I | M | 30 | 4 | Greece | MSM | 583 | 12.2 | A1 | T215C | Y181C | — |
| GR061 | I | M | 28 | 2 | Greece | MSM | 1086 | 0.2 | A1 | T215C | Y181C | — |
| GR075 | I | M | 38 | 1 | Greece | MSM | 633 | 1.3 | A1 | T215C | Y181C | — |
| GR105 | I | M | 35 | 2 | Albania | MSM | 350 | 33.0 | A1 | T215C | Y181C | — |
| GR117 | I | M | 48 | 3 | Greece | HSX | 154 | 23.0 | A1 | T215C | Y181C | — |
| GR144 | I | M | 36 | 2 | Greece | MSM | 305 | 109.0 | A1 | T215C | Y181C | — |
| GR146 | I | M | 50 | 2 | Greece | MSM | 496 | 12.0 | A1 | T215C | Y181C | — |
| GR083 | II | M | 32 | 3 | Greece | MSM | 228 | 10.0 | A1 | T215S | K103N | — |
| GR087 | II | M | 22 | 2 | Greece | MSM | 481 | 2.8 | A1 | T215S | K103N | N88S |
| GR107 | III | M | 25 | 1 | Greece | HSX | 238 | 1.5 | A1 | — | K103N | — |
| GR136 | III | M | 26 | 4 | Greece | MSM | 899 | 0.2 | A1 | — | K103N | — |
| GR143 | III | F | 31 | 8 | Russia | HSX | 382 | 0.7 | A1 | — | K103N | — |
| GR062 | IV | F | 38 | 2 | South Africa | HSX | 38 | 16.6 | C | — | Y181I | — |
| GR063 | IV | M | 40 | 2 | Greece | HSX | 495 | 0.3 | C | D67N | Y181I | — |
| GR033 | — | M | 29 | 12 | Greece | MSM | 378 | 16.2 | B | — | G190A | — |
| GR098 | M | 30 | 3 | Greece | MSM | 438 | 10.2 | A1 | T215C | Y181C | — | |
Indicates the laboratory code for each study subject.
F, female; M, male.
Indicates the duration from the first known positive HIV antibody test.
Country of birth of the study subjects. N/A, not available.
Defined according to the published list of mutations for surveillance to transmitted drug resistance as recommended by the World Health Organization.8
MSM, men who have sex with men; HSX, heterosexual contact; IDU, intravenous drug user, N/A, not available; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
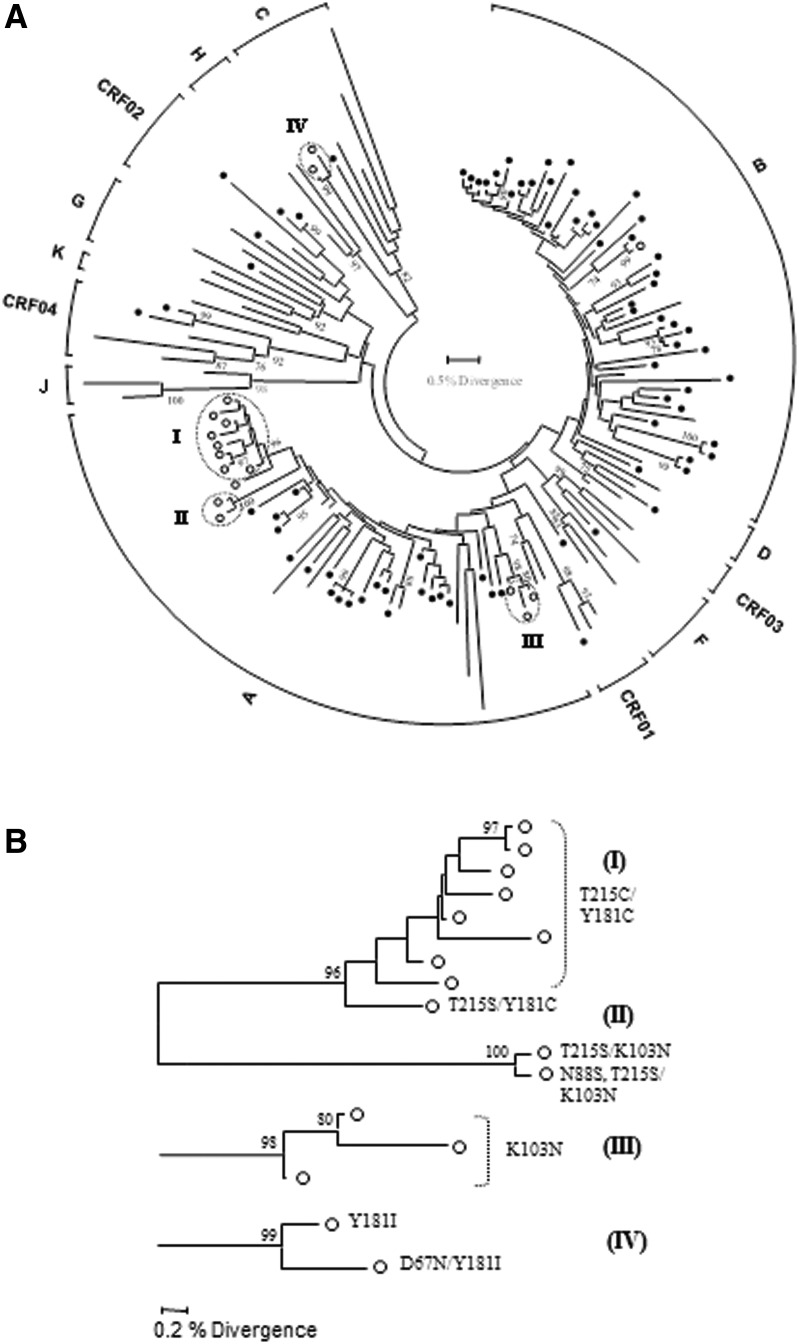
Phylogenetic analyses, presented in Fig. 1, revealed four transmission clusters highly supported by bootstrapping (>85%) and a mean genetic distance of <0.015 nucleotide substitutions per site. Three of the clusters included individuals infected with subtype A1 strains and one cluster with subtype C. The main cluster within subtype A1 (I) included eight men reporting having sex with men infected with dual-class RT-resistant strains carrying both T215C and Y181C mutations. The second subtype A1 cluster (II) involved two men who have sex with men (MSM) individuals, one with a dual-class RT-resistant strain carrying T215S and K103N and one with a triple-class-resistant strain carrying T215S, K103N, and N88S mutations. The third subtype A1 cluster (III) involved three individuals including a woman from Russia and two men from Greece, one of whom is MSM. The only subtype C cluster (IV) involved one heterosexual couple, a woman from South Africa and a man from Greece. Overall, 15 out of 17 study subjects (88.2%) infected with TDR strains were implicated in transmission clusters, 10 of whom (66.7%) were MSM and were also infected with subsubtype A1 strains.
FIG. 1.
(A) Neighbor-joining tree of pol (protease and partial reverse transcriptase) sequences, constructed as described in the text. The circular brackets on the periphery of the tree indicate the determined subtypes and circulating recombinant forms (CRF) as described in the text. The tips of reference sequences are shown with black lines and the patient sequences with circles; those with drug resistance mutations are indicated with open circles. Only consensus bootstrap values greater than 70% out of 1,000 replications are shown at several nodes. The scale at the middle of each tree is used to obtain the percent divergence between any two sequences. Clusters of sequences with transmitted drug resistance mutations (TDRM; indicated with open circles) that have a significant statistical support (>85% bootstrap support) for the branch subtending the cluster and a mean genetic distance of <0.015 nucleotide substitutions per site are indicated by dotted ovals. (B) The four observed clusters of drug-resistant strains are magnified for better viewing and the drug resistance mutations associated with each sequences are depicted at the edge of the branches.
In this study, the molecular epidemiology of HIV-1 infection and TDR in newly diagnosed patients in Northern Greece is presented. Ninety-eight newly diagnosed untreated patients, representing 63.7% of the antiretroviral-naive newly diagnosed HIV-1-seropositive patients at the database of the AIDS National Reference Laboratory of Northern Greece for the period 2009 to 2010, took part in this work, providing demographic and epidemiological characteristics as well as information on risk groups and drug use behavior. The subjects were predominantly young Greek men. The nationalities of the subjects who were not Greek demonstrate the influx of young people from Eastern Europe to the region and possibly its association with neighboring countries in the Balkans.10
From retrospective studies carried out in Northern Greece, the main subtypes found were subtype B and subtypes A.5,11–13 Phylogenetic subtyping of these sequences obtained in the pol region of the HIV-1 genome confirmed the frequent presence of subtype B and A strains in this population, but subtypes C, CRF02_AG, CRF04_cpx, CRF01_01, F1, and G were also found in significantly decreased frequencies. Interestingly, 41.1% of MSM were infected with subtype A1 strains, showing that this subtype has a significant prevalence in MSM in Northern Greece, and possibly illustrating spill-over among similar risk groups in the geographic region and neighboring countries.3,10 This would have to be confirmed by phylogenetic analysis with HIV-1 sequences from comparable risk groups from these countries and other regions in Greece.10,14,15
The results of the study showed that within the group tested, the prevalence of TDR to current HIV-1 antiretroviral drugs was 16.32%, which is significantly higher than known prevalence rates of TDR in newly diagnosed patients in other European countries,16 but is comparable to previously published prevalence rates of TDR in Northern Greece.13 The phylogenetic analysis of the sequences resulted in four clusters within TDR samples, with small genetic distances (<0.015) and high bootstrap values. Two of the four clusters included MSM infected with subtype A1 TDR strains. The main cluster included eight MSM individuals, seven Greeks and one immigrant from Albania, infected with dual-class RT-resistant strains, whereas the second subtype A1 cluster included two MSM individuals, one with a dual-class RT-resistant strain and one with a triple-class-resistant strain. Interestingly, 88.2% of the patients infected with TDR strains were implicated in transmission clusters, of whom 66.7% were MSM and were also infected with subsubtype A1 strains. Although further investigation would be necessary with a more extended sampling group to obtain a more complete picture of the molecular epidemiology of HIV-1 among MSM in Northern Greece, the results obtained in this prospective study could have an impact on the development of prevention strategies for TDR for the local setting.
Acknowledgments
Preliminary parts of this work were presented at the 10th European Meeting on HIV and Hepatitis, Barcelona, Spain, 2012 (P_20) and the 11th European Meeting on HIV and Hepatitis, Rome, Italy, 2013 (P_16). We thank all participating subjects from the National AIDS Reference Laboratory of Northern Greece and the Division of Infectious Diseases Division of AHEPA University Hospital of Thessaloniki, the Bioethics Committee of the Medical School of the Aristotle University of Thessaloniki for valuable assistance, M. Frangiscou for preliminary statistical analysis, and A. Andoniadis for helpful discussions.
This work was supported by financial support from the University of Cyprus and University of Thessaloniki and the Birch Biomedical Research LLC, USA (3416-25017) awarded to L.G. Kostrikis and by the Hellenic Center for Disease Control and Prevention (grant 81227).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Robertson DL, Anderson JP, Bradac JA, et al. : HIV-1 nomenclature proposal. Science 2000;288(5463):55–56 [DOI] [PubMed] [Google Scholar]
- 2.Thomson MM. and Najera R: Molecular epidemiology of HIV-1 variants in the global AIDS pandemic: An update. AIDS Rev 2005;7(4):210–224 [PubMed] [Google Scholar]
- 3.Abecasis AB, Wensing AM, Paraskevis D, et al. : HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniadou ZA, Hezka J, Kousiappa I, et al. : Cellular HIV-1 DNA levels are equivalent among drug sensitive and drug resistant strains in newly diagnosed and antiretroviral naive patients. AIDS Res Hum Retroviruses 2013[Epub ahead of print]; DOI: 10.1089/aid.2013.0160 [DOI] [PubMed] [Google Scholar]
- 5.Skoura L, Metallidis S, Pilalas D, et al. : High rates of transmitted drug resistance among newly-diagnosed antiretroviral naive HIV patients in Northern Greece, data from 2009–2011. Clin Microbiol Infect 2013;19(3):E169–172 [DOI] [PubMed] [Google Scholar]
- 6.Kousiappa I, Achilleos C, Hezka J, et al. : Molecular characterization of HIV type 1 strains from newly diagnosed patients in Cyprus (2007–2009) recovers multiple clades including unique recombinant strains and lack of transmitted drug resistance. AIDS Res Hum Retroviruses 2011;27(11):1183–1199 [DOI] [PubMed] [Google Scholar]
- 7.Kousiappa I, van de Vijver DA, Demetriades I, and Kostrikis LG: Genetic analysis of HIV type 1 strains from newly infected untreated patients in Cyprus: High genetic diversity and low prevalence of drug resistance. AIDS Res Hum Retroviruses 2009;25(1):23–35 [DOI] [PubMed] [Google Scholar]
- 8.Bennett DE, Camacho RJ, Otelea D, et al. : Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009;4(3):e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gifford RJ, de Oliveira T, Rambaut A, et al.: Phylogenetic surveillance of viral genetic diversity and the evolving molecular epidemiology of human immunodeficiency virus type 1. J Virol 2007;81(23):13050–13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanojevic M, Alexiev I, Beshkov D, et al. : HIV1 molecular epidemiology in the Balkans: A melting pot for high genetic diversity. AIDS Rev 2012;14(1):28–36 [PubMed] [Google Scholar]
- 11.Papa A, Papadimitriou E, Papoutsi A, Kiosses V, and Antoniadis A: HIV-1 subtypes and circulating recombinant forms (CRFs) in Northern Greece. Virus Res 2002;85(1):85–93 [DOI] [PubMed] [Google Scholar]
- 12.Papa A, Papadimitriou E, Papoutsi A, Malissiovas N, Kiosses VG, and Antoniadis A: Genetic variation of the protease and reverse transcriptase genes in HIV-1 CRF04_cpx strains. AIDS Res Hum Retroviruses 2002;18(9):677–680 [DOI] [PubMed] [Google Scholar]
- 13.Skoura L, Metallidis S, Buckton AJ, et al. : Molecular and epidemiological characterization of HIV-1 infection networks involving transmitted drug resistance mutations in Northern Greece. J Antimicrob Chemother 2011;66(12):2831–2837 [DOI] [PubMed] [Google Scholar]
- 14.Paraskevis D, Magiorkinis E, Magiorkinis G, et al. : Increasing prevalence of HIV-1 subtype A in Greece: Estimating epidemic history and origin. J Infect Dis 2007;196(8):1167–1176 [DOI] [PubMed] [Google Scholar]
- 15.Salemi M, de Oliveira T, Ciccozzi M, Rezza G, and Goodenow MM: High-resolution molecular epidemiology and evolutionary history of HIV-1 subtypes in Albania. PLoS One 2008;3(1):e1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vercauteren J, Wensing AM, van de Vijver DA, et al. : Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis 2009;200(10):1503–1508 [DOI] [PubMed] [Google Scholar]