Abstract
Background
Meta-analytic results of fear-conditioning studies in the anxiety disorders implicate generalization of conditioned fear to stimuli resembling the conditioned danger cue as one of the more robust conditioning markers of anxiety pathology. Due to the absence of conditioning studies assessing generalization in generalized anxiety disorder (GAD), results of this meta-analysis do not reveal whether such generalization abnormalities also apply to GAD. The current study fills this gap by behaviorally and psychophysiologically assessing levels of conditioned fear-generalization across adults with and without GAD.
Methods
Twenty-two patients with a DSM-IV-TR diagnosis of GAD and 26 healthy comparisons were recruited and tested. The employed generalization paradigm consists of quasi-randomly presented rings of gradually increasing size, with extreme sizes serving as conditioned danger cues (CS+) and conditioned safety-cues (CS−). The rings of intermediary size serve as generalization stimuli, creating a continuum-of-similarity between CS+ and CS− across which to assess response slopes, referred to as generalization gradients. Primary outcome variables included slopes for fear-potentiated startle (EMG) and self-reported risk ratings.
Results
Behavioral and psychophysiological findings demonstrate overgeneralization of conditioned fear among patients with GAD. Specifically, generalization gradients were abnormally shallow among GAD patients, reflecting less degradation of the conditioned fear response as the presented stimulus differentiated from the CS+.
Conclusions
Overgeneralization of conditioned fear, to safe encounters resembling feared situations, may contribute importantly to the psychopathology of GAD by proliferating anxiety cues in the individual’s environment that are then capable of evoking and maintaining anxiety and worry associated with GAD.
Keywords: stimulus generalization, fear-conditioning, fear-potentiated startle, generalized anxiety disorder, pathophysiology, interpretation bias
Introduction
Central to many etiological accounts of anxiety disorders is classical fear-conditioning (1): the evolutionarily conserved learning process through which a neutral conditioned stimulus (CS) acquires the capacity to elicit fear following its co-occurrence with an aversive unconditioned stimulus (US) (2). Because the neural substrates of fear-conditioning have been mapped in lower mammals (3–4), conditioning-based research in anxiety patients represents a particularly valuable approach to generating neurobiological insight on clinical anxiety. A first step in this line of work is to link specific conditioning abnormalities to anxiety disorders.
Meta-analytic results implicate overgeneralization of conditioned fear as one of the most robust conditioning abnormalities in the anxiety disorders (5). In overgeneralization, fear responses appropriate to a danger cue (CS+) are inappropriately evoked by perceptually-similar conditioned safety cues (CS−). Due to the absence of conditioning studies testing overgeneralization in generalized anxiety disorder (GAD) at the time of this meta-analysis, results do not reveal whether such abnormalities in conditioned generalization also apply to GAD. Additionally, the one lab-based study of fear generalization in GAD patients conducted after this meta-analysis found no behavioral or autonomic (pupillary response) evidence of overgeneralization in GAD patients (6). Substantial research and clinical findings linking GAD to overgeneralization, however, warrants further testing of this relation. Empirical evidence derives from the well-established link between GAD and both heightened intolerance of uncertainty (7) and increased tendencies to interpret ambiguous stimuli as threatening (8–10). Stimuli resembling the CS+, referred to as generalization stimuli (GS), constitute degraded versions of the original conditioned danger-cue and, as such, represent a more ambiguous and uncertain source of threat. It thus follows that individuals with GAD may be both more stressed by the threat uncertainty of GSs and more prone to interpret GSs as threatening, resulting in heightened anxious reactivity to GSs. Additionally, the clinical phenomenology of GAD seems consistent with a tendency toward overgeneralization. For example, a GAD patient with an anxiogenic preoccupation with family member’s risk for cancer, may not only become worried when confronted by encounters directly related to cancer (e.g., news of a relative with a malignancy, meeting a child with cancer), but may generalize this fear to stimulus events with only moderate relatedness to cancer (e.g., noticing the health section of the newspaper open on the counter, seeing a medical doctor on a TV show). This type of fear generalization is thought to proliferate anxiety cues in the individual’s environment (11) and, in so doing, may increase the persistence of anxiety and worry associated with GAD. Importantly, because of extensive animal research on conditioned fear and its generalization, confirming overgeneralization in GAD patients may aid in bridging the gap between basic and GAD-relevant clinical science by implicating neural substrates of conditioned generalization found by animal work in the pathophysiology of GAD.
The current study tests for overgeneralization in GAD patients with a previously validated, conditioned fear-generalization paradigm assessing fear-potentiated startle (EMG) and behavioral responses to both conditioned danger cues (CS+) and generalization stimuli parametrically varying in similarity to the CS+ (12,13). This paradigm affords assessment of downward slopes in conditioned responding as the presented stimulus differentiates from CS+, known as generalization gradients (2). The strength of generalization is captured by the steepness of gradients, with less steep downward slopes indicative of greater generalization. In both intact animals and healthy humans, generalization gradients form steep, quadratic slopes with highest levels of fear-conditioning to CS+, precipitous declines in responding to the closest two or three approximations of the CS+, and a leveling off of responses to the remaining generalization stimuli (12,14–16). By contrast, responses to CS+ and GSs in patients with GAD are predicted to deviate from this pattern, with less quadratic and more gradual generalization gradients, reflecting heightened levels of generalization.
Previous applications of this paradigm demonstrate overgeneralization of fear-conditioning reflected by abnormally gradual generalization gradients in patients with panic disorder (13) and PTSD (11). Such data are beginning to paint a picture of overgeneralization as a broad vulnerability marker cutting across traditional anxiety disorder categories. An additional aim of this study is to further assess the reach of overgeneralization by testing whether it extends to patients with GAD.
Methods and Materials
Participants
Participants included 22 patients with a diagnosis of GAD and 26 healthy comparisons matched for gender and age. For all patients GAD symptoms constituted the primary source of current discomfort and dysfunction. Table 1 displays sample characteristics for each group. Diagnostic and consenting procedures as well as inclusion-exclusion criteria for each group are included in Supplement 1.
Table 1.
Demographics and clinical characteristics across patient and control samples.
| Variable | GAD Patients (n = 22) |
Healthy Control (n = 26) |
Significancea | ||
|---|---|---|---|---|---|
| Mean | SD | SD | |||
| Age (years) | 8.66 | 29.27 | 10.43 | p =.20 | |
| STAI-State | 47.00 | 8.44 | 26.32 | 6.99 | p <.0001 |
| STAI-Trait | 52.5389 | 6.76 | 29.94 | 6.59 | p<.0001 |
| BDI | 10.68 | 6.39 | 2.33 | 2.37 | p<.0001 |
| N | % | N | % | Significancea | |
| Male Gender | 5 | 23% | 8 | 31% | p =.54 |
| Comorbidities | |||||
| Social Anxiety Dx | 10 | 45% | 0 | 0% | -- |
| Specific phobia | 1 | 5% | 0 | 0% | -- |
| Past MDD | 2 | 9% | 0 | 0% | |
| Ethnicity | |||||
| African American | 4 | 18% | 3 | 12% | -- |
| Caucasian | 16 | 73% | 18 | 69% | -- |
| Hispanic | 1 | 5% | 2 | 8% | -- |
| Asian Pacific | 0 | 0% | 3 | 12% | -- |
| Other | 0 | 0% | 0 | 0% | -- |
Two-tailed p values reflecting the significance of group differences derived from independent samples t-tests for all variables except gender which was assessed using the chi-square statistic.
STAI = Spielberger State/Trait Anxiety Inventory; BDI = Beck Depression Inventory, Dx = disorder, MDD = major depressive disorder.
Conditioned Generalization Paradigm
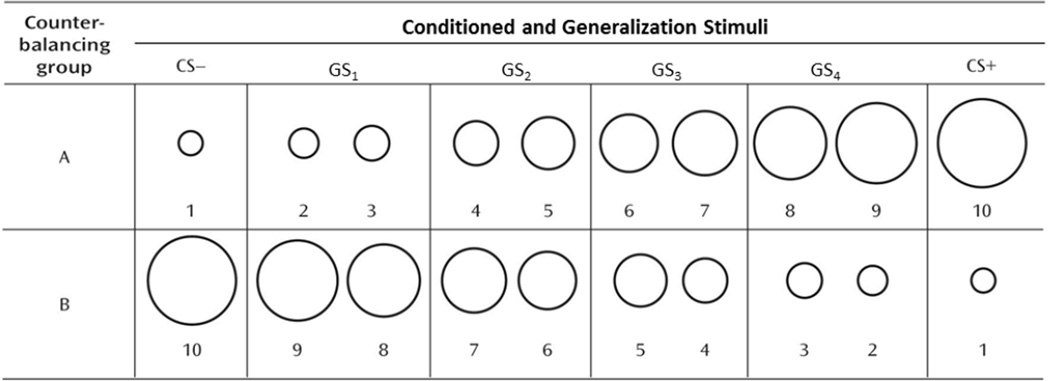
The paradigm used in this study was identical to the one we described in detail elsewhere (12), in which 10 rings of gradually increasing size (Figure 1) presented on a computer monitor serve as conditioned and generalization stimuli. The largest and smallest rings serve as the conditioned danger cue (CS+) and conditioned safety cue (CS–), the former paired and the latter unpaired with an aversive unconditioned stimulus. The eight intermediately sized rings serve as generalization stimuli (GSs) that form a continuum of size between the CS+ and CS−. As can be seen if Figure 1, the eight GSs were collapsed into four classes of GSs (GS1, GS2, GS3, GS4). All conditioned and generalization stimuli are presented for 8 seconds on a computer monitor. The unconditioned stimulus is a 100-msec electric shock delivered to the left wrist (3–5 mA) that was rated by participants as “highly uncomfortable but not painful.”
Figure 1.
Conditioning and generalization stimuli for counterbalancing Groups A and B. For half of participants, the largest ring was the CS+ and the smallest was the CS− (Group A), and for the other half this is reversed (Group B). The numbers 1–10 at the bottom of the rings label the stimuli from smallest (1) to largest (10). As was done by Lissek et al.[9]—to avoid an unduly large number of trials while maintaining a gradual continuum-of-size across rings—each of two intermediaries are collapsed into a single class of stimulus leaving four classes of GSs. For both counterbalancing groups A and B, these classes of GSs are numbered such that Class GS4 consists of the two rings closest in size to the CS+ (rings 8 and 9 for counterbalancing group A, rings 3 and 2 for group B), and Classes GS3 through GS1 consist of rings progressively increasing in similarity to the CS−. The diameter for the smallest ring (Ring #1) was 2.00 in and subsequent rings increased by 15% with Ring #2 increasing 15% from Ring#1 (2.30 in), Ring #3 increasing 30% from Ring #1 (2.60 in), Ring #4 increasing 45% from #1 (2.90 in), and so on. Such size increments resulted in ring diameters, from smallest to largest, of 2.00, 2.30, 2.60, 2.90, 3.20, 3.50, 3.80, 4.10, 4.40, 4.70. CS+ = conditioned stimulus paired with shock; CS− = conditioned stimulus unpaired with shock; GS1, GS2, GS3, GS4 = generalization stimulus classes 1–4.
This paradigm consists of three phases: habituation (startle habituation to nine startle probes), pre-acquisition, acquisition (presentation of CS− and CS+ only, with CS+ reinforced 75% of trials), and generalization (presentation of the CS−, CS+, and the eight GSs, with CS+ reinforced on 50% of trials). The trial types and frequencies for each phase are listed in Table 2. During each phase, half of the trials were followed by acoustic startle probes (40ms, 102 dBA) that occurred 4 or 5 seconds after onset of the conditioned or generalization stimulus. A balanced number of startle probes were presented during inter-trial-intervals.
Table 2.
Trial types and frequencies during pre-acquisition, acquisition, and generalization phases of the study.
| Conditioned and Generalization Stimuli | ||||||||
|---|---|---|---|---|---|---|---|---|
| CS+ | ||||||||
| Phase | CS− | GS1 | GS2 | GS3 | GS4 | Yes US | No US | ITI |
| Pre-acquisition | 6 | 0 | 6 | 6 | ||||
| Acquisition | 12 | 9 | 3 | 12 | ||||
| Generalization | 12 | 12 | 12 | 12 | 12 | 6 | 6 | 12 |
CS−=conditioned safety cue; CS+=conditioned danger cue; GS1, GS2, GS3, and GS4=generalization stimulus classes 1, 2, 3, and 4; US=unconditioned stimulus; ITI=intertrial intervals. During the generalization test, the CS+ continued to be reinforced with shock to avoid extinction of the conditioned response during the generalization sequence.
During trials without startle probes, behavioral ratings (perceived risk for shock) and associated response times were collected. Specifically, the question “Level of risk?” appeared above the stimulus 1 to 2 seconds after trial onset, which cued participants to rate their perceived likelihood of receiving a shock on a 3-point scale (1=no risk, 2=moderate risk, and 3=high risk). Further details on risk ratings and self-report measures as well as the startle EMG apparatus and EMG data processing can be found in Supplement 1.
Data Analysis
For acquisition, startle and behavioral data were analyzed with a 2 (Stimulus type: CS−, CS+) × 2 (Group: GAD patients, healthy comparisons) repeated measures analysis of variance (ANOVA). Startle and behavioral data at generalization were analyzed with a 6 (Stimulus type: CS−, GS1, GS2, GS3, GS4, CS+) × 2 (Group: GAD patients, healthy comparisons) repeated measures ANOVA. ANOVAs were computed using Wilks's lambda and were followed, when necessary, by either trend analyses, paired-samples t-tests, or independent samples t-tests. Quadratic trend analyses were particularly important for testing the shape of generalization gradients, with the a priori hypothesis that gradients of patients, but not healthy comparisons, would depart from the quadratic function found in healthy humans and intact animals (12,14). Alpha was set at .05 for all statistical tests.
Results
Pre-Acquisition
Neither main effects of stimulus-type nor Stimulus-type × Group interactions were significant for behavioral risk ratings and startle EMG (all ps<.47).
Acquisition
Startle-EMG and behavioral results at acquisition are displayed across groups in Figures 1a and 1b.
Startle EMG
A main effect of stimulus type was found, F(1,46)=46.09, p<.0001, and resulted from significant potentiation of startle to CS+ versus CS− in both GAD (Mean CS+=57.38 (SD=6.51), Mean CS−=52.22 (SD=4.97); t(21)=3.74, p=.001) and healthy groups (Mean CS+=56.05 (SD=4.99), Mean CS− = 49.15 (SD=3.51);t(25)=6.04, p<.0001). The Group × Stimulus-type interaction as not significant (p=.33).
Risk ratings
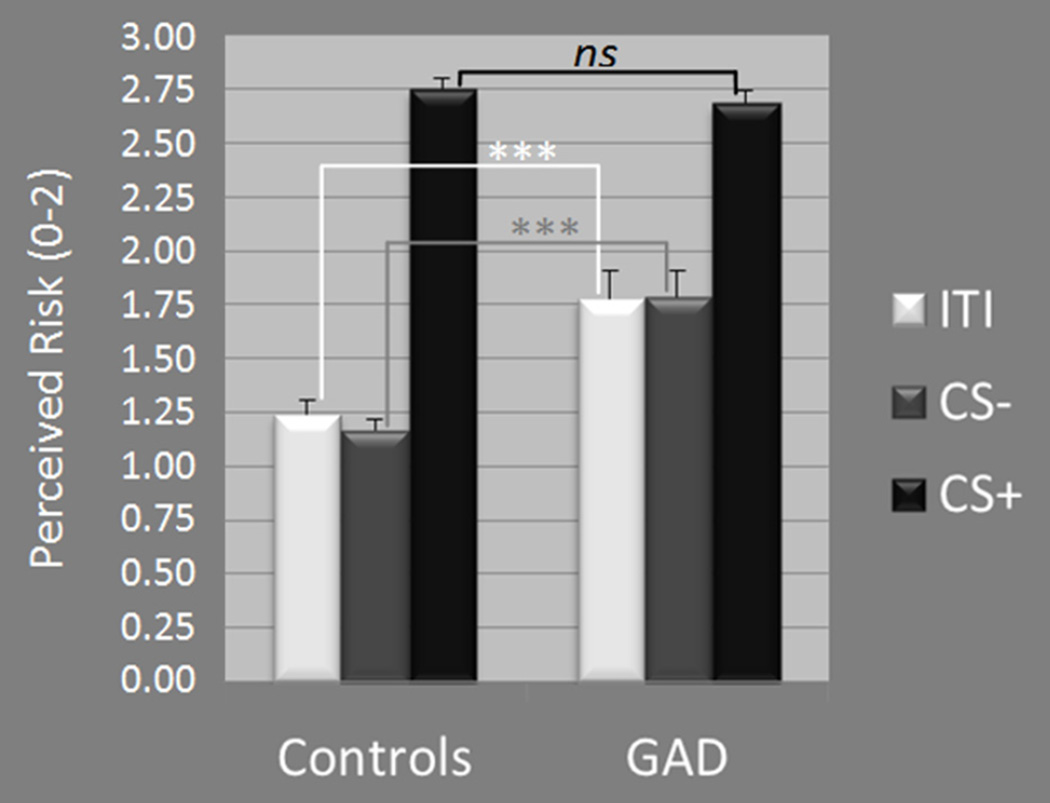
A main effect of stimulus-type, F(1,46)=250.22, p<.0001, was driven by increased risk ratings to the CS+ versus CS− in both GAD t(21)=6.62, p<.0001, and healthy groups, t(25)=21.46, p<.0001. Additionally, a Group × Stimulus-type interaction, F(1,46)=18.70, p<.0001, resulted from greater levels of reported risk among GAD patients versus healthy comparison to CS− (p<.0001) and ITI (p=.001), but equal levels across groups to CS+ (p=.41).
Response times
A main effect of stimulus-type was found, F(1,46)=8.88, p=.004, and reflected quicker response times to CS+ than CS−. No Stimulus-type × Group interaction was found (p=.38).
Retrospective anxiety
Conditioning to the danger cue was also apparent in self-reported anxiety as evidenced by a main effect of stimulus-type, F(1,46)=115.32, p<.0001, driven by greater reported anxiety to CS+ versus CS− in both GAD (p=.001) and control groups (p<.0001). Furthermore, the Group × Stimulus-type interaction was significant, F(1,46)=12.90, p=.001, and reflected greater anxiety in GAD patients versus controls to CS− (p<.0001) but not CS+ (p=.37).
Generalization Test
Startle EMG
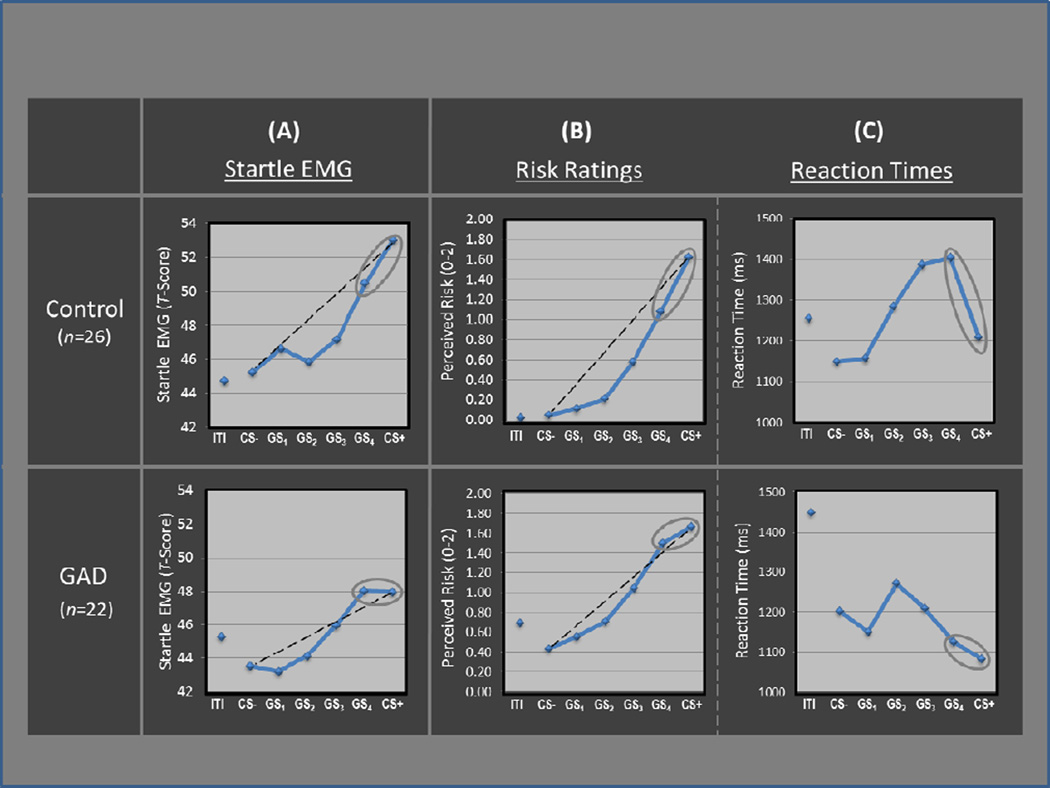
Generalization of fear conditioning was evidenced by main effects of stimulus type in both patients, F(5,17)=4.12, p=0.012, and healthy comparisons, F(5,21)=9.43, p<.0001), which derived from graded decreases in startle magnitude as the presented stimulus diverged from CS+ (see Figure 3a). The shape of this declining slope differed across groups, as reflected by a significant Group × Stimulus-type interaction, F(5,42)=2.35, p<.05, attributable to between-group differences in the quadratic component of their respective slopes (Group × Stimulus-type quadratic trend: F(1,46)=4.13, p<.05). Follow-up tests on the Group × Stimulus type quadratic interaction revealed a significant quadratic component in the generalization gradient of comparison subjects, F(1,25)=19.69, p =.0002, but not GAD patients, F(1,21)=.91, p>.35.
Figure 3.
Generalization results across groups and dependent variables including: (A) standardized startle EMG, (B) behavioral risk ratings (0=no risk, 1=some risk, 2=high risk), and (C) reaction times. Levels of responding for all indices are displayed across conditioned danger-cues (CS+), conditioned safety-cues (CS−), as well as four classes of generalization stimuli (GS1, GS2, GS3, GS4) forming a continuum of similarity between CS+ and CS−. Black dotted lines reflect linear decreases in responding from CS+ to CS− with which to visualize the deviation of gradients from linearity. Grey ellipses around the rightmost data points highlight more shallow response-slopes from CS+ to GS4 in patients versus controls across all indices. GAD=generalized anxiety disorder; ITI=inter-trial-interval.
Group differences in quadratic components are visualized in Figure 3a for GAD and healthy groups separately. The dotted lines represent hypothetical linear declines in startle potentiation from CS+ to CS−, with which to illustrate the presence and absence of a quadratic deviation from linearity among healthy controls and patients. Whereas healthy controls displayed a marked departure from linearity, characterized by a steep quadratic decline in fear-conditioning as the presented stimulus differentiates from CS+, the absence of a quadratic function in GAD patients is evidenced by substantially less deviation from linearity, indicating a more gradual decline in fear-conditioning as stimuli move down the continuum of similarity.
Additional evidence for over-generalization in GAD patients lies in response slopes from CS+ to GS4. Whereas conditioned fear drops precipitously from CS+ to GS4 in healthy comparisons, GAD patients display approximately equal levels to CS+ and GS4, suggesting complete generalization of conditioned fear from CS+ to its first approximation. This group difference in startle responding was confirmed by a significant 2 (Group: GAD, controls) × 2 (Stimulus: CS+, GS4) interaction, F(1,46) =3.83, p<.05, with significant declines from CS+ to GS4 in controls (p=.009) but not patients (p=.96).
Risk ratings
Main effects of stimulus-type where found in both patient and comparison groups (ps<0.0001), and resulted from downward generalization gradients in perceived risk as the presented stimulus differentiated from CS+ (see Figure 3b). Additionally, a significant Group × Stimulus-type quadratic trend emerged, F(1,46)= 9.90, p=.003, indicating group differences in the quadratic component of generalization gradients. Figure 3b includes dotted lines demarcating linear declines in risk ratings from CS+ to CS− with which to visualize group differences in quadratic departures from linearity. As can be seen, such departures are more substantial in healthy comparisons versus individuals with GAD—a group difference responsible for the resulting Group × Stimulus-type quadratic trend.
As was the case for startle data, group differences in generalization of risk ratings were most apparent when looking at response slopes from CS+ to GS4. Specifically, perceived risk of shock generalized more strongly from CS+ to GS4 in GAD patients relative to healthy comparisons as evidenced by a significant 2 (Group: GAD, controls) × 2 (Stimulus: CS+, GS4) interaction, F(1,46)=10.76, p=.002.
Response times
There was a main effect of stimulus type, F(5,42)=4.40, p=.003, with significant quadratic components found for both healthy comparisons, F(1,25)=11.64, p=.002, and GAD patients, F(1,21)=4.45, p<.05. As can be seen in Figure 3c, these quadratic components take the form of inverted U-shaped curves reflecting slower risk ratings for GSs with ambiguous signal value, and faster ratings for stimuli communicating more certain threat or safety information (i.e., CS+ and CS−). This pattern of results implicates reaction time as an index of threat uncertainty, with longer reaction times reflecting more uncertainty.
Group differences in reaction times across stimuli were evidenced by a Group × Stimulus-type interaction, F(5,42)=4.07, p=.005. This interaction was driven by significantly faster responses to GS4 in patients relative to comparison subjects (p<0.02), as group differences between all other stimulus classes were nonsignificant, and reaction times to GS4 versus CS+ were significantly longer for healthy comparisons (p=0.004) but not patients (p=.30), resulting in a significant 2 (Group: GAD, controls) × 2 (Stimulus: CS+, GS4) interaction, F(1,46)=4.21, p<.05. That response times for GS4 were not significantly different from CS+ among GAD patients suggests that patients were equally certain of risk for shock whether presented with the closest approximation of the danger cue or the danger cue itself.
Retrospective anxiety
Self-reported levels of anxiety, following the generalization test, reveal greater anxiety to the CS+ versus CS− in both GAD patients and healthy comparisons (both ps<.0001). Additionally, self-reported anxiety was higher in patients versus controls to CS− (p=.002) but not CS+ (p=.53).
Testing effects of social anxiety disorder in GAD patients
Because 10 of 22 GAD patients were diagnosed with comorbid social anxiety disorder (SAD), the influence of comorbid SAD on generalization results in GAD patients was examined with two sets of sub-analyses. In the first set, differences between GAD patients with and without comorbid SAD on behavioral and psychophysiological indices of generalization were tested using Group (2 levels: GAD-alone, GAD+SAD) × Stimulus-type (6 levels: CS−, GS1, GS2, GS3, GS4, CS+) interactions. Such analysis found no Group × Stimulus-type interactions for startle data or behavioral risk-ratings (all ps>.30), suggesting that comorbid SAD did not alter results in GAD patients. Because these null effects may have resulted from underpowered sample sizes, such effects should be interpreted with caution.
The second set of sub-analyses tested whether generalization effects in the GAD-alone patients were similar to those found in the total-GAD group. Again, statistics from this subanalysis are likely underpowered due to small sample size, and results should be interpreted cautiously. After excluding the 10 GAD patients with comorbid SAD, the Group × Stimulus interaction for startle EMG was reduced to a non-significant trend, F(5,32)=1.91, p=.117. Nevertheless, the pattern of results in the GAD-alone subjects remained the same as those for the total-GAD group with generalization gradients characterized by linear, F(1,11)=14.54, p=.003, but not quadratic components, F(1,11)=.52, p=.49. Additionally, GAD-alone subjects, versus healthy controls, continued to display less steep declines in startle responding from CS+ to GS4, F(1,36)=4.09, p=.05, indicating overgeneralization in GAD-alone patients. In terms of online risk ratings, after removing the 10 GAD patients with comorbid SAD, the Group (2 levels: GAD-alone, healthy controls) × Stimulus-type (6 levels: CS−, GS1, GS2, GS3, GS4, CS+) quadratic trend was reduced to nonsignificance (p=.18), though a trend for less steep declines from CS+ to GS4 remained in GAD-alone versus healthy controls, F(1,36)=3.50, p=.07. Thus, while the significance of group differences in generalization was generally reduced after dropping GAD patients with comorbid SAD, results continued to reflect overgeneralization in the GAD-alone group, suggesting that comorbid SAD was not responsible for the pattern of results found in the total-GAD sample.
Discussion
This study represents the first behavioral and psychophysiological demonstration of lab-based overgeneralization of fear-conditioning in generalized anxiety disorder. Whereas generalization gradients in healthy comparison fell along precipitous, quadratic declines in conditioned responding as the presented stimulus diverged in similarity from the CS+, gradients among GAD patients fell along less steep and less quadratic slopes of responding, indicating stronger transfer of fear to stimuli resembling the CS+. This heightened generalization in GAD patients was found whether indexing the generalization gradient with behavioral risk-ratings or psychophysiological assessments of fear-potentiated startle. Furthermore, analyses testing effects of SAD on levels of generalization in GAD patients suggest that results in GAD patients are not driven by comorbid SAD, although such analyses were likely underpowered.
Differential Response-Slopes from CS+ to GS4 Across Groups
In addition to group differences in the overall shape of generalization gradients, response slopes from CS+ to the closest approximation of the CS+ (GS4) among GAD patients, deviated consistently from such slopes in healthy comparisons across behavioral and psychophysiological measures. Specifically, slopes in startle magnitudes, risk-responses, and reaction times from CS+ to GS4 were steep in healthy comparisons, but nearly flat among GAD patients (see circled data points in Figure 3). Such findings indicate that those with, but not without, GAD behaviorally and psychophysiologically responded to the closest approximation of the CS+ much like they responded to the conditioned danger cue itself. That is, patients with GAD displayed virtually full generalization of fear from CS+ to the first approximation. By contrast, levels of responding to GS4 deviated significantly from responses to CS+ among healthy comparisons, indicative of less generalization. Whereas, this group effects for startle data and risk ratings directly reflect greater transfer of conditioned responding to GS4 among those with GAD, the group effect for reaction times suggest that GAD patients were equally certain of their risk for shock during CS+ and GS4, whereas healthy comparisons were significantly less certain of their risk during GS4 relative to CS+.
Relation to Past Generalization Findings in GAD
To date, the only published study testing generalization gradients in GAD patients found no abnormalities in behavioral or psychophysiological measures (6). The reason for the discrepancy with current results may result from methodological differences between the two studies. One key difference lies in the way behavioral data were collected. Though, like the current study, Greenberg et al. assessed behavioral generalization by recording participants’ perceived risk of shock to CS+ and GSs, these ratings were collected retrospectively at the end of the experiment, when shock was no longer possible. In the current study, behavioral risk ratings were collected online when shock was a real possibility. As discussed earlier, threat ambiguity signaled by GSs may elicit heightened fear in GAD patients through “what if” speculations (i.e., “I don’t think shock will follow the GS, but what if it does”). If shock is not a real possibility, as was the case in Greenberg’s study, GAD patients may be less likely to engage in this kind of anxiogenic counterfactual thinking, resulting in less overgeneralization of perceived risk. By contrast, in the current study, participant ratings occurred amidst real risks of shock which may increase “what if” speculations in GAD patients and increase their overgeneralization of risk ratings.
Toward a Neurobiology of Conditioned Overgeneralization
Owing to the cross-species relevance of fear-conditioned generalization, neural substrates of overgeneralization, of the kind presently found in GAD, are informed by neurobiological findings in animals. Among them are results suggesting a critical role for the hippocampus, with lesions of either hippocampus (17,18) or cortical inputs to the hippocampus (i.e., postrhinal and perirhinal cortex) (19) increasing generalization of fear from CS+ to resembling safety cues (i.e., CS−) in animals. These findings suggest that hippocampal activations are necessary for successful discrimination of CS+ from GSs, potentially attributable to the pattern separation function of the hippocampus (20), through which brain representations of resembling, yet distinct, sensory experiences are discriminated. Thus, one important candidate neural substrate of overgeneralization in GAD may be deficits in hippocampally-mediated pattern separation of GSs from CS+.
A further area implicated in generalization of classical conditioning in animals is the orbital prefrontal cortex (OPFC) (21). Specifically, rats with OPFC lesions generalize freezing behavior from a context paired with shock to an unpaired context, while intact animals display freezing only in the paired context. Such results suggest that OPFC activations are needed to inhibit fear to stimulus events resembling the CS+, and deficits in such activations among those with GAD may result in overgeneralization.
Contributions from the hippocampus and OPFC toward conditioned fear generalization in humans have been supported by recent fMRI evidence of generalization gradients instantiated in both anterior hippocampus and an area of the OPFC referred to as ventromedial prefrontal cortex (vmPFC) (22). Such findings support a proposed neural model of generalization in which GS presentations elicit hippocampally-mediated pattern separation of GS and CS+ brain representations, leading to activation of vmPFC, and culminating in inhibition of fear to GSs (i.e., successful discrimination and little generalization) (23). According to this model, overgeneralization in anxiety disorders may result from both reduced hippocampally-mediated pattern separation and reduced vmPFC activation in response to GSs. Specifically, anxiety patients may require greater dissimilarity between the CS+ and GS before the hippocampus initiates pattern separation and propogates activation to the fear inhibiting vmPFC. Additionally clinical abnormalities may lie in the vmPFC, whereby fear to the GS fails to be inhibited following “normative” hippocampally mediated pattern separation due to insufficient vmPFC reactivity to hippocampal inputs. This latter prediction receives support from Greenberg and colleagues (17) who found less steep increases in vmPFC activity as the presented GS differentiated from CS+ among those with versus without GAD.
Overgeneralization as a Phenomenological Constituent of GAD
A cardinal feature of GAD is excessive and persistent worry that future events will be negative and disastrous (24). Such worry is often expressed as catastrophic “what if” speculations (8), which serve to bias those with GAD toward threatening appraisals of unclear or ambiguous situations (i.e., interpretation bias) (8–10). That is, less objective threat information is required for someone with, versus without, GAD to interpret a stimulus-event as threatening. Stimuli resembling conditioned danger cues (i.e, GSs) include degraded threat information that may be sufficient to exceed the threshold for threat detection among those with, but not without GAD. In this way, overgeneralization of fear may be viewed as a special case of interpretation bias, in which safe stimuli with resemblance to feared situations are over-interpreted as threatening by those with GAD. This form of generalization-based interpretation bias may contribute to the psychopathology of GAD by transferring fear to situations resembling other anxiety-provoking situations, resulting in the undue proliferation of environmental cues capable of eliciting and maintaining anxious apprehension. Of note, the opposite direction of causality is also plausible. That is, worry and anxious states may predispose an individual with GAD to interpret ambiguous stimuli as threatening, resulting in overgeneralization. Unfortunately, the current study cannot determine the direction of causality and future longitudinal studies are needed to clarify this issue.
Overgeneralization as a Pathogenic Mechanism Cutting Across Traditional Anxiety-Disorder Categories
Abnormally shallow gradients of generalization, reflecting overgeneralization of conditioned fear, have recently been documented in panic disorder (13) and, preliminarily in, posttraumatic stress disorder (11). Such results, together with current findings in GAD, are beginning to paint a broader picture of overgeneralization as a conditioning abnormality operating across the anxiety disorders. Indeed, the shape of shallow generalization gradients across these three anxiety groups were extremely similar, with the exception of a more marked generalization of fear to the first approximation of the CS+ among GAD versus panic or PTSD patients. A common precipitant of overgeneralization in anxiety patients may be an underlying disposition toward reduced thresholds for threat reactivity, resulting in less danger information required for a stimulus to activate the fear system. That is, generalization stimuli with degraded resemblance to conditioned danger-cues may contain sufficient threat information to cross fear thresholds in those with, but not without, clinical anxiety. What may differentiate specific disorders is the type of conditioned danger cue from which fear is overgeneralized. For example, overgeneralization in PTSD, panic disorder, and GAD may be triggered by stimuli resembling trauma cues, panic cues, and features of worrisome situations, respectively. Though overgeneralization is precipitated by different classes of danger cues, once evoked, the underlying mechanics are likely shared across disorders. Studying these shared mechanics is consistent with the NIMH, Research Domain Criteria (RDoC) project, in which fundamental neural mechanisms of mental illness, are dimensionally assessed across disorders. In the current context, shared mechanics likely draw from the neural substrates of overgeneralization described above, and putatively include vmPFC hypo-reactivity to GSs and deficiencies in hippocampally-mediated pattern separation.
Supplementary Material
Figure 2.
Acquisition results across groups and stimulus-type including: (A) standardized startle-blink magnitudes and (B) self-reported risk of shock, where 0=no risk, 1=some risk, and 2=high risk. GAD = generalized anxiety disorder; CS+ = conditioned stimulus paired with shock; CS− = conditioned stimulus unpaired with shock; ITI = intertrial-interval. *p≤.05, ** p≤.01, ***p≤.001
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, and by a grant to SL from the Extramural Research Program of the National Institute of Mental Health [K99 MH080130].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The data in this paper were presented as part of the 3 symposium talks below:
Lissek, S. (November, 2012). Generalization of classically conditioned fear: A central yet understudied marker of clinical anxiety. In D. Hermans (Chair), Fear generalization as a crucial mechanism in the development of anxiety disorders: New insights and findings. A symposium presented at the Annual Meeting of the Association for Behavioral and Cognitive Therapies, National Harbor, Maryland.
Lissek, S. (October, 2012). The role of conditioned fear generalization in the anxiety disorders: A move toward studying both Pavlovian and instrumental contributions. In V. Miskovic (Chair), To fear or not to fear: Excitatory and inhibitory conditioning in the laboratory and the clinic. A symposium presented at the Annual Meeting of the Society for Psychophysiological Research, New Orleans, Louisiana.
Lissek, S. (March, 2011). Generalization of Pavlovian cue-shock conditioning in the anxiety disorders. In D.S. Pine (Chair), Dimensions of psychopathology: Implications for treatment and research in anxiety disorders. Presented as part of the ADAA Annual Research Symposium delivered at the 31st Annual Conference of the Anxiety Disorders Association of America, New Orleans, LA.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Mineka S, Zinbarg RA. Contemporary learning theory perspective on the etiology of anxiety disorders: It's not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Pavlov I. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 5.Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30:242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- 7.Dugas MJ, Marchand A, Ladouceur R. Further validation of a cognitive-behavioral model of generalized anxiety disorder: Diagnostic and symptom specificity. J Anxiety Disord. 2005;19:329–343. doi: 10.1016/j.janxdis.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Aikins DE, Craske MG. Cognitive theories of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:57–74. doi: 10.1016/s0193-953x(05)70206-9. [DOI] [PubMed] [Google Scholar]
- 9.Mineka S. The positive and negative consequences of worry in the aetiology of generalized anxiety disorder: A learning theory perspective. In: Yiend J, editor. Cognition, emotion and Psychopathology Theoretical, empirical and clinical directions. Cambridge University Press; 2004. pp. 29–48. [Google Scholar]
- 10.Roemer L, Orsillo SM, Barlow DH. Generalized anxiety disorder. In: Barlow DH, editor. Anxiety and its disorders: The nature and treatment of anxiety and panic. The Guilford Press; 2002. pp. 477–515. [Google Scholar]
- 11.Lissek S, Grillon C. Learning models of PTSD. In: Beck JG, Sloan DM, editors. The oxford handbook of traumatic stress disorders. New York: Oxford University Press; 2012. [Google Scholar]
- 12.Lissek S, Biggs AL, Rabin SJ, et al. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav Res Ther. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lissek S, Rabin S, Heller RE, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: Effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- 15.Blough D. Steady state data and a quantitative model of operant generalization and discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1975;104:3–21. [Google Scholar]
- 16.Hanson HM. Effects of discrimination training on stimulus generalization. Journal of Experimental Psychology. 1959;58:321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- 17.Solomon PR, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (orcytolagus cuniculus) following dorsal hippocampal ablation. Journal of Comparative and Physiological Psychology. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- 18.Wild JM, Blampied NM. Hippocampal lesions and stimulus generalization in rats. Physiology of Behavior. 1972;9:505–511. doi: 10.1016/0031-9384(72)90005-4. [DOI] [PubMed] [Google Scholar]
- 19.Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav Neurosci. 2002;116:479–488. [PubMed] [Google Scholar]
- 20.O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 21.Zelinski EL, Hong NS, Tyndall AV, Halsall B, McDonald RJ. Prefrontal cortical contributions during discriminative fear conditioning, extinction, and spontaneous recovery in rats. Exp Brain Res. 2010;203:285–297. doi: 10.1007/s00221-010-2228-0. [DOI] [PubMed] [Google Scholar]
- 22.Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nst096. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of pavlovian fear-learning: The case for conditioned overgeneralization. Depress Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text revision (DSM-IV-TR) Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.