Abstract
Circulating levels of microbial products such as lipopolysaccharide (LPS) are increased in HIV infection. Microbial translocation promotes obesity, insulin resistance, and dyslipidemia in other settings. We examined data from 178 subjects: an Indiana University (IU) cross-sectional study [N=49 on antiretroviral therapy (ART), N=47 not on ART], and a 24 week prospective study of ART initiation ACTG 5152s (N=82). Pearson correlations were used to describe relationships of plasma LPS levels and soluble CD14 (sCD14), a marker of monocyte activation, with metabolic and body composition measures. HOMA-IR (a measure of insulin resistance) and LPS were correlated for the combined cohorts (r=0.19, p=0.02), particularly in the 5152s ART-naive cohort (r=0.41, p<0.01). Triglycerides were correlated with LPS in the combined cohort (r=0.32, p<0.01), and all subsets excluding the IU on ART subset. There were negative correlations between sCD14 and high-density lipoprotein (HDL) cholesterol in all subjects (r=−0.21, p<0.01), as well as the IU subset not on ART (r=−0.32, p=0.04). Large particle HDL as measured by NMR spectroscopy, but not HDL cholesterol, was negatively correlated with LPS (r=−0.18, p=0.02), particularly among the IU subset receiving ART (r=−0.33, p=0.03). In the combined cohorts, sCD14 was negatively correlated with lean mass as well as trunk and limb fat. There is a relationship between microbial translocation markers and metabolic effects, particularly lipoproteins. During prolonged ART, microbial translocation was associated with an adverse effect on large HDL and thus may contribute to the increased cardiovascular disease risk observed during chronic treatment of HIV.
Introduction
Infection with HIV-1 leads to the depletion of CD4+ T cells in gut-associated lymphoid tissues (GALT), which is associated with the translocation of microbial products, such as lipopolysaccharide (LPS), across the mucosa of the gastrointestinal tract.1,2 Although successful treatment with antiretroviral therapy (ART) increases GALT CD4+ T cells, the numbers of these cells do not return to prior levels, even when viremia is completely suppressed.1,3 Likewise, successful ART has been associated with decreased levels of microbial products in the plasma, but again, there is failure to normalize to levels seen in HIV-seronegative persons.2,4
This translocation of microbial products into the plasma leads to activation of both innate and adaptive immune responses, with chronic monocyte stimulation.5,6 Soluble CD14 (sCD14) is released at higher levels from monocytes upon stimulation with LPS as well as other molecules, and serves as a marker for monocyte activation.7,8 The innate immune response is triggered by the interaction of Toll-like receptor 4 (TLR4) with LPS binding protein (LBP) bound to LPS or to an LPS/sCD14 complex, leading to the release of proinflammatory mediators and resulting in systemic inflammation.8,9
Studies of increased levels of sCD14 and LPS have shown associations with adverse metabolic outcomes.8,10,11 Higher levels of sCD14 in apparently healthy male and female subjects are associated with increased levels of fasting triglycerides.12 In HIV-infected patients, sCD14 levels correlated with other markers of inflammation, including interleukin 6, C-reactive protein, and D-dimer.4 In these subjects, sCD14 levels also correlated with increased risk of cardiovascular disease (CVD) events, and was an independent predictor of mortality.4,7 Baseline levels of LPS and sCD14 were significantly associated with progression of subclinical atherosclerosis over 144 weeks in ART-treated subjects.13 We recently reported impaired vascular endothelial function associated with greater LPS levels among HIV-infected subjects receiving long-term ART.14
In mice, mimicking the translocation of gut bacterial products into the circulation via subcutaneous infusion of LPS has been shown to increase fasting glucose and insulin levels, liver insulin resistance, and body weight. These changes were accompanied by increases in visceral and subcutaneous adipose deposition, and greater liver triglyceride content.10,15 Moreover, there is a positive correlation between insulin and LPS levels in healthy nondiabetics and higher circulating levels of LPS are seen in type II diabetic subjects compared to nondiabetic healthy subjects.15 Microbial translocation is thought to be a key factor in the pathogenesis of type II diabetes and insulin resistance.11,15,16
There has been limited investigation into the relationships between metabolic parameters or body composition and microbial translocation in HIV-infected patients. Therefore, it is of interest to examine whether increased translocation of bacterial products may be associated with HIV-related complications such as insulin resistance, lipid and lipoprotein disorders, body fat abnormalities, and bone loss.17
Materials and Methods
Study design and subject population
Data were collected from two separate cohorts, with analyses performed on the aggregate as well as individual cohorts and prespecified subsets. AIDS Clinical Trial Group (ACTG) 5152s18 was a substudy of a prospective clinical trial ACTG 514219 that investigated three different ART regimens as initial therapy. Thus, this 5152s cohort represented an ART-naive group in need of ART who subsequently initiated therapy for 24 weeks.
The Indiana University (IU) study was a single-site cross-sectional observational study of subjects recruited from local primary HIV care clinics with ART determined by the subjects' individual practitioner as part of routine care.20 This cohort represented two distinct groups: (1) stable subjects not receiving ART, with relatively well-maintained CD4 cell counts, and (2) subjects receiving prolonged ongoing ART, with 84% undetectable HIV RNA.14 In addition to analyses of all subjects (the combined cohorts consisting of baseline 5152s subjects plus the IU cohort), prespecified subset analyses included A5152s subjects at baseline and at week 24, IU subjects on ART, and IU subjects not on ART. Major exclusion criteria for both studies included known cardiovascular disease, diabetes mellitus, and current use of lipid-lowering medications.
The ACTG study was approved by the institutional review boards at participating ACTG sites. The IU study was approved by the Indiana University Institutional Review Board. In both studies written informed consent was provided, which included consent to having blood specimens saved for possible future analyses.
Assays
Plasma samples preserved with EDTA were stored at −70°C until the time of assay. LPS was quantified using the Endpoint Chromogenic LAL assay (Lonza, Basel, Switzerland) and sCD14 measurements were performed with the Quantikine ELISA from R&D systems (Minneapolis, MN) according to the manufacturer's instructions. For both assays all samples were analyzed in triplicate in the same laboratory. Lipids, lipoproteins by nuclear magnetic resonance (NMR), glucose, insulin, and inflammatory markers were measured as originally described.18,20 In both studies, insulin sensitivity was estimated by homeostasis model assessment-insulin resistance (HOMA-IR).21
Body composition testing
In both studies whole-body and regional dual x-ray absorptiometry (DXA) scans were performed, including measurements of lean mass, bone mineral density, and limb and trunk fat.20,22 DXA scans were done at baseline and week 48 in ACTG 5142.22 Additionally, in the IU study, a single-slice computed tomography (CT) of the abdomen at L4–5 was performed to estimate visceral and subcutaneous fat.20
Statistical analyses
Continuous variables are summarized by mean±standard deviation (SD) or median [interquartile range (IQR)] and categorical variables are summarized by frequency and percentage. Changes of LPS and sCD14 over 24 weeks for the A5152 cohort are tested by paired t test. Pearson correlation coefficients were used to characterize the correlation between metabolic/body composition variables and LPS and sCD14. The variables examined in both cohorts included regional and whole body DXA measures, standard lipid profile [total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, calculated low-density lipoprotein (LDL) cholesterol], lipoproteins by NMR spectroscopy (including LDL and total and large HDL particle concentrations), HOMA-IR, high-sensitivity C-reactive protein, soluble vascular cell adhesion molecule 1, adiponectin, CD4+ cell count, and levels of HIV RNA. Subjects who had any metabolic or body composition variable tested and who also had LPS or sCD14 measurements available were included for analysis. In the A5152s subjects, a week 24 DXA was not performed so the week 48 DXA variables were correlated with the week 24 LPS and sCD14 levels. A two-sided p-value of<0.05 was considered statistically significant. Analyses were not corrected for multiple comparisons in this exploratory study. All analyses were performed using SAS 9.2 (SAS Inc., Cary, NC).
Results
Subject characteristics
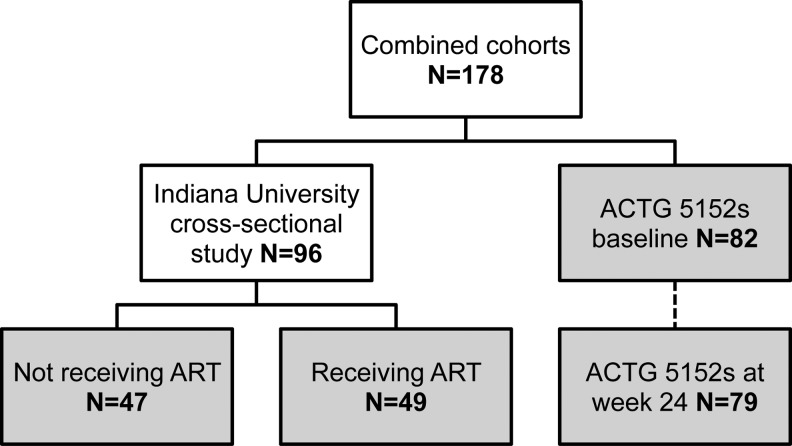
A total of 178 subjects were included in the analysis, including 96 from the IU study (47 not receiving ART, and 49 receiving ART) and 82 from ACTG 5152s (Table 1; Fig. 1). In the IU study, among subjects not on ART, the mean age was 37.0 years with 81% males, 60% white non-Hispanic, 38% black, and 2% Hispanic. Among subjects on ART in the IU study, the mean age was 40.3 years, with 65% males, 57% white non-Hispanic, 35% black, and 8% Hispanic. Subjects receiving ART had a mean of 39.6±29.5 months on treatment, with a median sCD14 of 1.67×106 pg/ml (IQR 1.53–1.97) and median LPS of 27.6 pg/ml (IQR 17.4–38.8). Subjects not on ART had a median sCD14 of 1.68×106 pg/ml (IQR 1.38–2.03) and LPS of 21.4 pg/ml (IQR 12.9–30.2).
Table 1.
Subject Characteristics
| 5152s baseline | 5152s week 24 | IU on drug | IU not on drug | |
|---|---|---|---|---|
| N | 82 | 79 | 49 | 47 |
| Age, years (mean) | 35.6±8.3 | — | 40.3±8.1 | 37.0±11.5 |
| Male sex | 76 (92%) | — | 32 (65%) | 38 (81%) |
| Race/ethnicity | ||||
| White non-Hispanic | 45 (55%) | — | 28 (57%) | 28 (60%) |
| Hispanic | 12 (15%) | — | 4 (8%) | 1 (2%) |
| Black | 23 (28%) | — | 17 (35%) | 18 (38%) |
| Unknown | 2 (2%) | — | ||
| Time on ART | — | 24 weeks | 39.6±29.5 months [46] | — |
| BMI, kg/m2 | 25.1 (22.8–27.7) [77] | 26.0 (23.5–28.4) [73] | 25.5 (21.7–28.6) [48] | 25.3 (22.4–28.0) [47] |
| HOMA-IR | 1.37 (0.78–2.33) [73] | 1.42 (0.02–2.18) [73] | 2.29 (1.60–2.76) [43] | 2.39 (1.96–3.41) [44] |
| Total cholesterol, mg/dl | 144 (128–163) [81] | 180 (159–217) [79] | 182 (164–221) [43] | 155 (136–169) [44] |
| Triglycerides | 113 (90–178) [81] | 177 (100–275) [79] | 129 (85–182) [48] | 99 (77–157) [47] |
| HDL cholesterol | 31 (26–36) [80] | 41 (34–50) [76] | 47 (35–55) [45] | 38 (28–45) [45] |
| LDL cholesterol | 88 (66–102) [78] | 96 (81–118) [68] | 104 (90–132) [45] | 95 (72–110) [44] |
| HDL particles, nmol/liter | 22.7 (19.9–26.1) [80] | 29.0 (25.2–33.2) [76] | 25.6 (22.8–28.8) [49] | 23.1 (19.5–26.3) [44] |
| Large HDL particles | 3.2 (2.0–4.9) [80] | 4.2 (1.6–7.3) [76] | 4.1 (2.0–7.3) [49] | 3.4 (1.8–4.4) [44] |
| LDL particles | 1124 (874–1298) [80] | 1268 (995–1602) [76] | 1103 (881–1317) [49] | 1060 (821–1352) [44] |
| CD4+ cells/mm3 | 245 (119–356) [78] | 376 (252–557) [79] | 464 (368–735) [48] | 335 (218–546) [47] |
| Log10 HIV RNA copies/ml | 4.93±0.63 [82] | 1.95±0.74 [79] | 2.85±0.72 [46] | 4.22±1.00 [44] |
| % HIV RNA <400 copies/ml | 0 [82] | 92 [79] | 85 [46] | 9 [44] |
| sCD14 (106 pg/ml) | 2.12 (1.81–2.57) [82] | 2.08 (1.60–2.55) [79] *p=0.4 | 1.67 (1.53–1.97) [49] | 1.68 (1.38–2.03) [42] |
| LPS (pg/ml) | 28.8 (16.5–42.0) [82] | 35.1 (17.8–56.6) [79] *p=0.02 | 27.6 (17.4–38.8) [47] | 21.4 (12.9–30.2) [40] |
p-value for the change from baseline to week 24.
Values are mean±SD or median (25–75% percentile). Numbers in brackets represent the number of subjects who had that variable measured.
ART, antiretroviral therapy; sCD14, soluble CD14; LPS, lipopolysaccharide; IU, Indiana University; BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
FIG. 1.
Numbers of subjects in the cohorts.
In ACTG 5152s, the mean age was 35.6 years, with 92% males, 55% white non-Hispanic, 28% black, 15% Hispanic, and 2% unknown. At 24 weeks, median CD4+ cell count rose to 376 cells/mm3 and mean log10 HIV-1 RNA dropped to 1.95 copies/ml with 92% having less than 400 HIV RNA copies/ml. Baseline median LPS was 28.8 pg/ml (IQR 16.5–42.0), which rose to 35.1 pg/ml (IQR 17.8–56.6) at 24 weeks (p=0.02).
Correlations between variables
There was a weak positive correlation between LPS and HOMA-IR in the entire group (r=0.19, p=0.02), possibly driven by the stronger correlation seen among treatment-naive participants in A5152s (r=0.41, p<0.01). LPS was positively correlated with triglycerides in the whole group (r=0.32, p<0.01) and in all subsets except for the IU subset not on ART. Triglycerides and sCD14 were also positively correlated among subjects not on ART from the IU study (r=0.35, p=0.02) but not in the combined cohorts or in other subsets.
A negative correlation was seen between sCD14 and HDL cholesterol in the whole group (r=−0.21, p<0.01), with the strongest correlation occurring among IU subjects not on ART. The whole group also showed a weak negative correlation between LPS and large HDL particle concentration (r=−0.18, p=0.02), which was strongest in the IU subset receiving ART (r=−0.33, p=0.03). The 5152s subset at week 24 of ART showed a similar, but not statistically significant, magnitude of correlation. There were no significant correlations observed between LPS or sCD14 and total HDL particle number (data not shown).
We found no significant correlation (p-values all>0.10) between body mass index (BMI) and either sCD14 or LPS levels (Table 2), with a single exception for the A5152s subjects with sCD14 at week 24 (r=−0.24, p=0.04). We further evaluated for a quadratic relationship between BMI and either sCD14 or LPS levels and no significant associations were detected in the full cohort or any subgroup (data not shown). Thus, we did not perform statistical adjustment for BMI for any of the metabolic variables.
Table 2.
Pearson Correlations Between Selected Metabolic/Body Composition Variables and Markers of Microbial Translocation
| All subjects | 5152s baseline | 5152s week 24 | IU on drug | IU not on drug | |
|---|---|---|---|---|---|
| Total N in group | 178 | 82 | 79 | 49 | 47 |
| HOMA-IR | |||||
| LPS | 0.19 [151] p=0.02 | 0.41 [73] p<0.01 | 0.06 [73] p=0.6 | −0.07 [41] p=0.6 | 0.22 [37] p=0.2 |
| sCD14 | −0.08 [155] p=0.3 | 0.1 [73] p=0.4 | 0.01 [73] p=1.0 | −0.10 [43] p=0.5 | −0.10 [39] p=0.5 |
| Triglycerides | |||||
| LPS | 0.32 [167] p<0.01 | 0.35 [81] p<0.01 | 0.81 [79] p<0.01 | 0.24 [46] p=0.1 | 0.33 [40] p=0.04 |
| sCD14 | 0.14 [171] p=0.07 | 0.13 [81] p=0.2 | 0.10 [79] p=0.4 | −0.09 [48] p=0.5 | 0.35 [42] p=0.02 |
| HDL cholesterol | |||||
| LPS | −0.12 [162] p=0.1 | 0.05 [80] p=0.7 | −0.01 [76] p=0.9 | −0.26 [43] p=0.09 | −0.08 [39] p=0.7 |
| sCD14 | −0.21 [166] p<0.01 | −0.08 [80] p=0.5 | 0.11 [76] p=0.3 | 0.18 [45] p=0.2 | −0.32 [41] p=0.04 |
| Large HDL particles | |||||
| LPS | −0.18 [165] p=0.02 | −0.13 [81] p=0.3 | −0.21 [76] p=0.07 | −0.33 [47] p=0.03 | −0.06 [37] p=0.7 |
| sCD14 | −0.04 [169] p=0.6 | −0.02 [81] p=0.9 | 0.24 [76] p=0.03 | 0.14 [49] p=0.3 | −0.29 [39] p=0.07 |
| BMI | |||||
| LPS | 0.02 [163] p=0.81 | −0.01 [77] p=0.90 | 0.15 [72] p=0.20 | 0.09 [46] p=0.55 | 0.02 [40] p=0.88 |
| sCD14 | −0.12 [167] p=0.12 | −0.12 [77] p=0.31 | −0.24 [72] p=0.04 | −0.04 [48] p=0.78 | −0.25 [42] p=0.11 |
| VAT by L4–5 CT scan | |||||
| LPS | 0.22 [82] p=0.051 | Not performed | Not performed | 0.20 [43] p=0.2 | 0.22 [39] p=0.2 |
| sCD14 | −0.09 [86] p=0.4 | −0.10 [45] p=0.5 | −0.06 [41] p=0.7 | ||
| Limb fat by DXAa | |||||
| LPS | −0.06 [168] p=0.9 | 0.02 [79] p=0.9 | 0.10 [70] p=0.4 | 0.11 [46] p=0.5 | −0.15 [40] p=0.4 |
| sCD14 | −0.32 [172] p<0.0001 | −0.03 [79] p=0.8 | −0.06 [70] p=0.6 | 0.03 [48] p=0.9 | −0.11 [42] p=0.5 |
| Trunk fat by DXAa | |||||
| LPS | −0.01 [164] p=0.9 | −0.04 [82] p=0.7 | 0.17 [70] p=0.2 | 0.17 [44] p=0.3 | 0.04 [38] p=0.8 |
| sCD14 | −0.34 [168] p<0.0001 | −0.01 [82] p=1.0 | −0.16 [70] p=0.2 | −0.02 [46] p=0.9 | −0.13 [40] p=0.4 |
| Total bone mineral densitya | |||||
| LPS | −0.01 [165] p=0.9 | 0.02 [79] p=0.9 | −0.02 [68] p=0.9 | 1.0 [46] p=1.0 | −0.14 [40] p=0.4 |
| sCD14 | 0.11 [169] p=0.16 | 0.25 [79] p=0.03 | −0.08 [68] p=0.5 | −0.01 [48] p=0.9 | −0.19 [42] p=0.2 |
| Total lean body massa | |||||
| LPS | −0.07 [168] p=0.38 | 0.003 [82] p=0.98 | 0.09 [70] p=0.44 | −0.05 [46] p=0.7 | 0.21 [40] p=0.2 |
| sCD14 | −0.42 [172] p<0.0001 | −0.16 [82] p=0.14 | −0.15 [70] p=0.21 | −0.09 [48] p=0.5 | 0.08 [42] p=0.6 |
For the 5152s subjects at week 24, these represent correlations with the week 48 DXA measurements.
Numbers in brackets represent the number of subjects with both variables measured. p-values of<0.05 are shown in bold.
sCD14, soluble CD14; LPS, lipopolysaccharide; HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein; VAT, visceral adipose tissue; DXA, dual x-ray absorptiometry; IU, Indiana University; ART, antiretroviral therapy; BMI, body mass index.
For the group as a whole, sCD14 correlated negatively with both trunk and limb fat as well as lean mass by DXA (Table 2). However, no correlations between these measures were seen in any of the subgroups. The 5152s subset did show a significant positive correlation between sCD14 and total bone mineral density at baseline (r=0.25, p=0.03), but this association was not observed at week 48 nor was it seen in the whole group or any other subset. LPS levels did not correlate with any body composition measures. There were no significant correlations between visceral fat by CT scan (performed only in the IU cohort) and either LPS or sCD14. Changes in regional fat and in total bone mineral density at week 48 in the 5152s cohort also did not correlate with translocation markers (data not shown).
There was no correlation between LPS and sCD14 levels in the whole group (r=0.11, p=0.16, N=169) nor in any subgroup (data not shown).
Discussion
Our results suggest a relationship between microbial translocation and metabolic effects, particularly affecting lipoproteins and lipids, including large HDL particles and triglycerides. The modest association between LPS levels and insulin resistance was most evident in those with more advanced HIV disease, i.e., those about to initiate ART in A5152s. Overall, LPS was more frequently associated with metabolic changes than sCD14, perhaps making it a generally better marker for these effects in this setting.
Triglyceride levels were consistently positively correlated with LPS levels for the group as a whole and in all subsets except in those receiving prolonged ART in the IU cohort. HDL cholesterol and LPS were not significantly correlated in any cohort or subset, but a significant negative correlation between large HDL particles measured by NMR spectroscopy and LPS was seen in the subset receiving prolonged ART. This association is of concern because greater levels of large HDL particles are associated with a reduced risk of CVD and of insulin resistance,23 and lower levels of large HDL are associated with greater coronary artery endothelial dysfunction.24 Moreover, baseline large HDL was the lipoprotein subclass most associated with decreased risk of incident coronary heart disease events in HIV-infected subjects in the SMART study.25 Together, this raises the possibility that persistent microbial translocation during prolonged ART may contribute to the increased CVD risk seen during chronic treatment of HIV in part due to adverse effects on HDL particles.
LPS in the plasma is transferred from HDL to LDL by LPS-binding protein and promotes the formation of LDL–HDL complexes. This may lead to dyslipidemia and HDL remodeling,26 and is a possible explanation of the correlation between HDL changes and bacterial product translocation. In addition, systemic inflammation increases LPS-binding protein levels, which may lead to perpetuation of the cycle of inflammation and dyslipidemia.26
Correlations between LPS and insulin resistance estimated by HOMA-IR were strongest among subjects in the group with the most advanced HIV disease and lowest CD4+ cell count, i.e., the 5152s baseline subset. Adipocytes may play a role in mediating the relationship between LPS levels and HOMA-IR. LPS binds Toll-like receptor 4 present on adipocytes, activating NF-κB and triggering the local release of inflammatory cytokines.27 This LPS-induced release of proinflammatory molecules from preadipocytes and macrophages may trigger insulin resistance in adipocytes.11
There was a significant negative correlation between sCD14 levels and lean mass and limb and trunk fat in the group as a whole. This suggests that greater levels of microbial translocation and/or monocyte activation may play a role in muscle and fat loss during HIV infection. However, a similar relationship was not detected in any of the subgroups. Therefore, it is not clear from our data if this is an effect seen primarily in treated, as opposed to untreated, HIV infection. Other than a single modest correlation between sCD14 and bone mineral density in one subgroup, we did not find evidence in our study that microbial translocation is a major determinant of bone loss in patients with HIV infection.
There was no relationship between LPS levels and sCD14, as has been reported by others.4,13,28 We observed that LPS was more frequently associated with lipoprotein and lipid abnormalities and insulin resistance, while sCD14 was more frequently associated with body composition variables. Soluble CD14 is released from monocytes by LPS stimulation and is considered a marker for monocyte activation,7,8 but the lack of correlation between LPS and sCD14 suggests these two markers may to a certain extent reflect different pathologic processes. Other possibilities include heterogeneity in interindividual responses to the effect of LPS on monocytes, variability in the rates of clearance of LPS from the circulation, poor correlation of fasting LPS levels with overall systemic LPS exposure, and release of sCD14 from other sources such as hepatocytes.29 There was a moderate difference in sCD14 levels between the ACTG subjects and IU subjects, which would be expected in more advanced HIV disease seen in the ACTG subjects as demonstrated by these subjects' lower CD4+ T cell counts.
This study was limited by the relatively small numbers of subjects in the subsets. The IU study was cross-sectional and did not evaluate changes in measurements over time, therefore it cannot establish the temporal sequence of changes in the markers explored. The ACTG 5152s study was longitudinal, but only of 24 weeks duration, so it may not have been able to identify changes that might have developed over a longer period of treatment with continued ART. In addition, the p-values for the correlations in this study were not corrected for multiple comparisons, therefore, the findings from this analysis should be considered exploratory and hypothesis generating. However, among the 90 correlations reported in this study, 16 have a p-value less than 0.05, which is much larger than what would be expected by chance (i.e., four significant correlations). We did not measure T-lymphocyte activation in this study, which could have provided additional information and could be an area for further investigation.
In the setting of HIV infection, microbial translocation may contribute to elevated levels of triglycerides and insulin resistance. Among HIV-infected individuals during prolonged ART, greater microbial translocation was associated with lower levels of large HDL particles. These metabolic effects may contribute to the increased risk of CVD seen in individuals during chronic HIV treatment. Greater levels of monocyte activation, as reflected by sCD14 levels, were associated with decreased lean and fat mass. Additional studies are needed to establish causal relationships, as well as investigating the possible metabolic and body composition benefits of interventions that reduce microbial translocation and/or monocyte activation in HIV-infected individuals.
Acknowledgments
This work was supported by grants AI091492, HL72711, RR00750, and RR16176 from the NIH and a pilot grant from the Southern California Clinical Translational Science Institute RR031986. The authors gratefully acknowledge the contributions of all the subjects and the members of the ACTG 5142 and 5152s protocol teams as well as the staff of the Indiana University General Clinical Research Center, and to Kathy L. Clayton and Gina-Bob Dubé for managing the references. Results were presented in part at the 13th International Workshop on Adverse Drug Reactions and Co-morbidities in HIV, Rome, Italy, July 2011.
Author Disclosure Statement
S.K.G. has received unrestricted research grants from Merck & Co., Janssen/Tibotec Therapeutics, and Gilead Sciences, has received compensation for one lecture in 2012 from Merck & Co. pertaining to HIV-related renal disease, and has received travel support from Gilead Sciences in 2011 to present data for a tenofovir-related study. J.H.S. serves on data and safety monitoring boards for Abbott, Lilly, and Takeda. M.P.D. receives research support from Gilead Sciences and Serono and has served as a consultant to Serono.
References
- 1.Brenchley JM, Price DA, and Douek DC: HIV disease: Fallout from a mucosal catastrophe? Nat Immunol 2006;7(3):235–239 [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Lederman MM, Hunt P, et al. : Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009;199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guadalupe M, Reay E, Sankaran S, et al. : Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003;77(21):11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203(6):780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12(12):1365–1371 [DOI] [PubMed] [Google Scholar]
- 6.Bukh AR, Melchjorsen J, Offersen R, et al. : Endotoxemia is associated with altered innate and adaptive immune responses in untreated HIV-1 infected individuals. PLoS One [Electronic Resource]. 2011;6(6):e21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, and Espevik T: Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: Correlation to disease progression and clinical events. Blood 1998;92(6):2084–2092 [PubMed] [Google Scholar]
- 8.Sun L, Yu Z, Ye X, et al. : A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 2010;33(9):1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffler A. and Scholmerich J: Innate immunity and adipose tissue biology. Trends Immunol 2010;31(6):228–235 [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Amar J, Iglesias MA, et al. : Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56(7):1761–1772 [DOI] [PubMed] [Google Scholar]
- 11.Manco M, Putignani L, and Bottazzo GF: Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010;31(6):817–844 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Real JM, Broch M, Richart C, Vendrell J, Lopez-Bermejo A, and Ricart W: CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab 2003;88(4):1780–1784 [DOI] [PubMed] [Google Scholar]
- 13.Kelesidis T, Kendall MA, Yang OO, Hodis HN, and Currier JS: Biomarkers of microbial translocation and macrophage activation: Association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012;206(10):1558–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blodget E, Shen C, Aldrovandi G, et al. : Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One 2012;7(8):e42624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creely SJ, McTernan PG, Kusminski CM, et al. : Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292(3):E740–747 [DOI] [PubMed] [Google Scholar]
- 16.Kelly CJ, Colgan SP, and Frank DN: Of microbes and meals: The health consequences of dietary endotoxemia. Nutr Clin Pract 2012;27(2):215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinspoon S. and Carr A: Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352(1):48–62 [DOI] [PubMed] [Google Scholar]
- 18.Torriani FJ, Komarow L, Parker RA, et al. : Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008;52(7):569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddler SA, Haubrich R, DiRienzo AG, et al. : Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008;358(20):2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubé MP, Shen C, Greenwald M, Mather KJ, and Gupta SK: Relationship of body composition, metabolic status, antiretroviral use, and HIV disease factors to endothelial dysfunction in HIV-infected subjects. AIDS Res Hum Retroviruses 2010;26(8):847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor DF, Treacher DF, and Turner RC: Homeostasis model assessment: Insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 22.Haubrich RH, Riddler SA, DiRienzo AG, et al. : Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009;23(9):1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, and Ridker PM: Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119(7):931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford MA, McConnell JP, Lavi S, et al. : Coronary artery endothelial dysfunction is positively correlated with low density lipoprotein and inversely correlated with high density lipoprotein subclass particles measured by nuclear magnetic resonance spectroscopy. Atherosclerosis 2009;207(1):111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duprez DA, Kuller LH, Tracy R, et al. : Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 2009;207(2):524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levels JHM, Marquart JA, Abraham PR, et al. : Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect Immun 2005;73(4):2321–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song MJ, Kim KH, Yoon JM, and Kim JB: Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006;346(3):739–745 [DOI] [PubMed] [Google Scholar]
- 28.Burdo TH, Lo J, Abbara S, et al. : Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204(8):1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meuleman P, Steyaert S, Libbrecht L, et al. : Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta 2006;366(1–2):156–162 [DOI] [PubMed] [Google Scholar]