Abstract
We examined the involvement of membrane microdomains during human luteinizing hormone (LH) receptor recovery from receptor desensitization after removal of bound hormone. Lateral motions of individual desensitized LH receptors expressed on the surface of Chinese hamster ovary (CHO) cells and transient association of these receptors with detergent-resistant membrane (DRM) microdomains isolated using isopyncnic sucrose gradient ultracentrifugation were assessed. Single particle tracking experiments showed untreated individual LH receptors to be confined within cell-surface membrane compartments with an average diameter of 199 ± 17 nm and associated with membrane fractions characteristic of bulk plasma membrane. After brief exposure to human chorionic gonadotropin (hCG), LH receptors remained for several hours desensitized to hCG challenge. Throughout this period, significantly increased numbers of LH receptors were confined within smaller diameter (<120 nm) membrane compartments and associated with detergent-resistant membrane (DRM) fragments of characteristically low density. By 5 hours, when cells again produced cAMP in response to hCG, unoccupied LH receptors were found in larger 169 ± 22 nm diameter cell-surface membrane compartments and >90% of LH receptors were again found in high density membrane fragments characteristic of bulk plasma membrane. Taken together, these results suggest that, during recovery from LH receptor desensitization, LH receptors are both located with DRM lipid environments and confined within small, mesoscale (80–160 nm) cell-surface compartments. This may reflect hormone-driven translocation of receptors into DRM and formation there of protein aggregates too large or too rigid to permit effective signaling. Once bound hormone is removed, receptor structures would have to dissociate before receptors can again signal effectively in response to hormone challenge. Moreover, such larger protein complexes would be more easily constrained laterally by membrane structural elements and so appear resident in smaller cell surface compartments.
Keywords: Luteinizing hormone receptor, luteinizing hormone, receptor desensitization, human chorionic gonadotropin, single particle tracking, plasma membrane microdomains, lipid raft, lateral diffusion
Introduction
Desensitization of human luteinizing hormone (LH) receptors is a key regulatory event in the mammalian reproductive cycle. After binding the glycoprotein hormone LH or its homolog human chorionic gonadotropin (hCG), LH receptors become unresponsive to further hormone challenges. Desensitization is a common feature shared by many G protein-coupled receptors (GPCRs) including the well-characterized β2-adrenergic receptor (β2-AR) [1]. LH receptors become desensitized in human ovarian follicles in response to the mid-cycle LH surge that promotes ovulation and, in human corpora lutea, in response to elevated levels of hCG during pregnancy. Desensitization of LH receptors is followed by a decrease in intracellular cyclic adenosine monophosphate (cAMP) levels and appears to be functionally important for oocyte meiosis [2].
We have shown previously that LH receptor association with detergent-resistant membrane (DRM) microdomains requires both binding of ligand and formation of a functional hormone-receptor complex [3]. Likewise, confinement of individual LH receptors in small membrane microdomains is dependent on hCG concentration [4]. Unlike the β2-AR, which is rapidly sequestered in membrane microdomains [5] and then internalized, desensitization and resensitization of LH receptors occurs over several hours without an apparent decrease in receptor density at the cell surface [6; 7] and does not appear to involve receptor phosphorylation [8]. However, neither the fate of individual LH receptors desensitized by hCG nor the possible association of LH receptors with DRM microdomains has been examined during LH receptor recovery from desensitization. Here we examine whether, following transient hCG exposure and subsequent removal of hormone, individual LH receptors are confined in membrane compartments that differ from compartments containing hormone-responsive receptors.
To evaluate the motions of individual LH receptors, single particle tracking (SPT) methods were used. Unlike fluorescence recovery after photobleaching (FRAP) and related approaches that measure the average lateral diffusion of large populations of LH receptors [9], SPT monitors lateral diffusion of individual LH receptors on viable cells. Expressing LH receptors with the extracellular FLAG epitope permits evaluation both of LH receptor diffusion rates during receptor recovery from hormone desensitization and of the relative number of LH receptors confined in smaller mesoscale compartments [10].
We also isolated detergent-resistant membrane fragments (DRM) from CHO cells where LH receptors had been desensitized by transient exposure to hCG followed by removal of bound hormone. DRM or rafts are a highly heterogeneous group of membrane microdomains that are defined by their ability to “float” in sucrose gradients. Although the relationship between rafts isolated using isopycnic sucrose gradient centrifugation and structures present on living cells remains a topic of debate [11], there is agreement that “rafts” observed in vitro may be transiently organized in vivo membrane structures that range in size from small collections of membrane lipids and proteins to larger, >10 nm-scale entities [12]. These membrane regions are enriched in cholesterol and sphingomyelin [13] and may, on intact cells, fuse with one another to form larger signalling platforms [14] or become nested in actin-delineated membrane compartments due to interactions between components of these microdomains and the actin cytoskeleton [10]. Fusion of membrane compartments or nesting of membrane microdomains in actin-delineated compartments may explain the formation of large, microscopically-visible, lipid-protein complexes following binding of ligand to LH receptors [6; 15; 16]. Inclusion of LH receptors in rafts as well as confinement of the receptor in membrane microdomains is of interest because the slow dissociation of multi-component complexes on the membrane may regulate the availability of molecules needed for receptor-mediated signalling and may explain, at least in part, the long time course of LH receptor recovery from desensitization following the LH surge.
Materials and Methods
Materials and cell culture
A stable CHO cell line expressing human LH receptor N-terminally coupled to the FLAG epitope was prepared as previously described [3]. Cells expressing FLAG-tagged LH receptors were maintained in medium in high glucose Dulbecco’s Modification of Eagle’s Medium (MEM) (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units of penicillin/mL, 100 μg streptomycin/mL (Gemini Bio-Products, Woodland, CA), 400 μg/mL G418 sulfate and non-essential amino acid solution (Sigma-Aldrich, Inc., St. Louis, MO). Cells were grown in 5% CO2 at 37 °C in a humidified environment. Geneticin (G418 sulfate) was purchased from Mediatech, Inc. (Manassas, VA). Intact, highly pure hCG (Fitzgerald Industries, Inc., Concord, MA) was prepared in 1x PBS. Forskolin, pFLAG vector and monoclonal anti-FLAG antibody directed against the FLAG epitope tag were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). 40 nm gold colloid was obtained from Ted Pella, Inc. (Redding, CA). Intracellular cAMP was measured using a TiterFluor Direct Cyclic AMP Enzyme Immunoassay Kit purchased from Assay Designs (Ann Arbor, MI).
LH receptor desensitization
Receptor desensitization was induced by treating cells with 100 nM hCG for 30 minutes beginning at time 0. A pH 3 buffer was then used to remove hCG from receptors as previously described [6; 7; 17]. Incubating CHO cells with hCG and removing the hormone typically took approximately 45 min regardless of whether cells were in solution or grown on coverslips. Except as noted, cells were not subsequently re-exposed to hCG. Previous work has shown that LH receptors desensitized using this method remain localized at the cell surface [6] without significant internalization [4] Moreover, previously desensitized LH receptors are able to bind fresh hCG after initial hCG treatment and removal [6].
cAMP assays
Over the 5 hr period during which receptors recovered from hormone-induced desensitization, we examined the confinement of individual LH receptors in small membrane compartments on viable cells or the association of receptors with DRM fragments. To evaluate LH receptor desensitization and recovery from desensitization, intracellular cAMP levels were assessed at 1 hr intervals following initiation of LH receptor desensitization at time zero. At times of 1 to 5 hrs after the initial introduction of hCG at time 0, fresh 100 nM hCG was added to cell suspensions for 30 minutes prior to measuring cAMP levels using the TiterFluor Direct Cyclic AMP Enzyme Immunoassay Kit from Assay Designs (Ann Arbor, MI). In otherwise untreated cells, cAMP levels in response to hCG were compared with cAMP response to 20 nM forskolin. We used the lowest possible concentration of forskolin that produced a maximal cAMP response in CHO cells. The cAMP response to 100 nM hCG was typically low, presumably because phosphodiesterase inhibitors were not used. These were omitted to reduce the possibility of unintended receptor redistribution in the plasma membrane and subsequent effects on LH receptor-mediated signaling. When phosphodiesterase inhibitors are present, it is difficult to determine whether receptor association with DRM fragments or confinement in membrane compartments results from brief hCG exposure or, alternatively, from effects of the phosphodiesterase inhibitor on membrane structure. We have, for example, examined effects of vanadium compounds that are known to inhibit phosphodiesterase activity and shown that these compounds alone result in insulin receptor association with DRM fragments [18; 19].
Single particle tracking of individual FLAG-tagged LH receptors
We examined the effects of receptor desensitization on the lateral dynamics and confinement of individual LH receptors in cell-surface plasma membrane compartments using single particle tracking methods similar to those previously described by Kusumi and colleagues [20]. CHO cells expressing FLAG-tagged human LH receptors were grown on coverslips to 50–75% confluence. Coverslips were placed in 60 cm2 petri dishes, washed with PBS prior to addition of 100 nM hCG for 30 minutes at 37 °C. Hormone-treated cells were then washed with fresh PBS to remove unbound hormone and subsequently treated with low pH buffer to remove receptor-bound hormone as described above. To identify FLAG-LHR, 40 nm nanogold particles were conjugated with a mixture of anti-FLAG monoclonal antibody and bovine serum albumin (BSA) at the lowest possible total protein concentration, typically 40 μg/mL, needed to stabilize the gold colloid. The ratio of antibody to BSA, typically 1:100 by weight, was selected to provide approximately 10–20 gold particles per cell. When cells were preincubated with a 10-fold excess of anti-FLAG antibody, no cell-bound gold particles were detected indicating that the binding of anti-FLAG antibody was specific for FLAG-tagged LH receptors.
Individual gold nanoparticles were imaged by differential interference contrast using a 1.4 N.A. 63x oil objective in a Zeiss Axiovert 135 TV inverted microscope. Images were acquired using a Dage IFG-300 camera and were recorded for two minutes (3600 frames) at approximately 30 nm/pixel under the control of Metamorph software (Molecular Devices Corp.). Trajectories for individual gold particles were segmented into compartments by calculation of statistical variance in particle position within windows of varying duration [21]. These results were analyzed using custom analysis programs to yield average compartment sizes and corresponding residence times for individual particles. Effective macroscopic diffusion coefficients (D) were then calculated as the square of the compartment diagonal divided by four times the residence time in the compartment as previously described [22].
Discontinuous sucrose-gradient isopycnic ultracentrifugation and Western blotting
The possible association of LH receptors with membrane rafts during receptor recovery from desensitization was explored by separating membrane fragments according to buoyant density by discontinuous sucrose gradient isopycnic ultracentrifugation using previously published methods [23]. Approximately 5 × 107 CHO cells expressing LH receptors with N-terminal FLAG epitopes were removed from cell suspensions treated with 100 nM hCG for 30 minutes at 37 °C and subsequently treated with low pH buffer to remove bound hormone from the receptor. A 40% sucrose solution containing membrane fragments was then layered within a discontinuous sucrose gradient formed from equal volumes of sucrose solutions containing 10% – 80% sucrose. After centrifugation of the sucrose gradient at 175,000 × g for 20 hours at 4 °C in a Beckman Coulter Optima XL-80K Preparative Ultracentrifuge (Beckman Coulter, Inc., Fullerton, CA), eighteen equal fractions were collected from the top of the gradient (10% sucrose) downward. FLAG-tagged LH receptors were identified in each fraction using anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, Inc., St. Louis, MO). For each treatment condition, the relative amount of receptor in each of the 18 fractions was measured using a Bio-Rad GS-800 calibrated densitometer (Bio-Rad Laboratories, Inc., Hercules, CA). To evaluate possible significant loss of receptors from membrane fragments in the above experiments, the optical densities of bands in fractions 1–18 were summed and expressed as a percentage of the total amount of receptor recovers for an equivalent number of untreated cells in which membrane fragments had been separated on sucrose gradients.
Statistical Analysis of Data
Data are presented either as the arithmetic mean ± 1 standard error of mean (SEM) or arithmetic mean ± 1 standard deviation (SD) as indicated in the figure legends. Significance was assessed using Student’s t-tests and p values are indicated in both tables and figure legends.
Results
Transient exposure to hCG desensitizes LH receptors
To evaluate LH receptor signalling competency following transient exposure to hCG, intracellular cAMP levels were assessed at 1 hr intervals for up to 5 hrs following the initial introduction of hCG at time 0. Fresh 100 nM hCG was introduced to cells for 30 minutes at 37 °C prior to initiating measurements of intracellular cAMP levels. At times 1, 2, 3 or 4 hours following the initiation of LH receptor desensitization at time 0, hCG challenge did not cause significant increases in cAMP over basal levels. At 5 hours following initiation of LH receptor desensitization, hormone challenge resulted in an average 3.5-fold increase in cAMP which was comparable to the cAMP response observed in otherwise untreated cells exposed to 100 nM hCG (Table 1). However, significant variations in cAMP levels at this time suggested that the time required for recovery from LH receptor desensitization was highly variable.
Table 1.
Recovery of FLAG-LH receptor cAMP response to hCG after desensitizationa
| Treatment | Recovery time before measurement | cAMP conc Treatment (pmol/mL)b | n |
|---|---|---|---|
| None | - | 14 ± 2.71 | 8 |
| 100 nM hCG | - | 33 ± 142 | 5 |
| 20 nM forskolin | - | 49 ± 182 | 5 |
| 100 nM hCG; remove hormone from receptors via low pH wash; allow indicated recovery time before measurement | 1 hr | 11 ± 2.41 | 6 |
| 2 hr | 6.9 ± 2.91 | 2 | |
| 3 hr | 3.0 ± 0.71 | 3 | |
| 4 hr | 3.6 ± 0.11 | 2 | |
| 5 hr | 49 ± 46 | 6 |
Data shown are the means ± S.E.M. of the indicated numbers n of experiments.
Values with superscripts 1 and 2 differ significantly (p<0.05).
Desensitized LH receptors are confined in mesoscale cell-surface membrane compartments
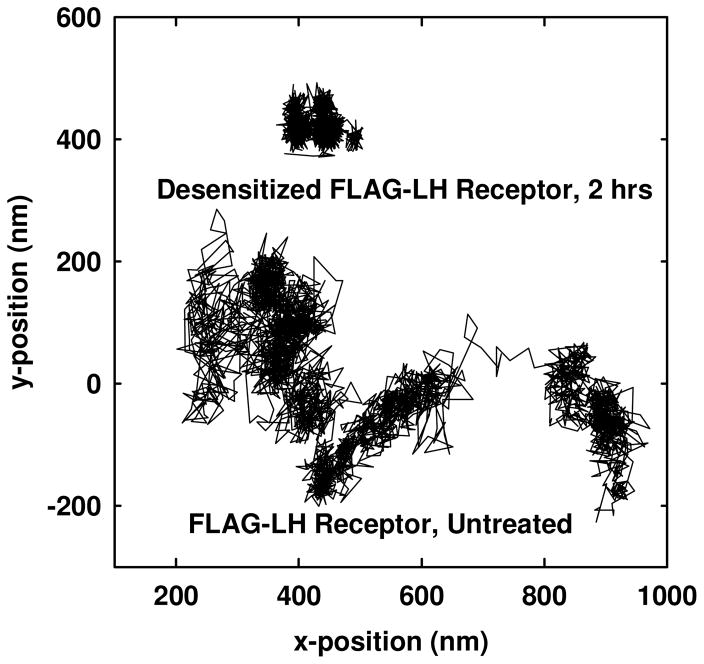
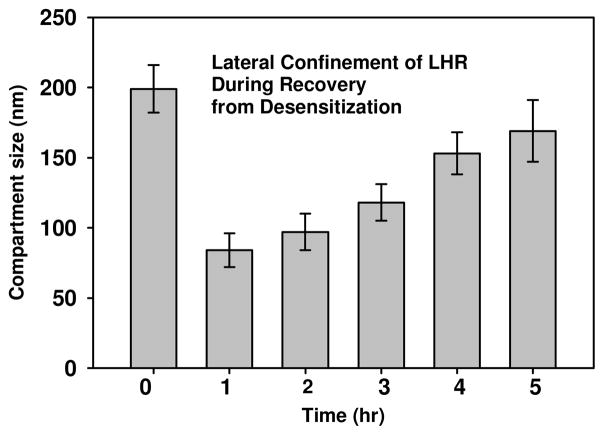
We used single particle tracking methods to assess the lateral motions of individual LH receptors during recovery from desensitization. LH receptors desensitized by hCG were found to be confined in small mesoscale membrane compartments with an average diameter (± standard deviation) of 84 ± 12 nm (Table 2). These compartments are significantly smaller than the 199 ± 17 nm diameter regions that LH receptors occupy on untreated cells. Figure 1 illustrates the differences in the track of an individual FLAG-LH receptor on an untreated cell and on a cell where receptors had been desensitized 2 hrs earlier by brief hCG exposure. The time evolution of receptor confinement during recovery from desensitization is seen most clearly in Figure 2. A significant fraction of the desensitized receptors evaluated remained confined in compartments with less than 100 nm diameters and exhibited restricted lateral diffusion for up to 3 hours after the initial hCG exposure at time 0. By 5 hours following desensitization, the LH receptors again exhibited relatively unconfined lateral diffusion in large compartments with diameters of 169 ± 22 nm.
Table 2.
Single particle tracking of human FLAG-LH receptors on individual CHO cells labeled with gold-conjugated anti-FLAG antibodya.
| Treatment | Recovery time before measurement | Compartments per 2 min trajectory | D0–1b (10−11 cm2sec−1) | Dmc (10−11 cm2sec−1) | Confinement time (sec) | Compartment size (nm) | Number of particles analyzed |
|---|---|---|---|---|---|---|---|
| None | - | 5 ± 21 | 6.2 ± 2.61 | 0.9 ± .11 | 22 ± 151 | 199 ± 171 | 20 |
| 100 nM hCG; remove hormone from receptors via low pH wash; allow indicated recovery time before measurement | 1 hr | 5 ± 21 | 2.2 ± 0.962 | 0.2 ± 0.22 | 19 ± 151 | 84 ± 122 | 10 |
| 2 hr | 6 ± 21 | 3.5 ± 1.52 | 0.2 ± 0.22 | 19 ± 121 | 97 ± 132 | 10 | |
| 3 hr | 6 ± 21 | 3.4 ± 2.42 | 0.3 ± 0.42 | 18 ± 111 | 118 ± 132 | 20 | |
| 4 hr | 4 ± 21 | 3.6 ± 1.72 | 0.5 ± 0.72 | 23 ± 141 | 153 ± 152 | 20 | |
| 5 hr | 5 ± 21 | 4.0 ± 1.92 | 0.6 ± 0.81 | 20 ± 121 | 169 ± 221 | 10 |
Data shown are the means ± standard deviations for the indicated numbers of experiments. Values in each column with superscripts 1 and 2 differ significantly (p<0.05)
Diffusion coefficient within compartment calculated from the first two points of mean square displacement vs. time plot as described by Daumas et al. [37].
Diffusion coefficient between compartments calculated as Dm = Lr2/4t from compartment size (Lr) and particle residence time (t) as described by Saxton [22].
Average particle residence time within a compartment.
Average size of an individual compartment calculated as previously described [21].
Figure 1.
Representative trajectories for a single untreated FLAG-tagged LH receptor and a single desensitized FLAG-tagged LH receptor stably expressed on CHO cells. Each trajectory represents data obtained during a single experiment over approximately 2 minutes. The average compartment diameter of the desensitized receptor after 2 hours is significantly smaller compared to the average compartment diameter of the untreated receptor. Individual compartments within a given particle trajectory were identified as described in Materials and Methods.
Figure 2.
Average sizes of compartments confining individual FLAG-LHR during recovery from desensitization at time 0. After the substantially reduced compartment size accompanying initial desensitization, compartment sizes increase more-or-less continuously over time and, by 5 hr, approach the value for untreated receptors.
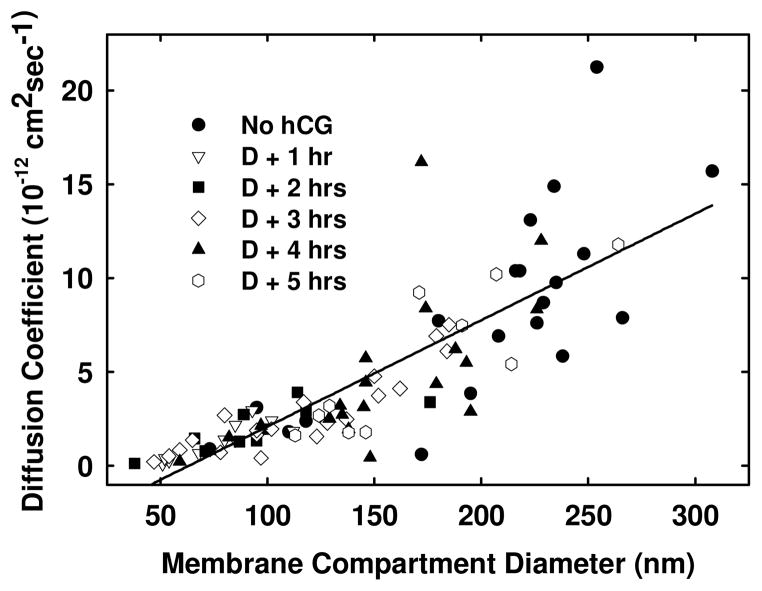
In general, as receptors accessed increasingly larger compartments, their rate of diffusion also increased (Figure 3), apparently reflecting reduced compartment size. The distributions of LH receptor diffusion coefficients and compartment sizes before and after LH receptor desensitization are shown in Figure 3. At early times following desensitization of receptors, the average rate of lateral diffusion was slower (Table 2) and there was comparatively little variation in diffusion coefficients for the various receptors studied. The sizes of compartments containing LH receptors were similarly reduced during desensitization. 3–4 hrs after desensitization, the range of diffusion coefficients and compartment sizes containing desensitized LH receptors increased. By 5 hrs, when cells appeared to be fully responsive to hCG challenge, the distribution of LH receptors and sizes of receptor-containing microdomains were comparable to those seen for untreated CHO cells.
Figure 3.
The relationship between average compartment size diameter and diffusion coefficients for CHO cells expressing human FLAG-LH receptors. Cells were treated 100 nM hCG then with low pH buffer to remove bound hormone. Individual LH receptors were then examined over 1–5 hrs using SPT. Desensitized receptors that appear in significantly smaller compartments also exhibit slower diffusion rates for several hours when compared to untreated receptors. This is presumably a consequence of approximately constant confinement times for receptors, independent of compartment size. The solid line shows the simple linear regression line that fits these data points. The figure legend identifies (●) −hCG, (○) desensitized + 1hr, (▼) desensitized + 2hr, (□) desensitized + 3hr, (■) desensitized + 4hr, (▫) desensitized + 5hr.
LH receptors associate with detergent-resistant membrane fractions following desensitization
To determine whether FLAG-LH receptors remain associated with membrane rafts during recovery from desensitization, we isolated DRM fractions using sucrose gradient isopycnic ultracentrifugation at 1 hr intervals following initiation of transient hCG exposure at time 0. The distribution of receptors in rafts and non-raft membrane fractions is shown in Table 3. Prior to hormone treatment, 96 ± 1% of untreated LH receptors appeared in high-density sucrose fractions, i.e. those containing >40% sucrose. Following LH receptor desensitization, a significant portion of LH receptors appeared in low-density gradient fractions (<40% sucrose). For example, at 2 hours following desensitization, 70% of LH receptors were located in low-density sucrose fractions. A significant portion of receptors appeared in low density gradient fractions for as long as 4 hours following desensitization. Not until 5 hours following desensitization were over 90% of LH receptors again found in high-density membrane fractions characteristic of the bulk membrane (Table 3).
Table 3.
Distribution of FLAG-LH receptors on CHO cells in low and high density sucrose fractions.a
| Treatment Treatment |
Recovery time before measurement | low density sucrose (<40%)b | high density sucrose (>40%)b | n |
|---|---|---|---|---|
| None | - | 4 ± 11 | 96 ± 11 | 5 |
| 100 nM hCG; remove hormone from receptors via low pH wash; allow indicated recovery time before measurement | 1 hr | 46 ± 52 | 54 ± 52 | 5 |
| 2 hr | 70 ± 172 | 30 ± 172 | 3 | |
| 3 hr | 48 ± 142 | 52 ± 142 | 3 | |
| 4 hr | 46 ± 32 | 54 ± 32 | 3 | |
| 5 hr | 9 ± 21 | 91 ± 21 | 6 |
Data shown are the means ± S.E.M. of the indicated numbers n of experiments.
Values with superscripts 1 and 2 differ significantly (p<0.05).
Figure 4 shows the distribution of buoyant densities of FLAG-tagged LH receptors isolated from cells at 1–5 hrs following initial hormone treatment. The receptor distribution between low-buoyancy and high-buoyancy membrane fragments changes during recovery from receptor desensitization over 5 hrs and, by 5 hr after initiation of desensitization, most receptors are no longer present in DRM-membrane fragments. In addition, the relative number of LH receptors recovered 1, 2, 3, 4, and 5 hrs after initiation of receptor desensitization, estimated by integrating band intensities such as shown in Figure 4, was 163%, 105%, 178%, 157% and 91 % of the amount of receptor recovered at time 0 from otherwise untreated cells. Fluctuations in these relative recoveries of receptors are likely due to variations between the two membrane preparations compared in each recovery measurement.
Figure 4.
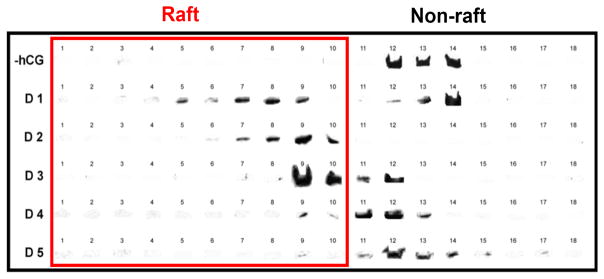
Western blots from a single experiment in which human FLAG-LH receptors were desensitized by brief exposure to 100 nM hCG and cells samples were removed at 1 hr intervals for preparation of membrane fragments. Membrane fragments from CHO cells with otherwise untreated LH receptors (−hCG) and FLAG-LH receptor recovery from desensitization was examined at hourly time points (D1, D2, D3, D4, D5) following initiation of receptor desensitization at time 0. Raft fractions are indicated as fractions 1 to 10 and contain sucrose concentrations of 10% to 39%. Non-raft fractions are indicated as fractions 11 to 18 and contain sucrose concentrations of 40% to 80%.
Discussion
The β2-adrenergic receptor (β2-AR) has served as a model for GPCR desensitization and its signal transduction has been well characterized. β2-ARs are desensitized and internalized within seconds to minutes and, after removal of ligand, approximately 20 min are needed for receptor recovery from the desensitized state [24]. Like many GPCRs, desensitization of the β2-AR depends on phosphorylation of the C-terminal domain and/or 3rd intracellular loop by two different classes of serine/threonine kinases, the second messenger-dependent kinases such as PKA, and the G protein-coupled receptor kinases (GRK) [24]. It has been suggested that phosphorylation of these intracellular residues on the receptor may promote interactions between the receptor and regulatory molecules such as β-arrestin leading to desensitization of the receptor and rapid internalization via clathrin-coated pits [5].
The mechanism involved in LH receptor desensitization differs significantly from that of β2-AR. Previous work has shown that phosphorylation of the LH receptor is not necessary for receptor desensitization [8]. Moreover, receptors become aggregated in large, microscopically visible clusters [25] and are not internalized over several hours. LH receptor aggregation appears to occur in response to ligand binding; small groups or microaggregates of LH receptors were observed on the surface of rat luteal cells following exposure to high concentrations of ovine luteinizing hormone [26] and immunofluorescence studies of LH receptor on rat granulosa cells also show the formation of large, punctate cell-surface structures following hCG treatment [16]. Similarly, time-resolved phosphorescence anisotropy measurements on porcine follicular membranes also demonstrate that desensitized LH receptors organize into large complexes exhibiting rotational correlation times approximately 3-fold slower than signaling-competent LH receptors [15]. Moreover, measurements of fluorescence resonance energy transfer (FRET) between fluorescein isothiocyanate- and tetramethylrhodamine isothiocyanate-tagged hCG bound to LH receptors on porcine granulosa cell membranes showed that desensitized LH receptors exhibited a 2.4-fold increase in FRET compared to actively signaling receptors.
Although phosphorylation of LH receptors may not be critical for receptor activation, phosphorylation and dephosphorylation events may have profound effects on downstream receptor-mediated signaling [27]. It is interesting to note here that phosphodiesterases are reported to be associated with membrane rafts and may also interact with membrane complexes containing the LH receptor through their interactions with β-arrestin or with scaffolding proteins that anchor phosphodiesterases to membrane-associated complexes (reviewed in [28]). The formation of large complexes that minimally include multiple copies of the LH receptor, β-arrestin and phosphodiesterase would account for the slow rotational diffusion observed for desensitized LH receptors [29] and the physical size of such complexes could account for increased confinement of the desensitized LH receptor in membrane microdomains as a result of interactions between the complex and cytoskeletal components.
The loss of hCG responsiveness following brief exposure of cells to hormone does not seem to arise from LH receptor internalization on the timescale of these experiments. This is seen most clearly in the representative experiment presented in Figure 4 where the total number of LH receptors recovered over the time course of receptor recovery following desensitization remains at values greater that 90% of the initial receptor number at time 0. These results are consistent with previous studies using a phosphorescent probe coupled to hCG to label LH receptors on cells briefly treated with unlabeled hCG where comparable levels of tagged hormone bound cells at 1 and 5 hrs followed receptor desensitization at time 0 [6]. Finally, single cell measurements of LH receptor density using fluorescence correlation spectroscopy [4] which directly measures receptor surface density show no evidence of receptor internalization for hCG-treated KGN cells or untreated CHO cells expressing YFP-LH receptors over 2 hrs, the time interval in which hCG-treated cells become non-responsive to subsequent hCG challenge.
Average values for lateral dynamics of LH receptors in single particle tracking studies agree well with previous studies using ensemble methods. In fluorescence recovery after photobleaching (FRAP) measurements, desensitized LH receptors remain laterally immobile, and presumably aggregated, for approximately 5 hr [6]. These FRAP results indicate that LH receptors associate into large receptor complexes during desensitization. However, as in all FRAP studies, such results describe physical properties of receptor populations rather than individual receptors. In SPT experiments, when cells were treated with 100 nM hCG, a concentration sufficient to saturate available LH receptors, the average diffusion rate of individual LH receptors is approximately 1–2 × 10−12cm2sec−1. This agrees with previous measurements of lateral diffusion for LH receptors on ovine luteal cells [31] and cell lines stably expressing LH receptors covalently coupled to visible fluorescent proteins [25] where most, if not all, LH receptors appeared laterally immobile on the timescale of FRAP experiments after binding either LH or hCG. In these experiments, diffusion coefficients were estimated to be less than 10−12cm2sec−1 [32], a practical detection limit for FRAP measurements. Moreover, individual desensitized LH receptors that exhibit slow lateral diffusion also were confined in small membrane microdomains.
Although the overall number of receptors per cell is comparatively low, desensitized LH receptors on viable cells may be highly concentrated on in specific membrane locations during and after signaling. Unliganded EGF receptors, as an example, have been shown, using spatial intensity distribution analysis, to exist as a heterogeneous mixture of monomers and dimers [33] and can also form larger receptor clusters that diffuse as a single unit [34]. Extracting cholesterol from the plasma membrane using methyl-β-cyclodextrin produces more highly clustered EGF receptors, presumably due to a decrease in the cholesterol-enriched membrane microdomains preferred by receptors and subsequent concentration of receptors in remaining domains. As Kusumi and coworkers have suggested, the concentration of membrane proteins in detergent-resistant membrane microdomains may be accompanied by nesting of these membrane microdomains in larger membrane compartments formed by interactions between membrane proteins and cytoskeletal components [10]. Moreover, LH receptors within these nested compartments may be present as dimers or small aggregates [6] as well as in large, heterogeneous complexes as suggested by rotational diffusion studies of LH receptors [29; 35].
Because resensitized LH receptors are laterally mobile in the bulk membrane when once again capable of responding to hormone, recovery from desensitization appears to involve release of individual receptors or small groups of receptors into the bulk membrane. Moreover, if dissociation of cluster components occurs progressively from the outer edges of the cluster or by progressive fragmentation of large clusters to form smaller clusters rather than as a result of spontaneous dissociation of all the cluster components, there should be a broadening of the range of diffusion coefficients and compartment sizes containing LH receptors at later times after receptor desensitization as was in fact observed. The relative number of LH receptors appearing in the bulk membrane would also increase as receptors moved out of clusters and were no longer preferentially associated with detergent-resistant membrane microdomains as was also observed. If transit of LH receptors into and out of membrane microdomains is important in LH receptor activation, desensitization and recovery from desensitization, the lipid composition of the plasma membrane may also affect receptor-mediated signaling. This may be important in the extent and time-course of desensitization of the LH receptor following the LH surge when FSH- and LH-driven changes in lipid metabolism affect synthesis of cholesterol and other membrane lipids [36].
Conclusions
These results indicate that, during recovery from LH receptor desensitization, LH receptors are both located with DRM lipid environments and confined within small, mesoscale (80–160 nm) cell-surface microdomains. This may reflect hormone-driven translocation of receptors into DRM and formation there over time of protein aggregates too large or too rigid to permit effective signaling. Once bound hormone is removed, such receptor structures would have to dissociate before receptors can again signal in response to hormone challenge. Moreover, such larger protein complexes would be more easily constrained laterally by membrane structural elements and so appear resident in smaller cell surface compartments. The time course of this apparent confinement would thus parallel that of receptor association of DRM and of functional receptor resensitization.
Acknowledgments
We thank Dr. Ying Lei for constructing the FLAG-tagged LH receptor expressing CHO cell line used here. This work was supported, in part, by NIH grant R03 HD41980 (D.A.R.), by the U.S.D.A. Animal Health and Disease Program at Colorado State University (D.A.R.) and by NSF grant MCB-1024669 (B.G.B).
References
- 1.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. β2-Adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. Journal of Biological Chemistry. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 2.Downs SM, Hunzicker-Dunn M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Developmental Biology. 1995;172:72–85. doi: 10.1006/dbio.1995.0006. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Lei Y, Liu J, Cahill ME, Hagen GM, Barisas BG, Roess DA. Luteinizing hormone receptors translocate to plasma membrane microdomains after binding of human chorionic gonadotropin. Endocrinology. 2006;147:1789–95. doi: 10.1210/en.2005-1046. [DOI] [PubMed] [Google Scholar]
- 4.Wolf-Ringwall AL, Winter PW, Liu J, Van Orden AK, Roess DA, Barisas BG. Restricted lateral diffusion of luteinizing hormone receptors in membrane microdomains. Journal of Biological Chemistry. 2011;286:29818–29827. doi: 10.1074/jbc.M111.250969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Progress in Neurobiology. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 6.Horvat RD, Barisas BG, Roess DA. Luteinizing hormone receptors are self-associated in slowly diffusing complexes during receptor desensitization. Molecular Endocrinology. 2001;15:534–542. doi: 10.1210/mend.15.4.0622. [DOI] [PubMed] [Google Scholar]
- 7.Dix CJ, Schumacher M, Cooke BA. Desensitization of tumor Leydig cells by lutropin: evidence for uncoupling of the lutropin receptor from the guanine nucleotide-binding protein. Biochemistry Journal. 1982;202:739–745. doi: 10.1042/bj2020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamm MLG, Hunzicker-Dunn M. Phosphorylation-independent desensitzation of the luteinizing hormone/chorionic gonadotropin receptor in porcine follicular membranes. Molecular Enocrinology. 1994;8(11):1537–1546. doi: 10.1210/mend.8.11.7877622. [DOI] [PubMed] [Google Scholar]
- 9.Roess DA, Niswender GD, Barisas BG. Cytochalasins and colchicine increase the lateral mobility of human chorionic gonadotropin-occupied luteinizing hormone receptors on ovine luteal cells. Endocrinology. 1988;122:261–269. doi: 10.1210/endo-122-1-261. [DOI] [PubMed] [Google Scholar]
- 10.Kusumi A, Shirai YM, Koyama-Honda I, Suzuki KGN, Fujiwara TK. Hierarchical organization of the plasma membrane: Investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Letters. 2010;584:1814–1823. doi: 10.1016/j.febslet.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 12.Pike LJ. Rafts defined: a report on the Keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. The Journal of Cell Biology. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews, Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 15.Hunzicker-Dunn M, Barisas BG, Song J, Roess DA. Membrane organization of luteinizing hormone receptors differs between actively signaling and desensitized receptors. J Biol Chem. 2003;278:42744–42749. doi: 10.1074/jbc.M306133200. [DOI] [PubMed] [Google Scholar]
- 16.Amsterdam A, Berkowitz A, Nimrod A, Kohen F. Aggregation of luteinizing hormone receptors in granulosa cells: a possible mechanism for desensitization to the hormone. Proc Nat Acad Sci (USA) 1980;77:3440–3445. doi: 10.1073/pnas.77.6.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Yague J, Rodriquez MC, Segaloff DL, Ascoli M. Truncation of the cytoplasmic tail of the lutropin/choriogonadotropin receptor prevents agonist-induced uncoupling. Journal of Biological Chemistry. 1992;267:7217–7220. [PubMed] [Google Scholar]
- 18.Roess DA, Smith SML, Winter P, Zhou J, Dou P, Baruah B, Trujillo AM, Levinger NE, Yang X, Barisas BG, Crans DC. Effects of vanadium-containing compounds on membrane lipids and on microdomains used in receptor-mediated signaling. Chemistry & Biodiversity. 2008;5:1558–1570. doi: 10.1002/cbdv.200890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter PW, Al-Qatati A, Wolf-Ringwall AL, Schoeberl S, Chatterjee PB, Barisas BG, Roess DA, Crans DC. The anti-diabetic bis(maltolato)oxovanadium(iv) decreases lipid order while increasing insulin receptor localization in membrane microdomains. Dalton Transactions. 2012;41:6419–6430. doi: 10.1039/c2dt30521f. [DOI] [PubMed] [Google Scholar]
- 20.Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophysical Journal. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barisas BG, Smith SM, Liu J, Song J, Hagen GM, Pecht I, Roess DA. Compartmentalization of the Type I Fc epsilon receptor and MAFA on mast cell membranes. Biophysical Chemistry. 2007;126:209–217. doi: 10.1016/j.bpc.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Saxton MJ. Single-particle tracking: the distribution of diffusion coefficients. Biophysical Journal. 1997;72:1744–1753. doi: 10.1016/S0006-3495(97)78820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei Y, Hagen GM, Smith SM, Liu J, Barisas G, Roess DA, Barisas BG, Song J, Pecht I, Cahill ME. Constitutively-active human LH receptors are self-associated and located in rafts. Mol Cell Endocrinol. 2007;260–262:65–72. doi: 10.1016/j.mce.2005.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefkowitz R. G protein-coupled receptors. The Journal of Biological Chemistry. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 25.Horvat RD, Nelson S, Clay CM, Barisas BG, Roess DA. Intrinsically fluorescent luteinizing hormone receptor demonstrates hormone-driven aggregation. Biochem Biophys Res Comm. 1999;255:382–385. doi: 10.1006/bbrc.1999.0185. [DOI] [PubMed] [Google Scholar]
- 26.Luborsky J, Slater W, Behrman H. Luteinizing hormone (LH) receptor aggregation: modification of ferritin-LH binding and aggregation by prostaglandin F2α and ferritin-LH. Endocrinology. 1984;115:2217–2225. doi: 10.1210/endo-115-6-2217. [DOI] [PubMed] [Google Scholar]
- 27.Linderman JJ. Modeling of G-protein-coupled receptor signaling pathways. Journal of Biological Chemistry. 2009;284:5427–5431. doi: 10.1074/jbc.R800028200. [DOI] [PubMed] [Google Scholar]
- 28.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiological Reviews. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 29.Hunzicker-Dunn M, Barisas G, Song J, Roess DA. Membrane organization of luteinizing hormone receptors differs between actively signaling and desensitized receptors. J Biol Chem. 2003;278:42744–9. doi: 10.1074/jbc.M306133200. [DOI] [PubMed] [Google Scholar]
- 30.Nimrod A, Bedrak E, Lamprecht SA. Appearance of LH-receptors and LH-stimulable cyclic AMP accumulation in granulosa cells during follicular maturation in the rat ovary. Biochemical and Biophysical Research Communications. 1977;78:977–984. doi: 10.1016/0006-291x(77)90517-4. [DOI] [PubMed] [Google Scholar]
- 31.Niswender GD, Roess DA, Sawyer HR, Silvia WJ, Barisas BG. Differences in the lateral mobility of receptors for luteinizing hormone (LH) in the luteal cell plasma membrane when occupied by ovine LH versus human chorionic gonadotropin. Endocrinology. 1985;116:164–169. doi: 10.1210/endo-116-1-164. [DOI] [PubMed] [Google Scholar]
- 32.Dragsten P, Henkart P, Blumenthal R, Weinstein J, Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proceedings of the National Academy of Science (USA) 1979;76:5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godin AG, Costantino S, Lorenzo L-E, Swift JL, Sergeev M, Ribeiro-da-Silva A, De Koninck Y, Wiseman PW. Revealing protein oligomerization and densities in situ using spatial intensity distribution analysis. Proceedings of the National Academy of Sciences (USA) 2011;108:7010–7015. doi: 10.1073/pnas.1018658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saffarian S, Li U, Elson E, Pike L. Oligomerization of the EGF receptor investigated by life cell fluorescence intensity distribution analysis. Biophysical Journal. 2007;93:1021–1031. doi: 10.1529/biophysj.107.105494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roess DA, Brady CJ, Barisas BG. Biological function of the LH receptor is associated with slow receptor rotational diffusion. Biochim Biophys Acta. 2000;1464:242–50. doi: 10.1016/s0005-2736(00)00159-0. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan H-Y, Zeleznik AJ, Richards JS. FSH and FOXO1 Regulate Genes in the Sterol/Steroid and Lipid Biosynthetic Pathways in Granulosa Cells. Molecular Endocrinology. 2009;23:649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]