Abstract
The a-wave of the electroretinogram (ERG) reflects the response of photoreceptors to light, but what determines the exact waveform of the recorded voltage is not entirely understood. We have now simulated the trans-retinal voltage generated by the photocurrent of dark-adapted mammalian rods, using an electrical model based on the in vitro measurements of Hagins et al. (1970) and Arden (1976) in rat retinas. Our simulations indicate that in addition to the voltage produced by extracellular flow of photocurrent from rod outer to inner segments, a substantial fraction of the recorded a-wave is generated by current that flows in the outer nuclear layer (ONL) to hyperpolarize the rod axon and synaptic terminal. This current includes a transient capacitive component that contributes an initial negative “nose” to the trans-retinal voltage when the stimulus is strong. Recordings in various species of the a-wave, including the peak and initial recovery towards the baseline, are consistent with simulations showing an initial transient primarily related to capacitive currents in the ONL. Existence of these capacitive currents can explain why there is always a substantial residual transient a-wave when post-receptoral responses are pharmacologically inactivated in rodents and nonhuman primates, or severely genetically compromised in humans (e.g. complete congenital stationary night blindness) and nob mice. Our simulations and analysis of ERGs indicate that the timing of the leading edge and peak of dark-adapted a-waves evoked by strong stimuli could be used in a simple way to estimate rod sensitivity.
Keywords: Electroretinogram, a-wave, rod photocurrent, rod photoreceptor, retinal currents, simulated photocurrent
Introduction
The initial negative deflection of the corneal electroretinogram (ERG) that appears when the light stimulus exceeds some minimal strength (Fig. 1A), was first designated the “A” deflection by Einthoven and Jolly (1908). They supposed that this deflection represented a brief transient component of the retinal response that was essentially complete before the onset of a slower and longer-lasting positive component that appeared in the ERG as the b-wave. Although alternative suggestions were made by subsequent investigators (e.g. Waller, 1909), this remained the generally accepted interpretation until Granit (1932) examined the effect of anaesthetics and various experimental interventions on the different “waves” (so named in Hartline, 1925) of the mammalian ERG. Granit’s (1932, 1933) observations of the differential effects of ether and circulatory and respiratory disturbance upon the ERG of decerebrate cats led him to conclude that the a-wave did not directly reflect the existence of an intrinsically brief negative response. Instead it resulted from the cancellation of the later part of a relatively prolonged negative component of short latency by a larger, more labile and slightly delayed positive component. Granit was unable to determine where the positive and negative components originated and simply referred to them as being generated by distinct processes that he called PII and PIII. While Granit’s work was accepted as establishing that the initial negative-going deflection of the ERG was provided by the leading edge of PIII, the retinal origin of PIII remained for some time a matter for speculation (Granit, 1947). Eventual resolution was achieved by recordings made with microelectrodes in retinas of intact cat eyes (see Brown, 1968) that confirmed that PIII was developed across the photoreceptor layers of the retina and by microelectrode recordings in rat retinal slices in vitro (Penn and Hagins, 1969) that demonstrated that the photocurrent originated in the rod outer segments. The intraretinal microelectrode studies in the intact cat eye also indicated a post-receptoral origin for PII (b-wave) across the inner nuclear layer where bipolar cells are located.
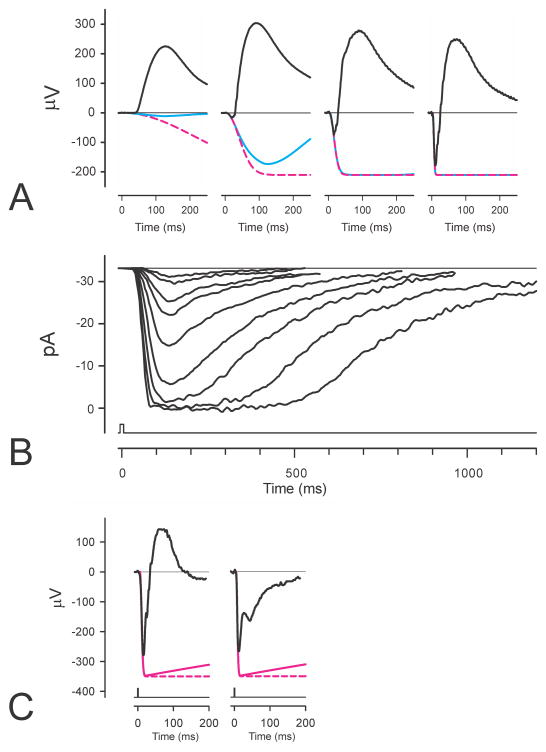
Fig. 1.
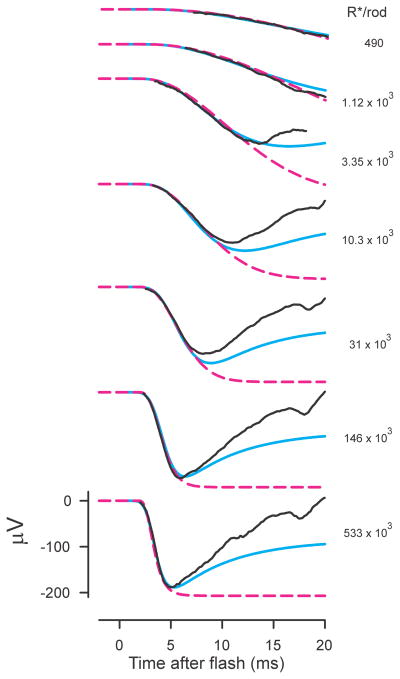
ERGs and photocurrents. A) ERGs (black lines) from anesthetized macaque to blue flash stimuli giving 10, 320, 2600, 55000 R*/rod. Recording methods described by Robson et al. (2003); bandwidth of recordings was 0 – 300Hz. Blue lines show simulations of a 3-stage filter model described in the text and dashed magenta lines show simulations of a basic Lamb and Pugh model. B) Suction electrode recordings (bandwidth 0 – 50 Hz) of the outer-segment photocurrent of a macaque rod to stimuli ranging from about 3 to 860 R*/rod (redrawn from Fig. 2 of Baylor et al. 1984). C) Recordings (bandwidth 0.1 – 300 Hz; stimulus 44.2 cd s m−2) of ERGs of a normal human (left) and a patient with complete CSNB (right) redrawn from Miyake et al. (1994). Dashed magenta lines show the prediction of a Lamb and Pugh model fitted to the leading edge of the a-wave while the solid magenta line shows how this would have been recorded by a system with the same high-pass filter that was used for recording the ERGs.
Identification of the leading edge of the a-wave with onset of the photoreceptor response prompted Fulton and Rushton (1978), in a study of light and dark adaptation, to use the “slope” of a human subject’s ERG a-waves as an objective indicator of rod response and hence of rod sensitivity. However, based on the more specific assumption that the PIII component of the ERG is a direct reflection of rod photocurrent and would have the same timecourse, Hood and Birch (1990a) suggested that the slope of the a-wave would be more appropriately interpreted by taking into account the information that had by then become available about the photocurrent responses of primate rods recorded in vitro using a suction electrode technique by Baylor et al. (1984) (Fig. 1B). In particular, Hood and Birch showed how the slope of the a-wave depended on stimulus strength (as originally reported by Van Norren and Valeton, 1979) in the way that would be predicted by the model that Baylor et al. had used to describe the time course of the photocurrent response of rod outer segments to flashes of light.
Hood and Birch (1990b, 1990c) subsequently examined in more detail the extent to which a model comprising a low-pass filter with multiple stages followed by a saturating non-linearity that well describes primate rod outer-segment photocurrent could also describe the a-waves of ERGs from both normal and abnormal human subjects. They concluded that “over a wide range of flash energies, the amplitude of the leading edge of the a-wave of the human ERG varies with time and flash energy in ways predicted by the model of the light-induced response of the mammalian rod” and that “the capability for recording the electrical activity of human photoreceptors in vivo opens new avenues for assessing normal and abnormal receptor activity in humans.”
The blue lines in Fig. 1A that show the fit of a similar filter model to the one used by Hood and Birch provide an illustration of the ability of such models to describe the initial rising phase of primate (macaque) as well as human a-waves over a wide range of stimulus energies. However, although such models can provide a good fit to a large part of the leading edge of the a-wave, they leave the later return of the ERG towards the baseline, and ultimately to some positive level, to be explained as the result of the slower development of a large positive-going signal, PII, from a post-receptoral source. While there is little doubt that this is the correct explanation for what is seen for responses to a weak stimulus. Hood and Birch (1990b; 1992) assumed that this explanation was appropriate even when the stimulus was strong. They argued that once the model parameters for PIII were ascertained by fitting the leading edge of the a-wave evoked by a strong stimulus, the timecourse of the positive-going component, PII, could be obtained by subtracting the predicted PIII from the recorded ERG. Their quantitative analyses of the waveform of PII were based on this assumption.
These early studies of Hood and Birch set the scene for the later widespread adoption of the modelling approach to the characterization of rod function in both normal and abnormal human and other mammalian species. However, at just the time that the power of this approach was first being appreciated (e.g. Breton and Montzka, 1992), Lamb and Pugh (1992) published a somewhat simpler model of the rod photocurrent generator that was based on a consideration of the steps involved in the transduction cascade within the photoreceptors. To make the modelling simpler, Lamb and Pugh restricted their attention to the generation of photocurrent at short times after a brief but strong stimulus. This made it possible for them to limit their analysis to the activation steps of the cascade and to ignore the inactivation and recovery processes that operate on a much longer timescale. They reported that the early part of the flash response can be understood as the result of three successive integrations, each related to one of the three main stages of the transduction cascade. They also assumed that the effect of the several other minor steps involved could be lumped together and represented by a short fixed time (transport) delay. As a result of making these approximations and adopting these restrictions, Lamb and Pugh were able to show that in response to a strong brief flash of light the rod photocurrent would, after a short delay, rise to a plateau as a Gaussian function of time whose time constant was inversely proportional to the square root of the stimulus energy.
The applicability of this model to describing the rise of photocurrent was first demonstrated for amphibian rods recorded in vitro (Lamb and Pugh, 1992; Pugh and Lamb, 1993) but shortly afterwards it was shown to provide a good description of the rising phase of photocurrent in primate (human) rods in vitro (Kraft et al., 1993) and subsequently in the rods of several other species.
Demonstrations of the applicability of the Lamb and Pugh formulation to rod photocurrent were rapidly followed by demonstrations of its ability to describe the leading edge of human ERG a-waves (Hood and Birch, 1993a; Breton et al., 1994; Cideciyan and Jacobson, 1996; Smith and Lamb, 1997). Indeed, Hood and Birch found that they could obtain a better fit with the Lamb and Pugh model than with the filter models they had previously used. These demonstrations and the basic simplicity of the approximation introduced by Lamb and Pugh led to extensive use of methods that fitted recorded a-waves with the simple Lamb and Pugh model to characterize rod function in several mammalian species as well as in many retinal diseases. The dashed magenta lines show fits of the Lamb and Pugh model to the macaque ERGs illustrated in Fig. 1A. For the stronger stimuli and for the duration of these records, there is no real difference between the predictions of this simple approximation and those of the filter model. However, for stimuli that are not strong enough to saturate the response in a few milliseconds, the Lamb and Pugh approximation is only good for a relatively short time after the stimulus flash. While these limitations make the Lamb and Pugh model inappropriate for dissecting components of the later part of ERGs, it has been seen as particularly appropriate for quantitative characterization of photoreceptor function in both clinical and research situations.
The assumptions that the PIII component of the ERG has the same waveform as that of the rod outer-segment current, and that the deviations of the ERG from the waveform of PIII after the first few milliseconds result from the intrusion of a positive PII component of post-receptoral origin are basic for the modelling approach to the analysis of the a- and b-waves originally introduced and later codified by Hood and Birch (2006). While these assumptions are intuitively appealing and will be closely approximated under certain conditions, there are a number of observations that suggest that they may not be appropriate in all circumstances, particularly when the stimulus is very strong.
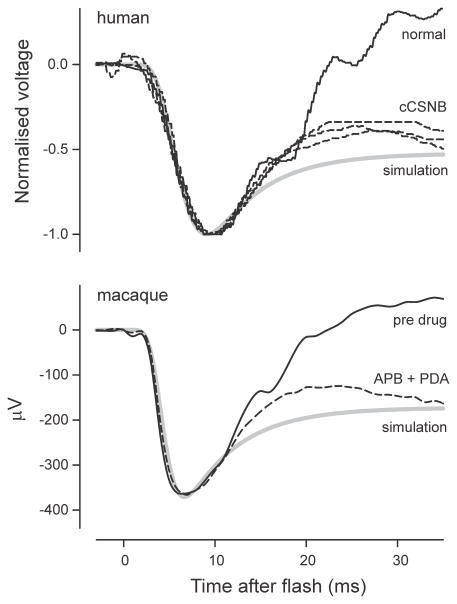
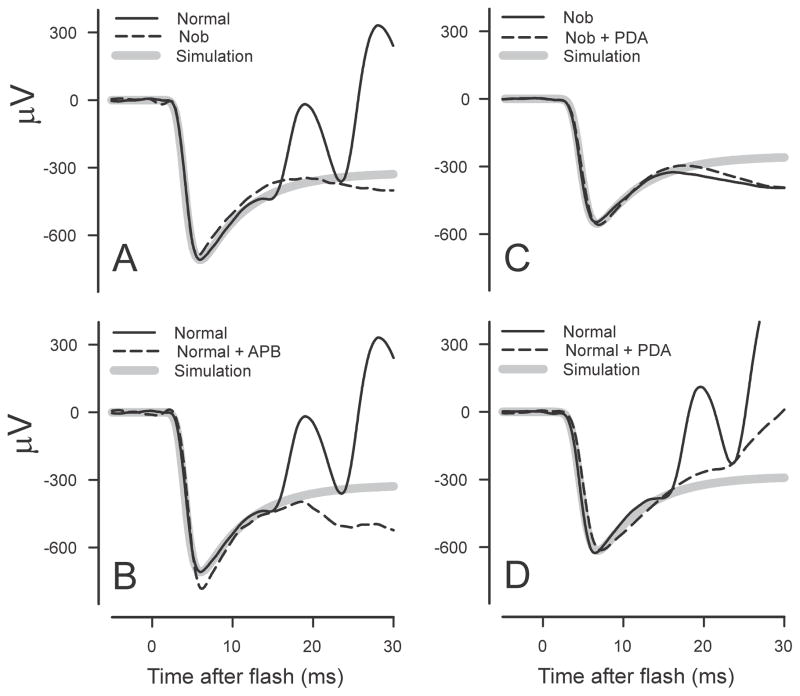
Perhaps the most obvious indication that there may be some problems with the basic assumptions is seen by examining the waveform of ERGs obtained in abnormal situations in which the b-wave is absent or severely reduced in amplitude. Such ERGs are seen in experimental animals when the activity of post-receptoral cells has been blocked pharmacologically (e.g. in macaques, Jamison et al., 2001), in mutant animals in which there is no transmission from photoreceptors to On-bipolar cells (e.g. in nob (Nyxnob) mice, Pardue et al., 1998) and in humans with complete congenital stationary night blindness (CSNB, e.g. Miyake et al., 1986).
A typical example of an ERG obtained from a patient with complete CSNB in response to a stimulus of 44.2 cd s m−2 (about 50,000 R*/rod) is shown in Fig. 1C together with the ERG of a normal subject in response to the same strong stimulus (redrawn from Fig. 3 of Miyake et al., 1994). Also shown in the figure (solid magenta lines) is the predicted rod component obtained by fitting the leading edge of each ERG with a Lamb and Pugh model. It should be noted that the predicted PIII component that putatively forms a part of the recorded ERG (solid magenta line) does not have the horizontal plateau that is the immediate prediction of the model (dashed magenta lines). This is because the clinical ERGs that are illustrated here were recorded with 0.1 to 300Hz filtering which would not only affect the waveform of the ERG but also that of the prediction to give the slight recovery toward baseline (“droop”), that is shown. [Clinical ERGs are commonly recorded with a 1 or 2 Hz high-pass filter which would result in a very much larger “droop” effect]. Neither of the ERGs in Fig. 1C shows any sign of the a-wave peak having become flattened as might be expected even in the normal ERG when the rising phase becomes very fast while, more significantly, a major part of the recovery of the ERG towards the baseline is still present in the CSNB record. Similar “negative ERGs” with a more or less normal initial negative transient followed by a negative plateau at a level about half that of the preceding negative peak can be seen in many other retinal disorders e.g. melanoma associated retinopathy (MAR), juvenile retinoschisis, quinine toxicity (e.g. see Weleber and Francis, 2006; Audo et al., 2008) where transmission from photoreceptors to the inner retina may be assumed to have been compromised.
The failure of removal or absence of PII to convert the ERG to the form predicted by any of the commonly used photocurrent models has previously been reported but not yet satisfactorily explained. Jamison et al. (2001) studied the effect of pharmacologically blocking all post-receptoral neurotransmission with glutamate analogues, L -2-amino-4-phosphonobutyric acid (APB) and cis-piperidine-2,3-dicarboxylic acid (PDA) on the dark-adapted macaque ERG. They saw that the initial recovery towards the baseline of the isolated photoreceptor responses to strong stimuli survived the blockade and suggested “the possibility that voltage gated channels on the primate photoreceptor contribute to this positive recovery phenomenon” though it was not made clear how this would be mediated. Kang Derwent and Linsenmeier (2001) recorded ERG as well as intraretinal signals in dark-adapted cats and also concluded that the initial recovery of the ERG from the a-wave peak was not the result of post-receptoral activity that could be blocked with glutamate analogues. However, they then went on to show that blocking the hyperpolarization-activated cyclic nucleotide-gated (HCN)Ih channels, thought to be located in the membrane of the photoreceptor inner segments, with various agents had no more than a slight effect on the initial a-wave recovery. They concluded that “the recovery of the a-wave following the trough is generated largely, if not exclusively, by the inner segment of the rod photoreceptors” though they did not have strong evidence about its cellular mechanism.
These studies, and others, have all assumed that the failure of a photocurrent model fitted to the leading edge of the a-wave to predict the form of the later part of the flash ERG when there is no positive post-receptoral component present results from some unidentified process that causes the later retinal photocurrent in vivo to differ from that predicted by a model of the outer-segment photocurrent that is applicable in vitro. A basically similar conclusion was reached by Arden (1976, 1977) who recorded voltages generated across various retinal layers of isolated rat retina using microelectrodes. He showed that although the source of the rod photocurrent was the outer segment and the major sink for this current was the inner segment, a large part of the ERG attributable to the extracellular flow of photocurrent was developed across the outer nuclear layer (ONL), the layer containing rod axons and nuclei. This had previously been shown to be the case in rat retinal slices (Penn & Hagins, 1969); however Arden also noted that when the stimulus was strong the voltage developed across the ONL had a waveform that was different from that developed across the layer of outer and inner segments. Whereas this latter voltage had the simple waveform that is associated with outer-segment photocurrent (Fig. 1B), the voltage across the ONL in response to a strong flash of light had a brief initial transient or “nose”. Arden’s observations indicated that the “complexity” that resulted in the “nose” was “located in the nuclear region of the photoreceptor” and suggested that this might reflect the existence of a slowly developing voltage-sensitive membrane conductance in this region that would reduce the extracellular current at later times.
The possibility that it is primarily the earlier rather than the later part of the ERG whose waveform is different from that of the outer segment photocurrent (i.e. that the “nose” is provided by some process that transiently increases the trans-retinal voltage at early times, rather than by a later process that attenuates it) has not previously been considered but is not obviously inconsistent with observations that have been reported. With this in mind, we will consider specifically the factors in the outer retina of mammals that determine the timecourse of PIII for both weak and strong stimuli. Our analysis will be based on the hypothesis that the timecourse of the outer segment photocurrent of rods in vivo is correctly predicted by a model of the filter type that describes the current in vitro but, importantly, that the timecourse of the voltage that is developed across the retina and is recorded as the ERG will be altered as a result of the electrical properties of the rods that modify the currents in and around their axons as well as outer and inner segments. More specifically, we shall show, in simulations, that capacitive currents, not fully considered in previous models of PIII, effectively provide an additional transient component to trans-retinal voltage that is manifest as the “nose” of recorded ERGs evoked by strong stimuli. We shall then show that these simulations can provide a good description not only of the entire leading edge of the a-wave of ERG recordings made in various different studies of several different species but also, for strong stimuli, the time of the peak and the initial recovery towards the baseline.
For the photocurrent portion of the model, in place of using the Lamb and Pugh model that defined only the leading edge of the photocurrent, we will use a model of the filter type that accounts for both recovery and activation of the three major stages of transduction in the outer segment. In its original form this model was applied by Robson et al. (2003) to the photocurrent in macaque retina derived using the ERG paired-flash (test and probe) technique of Pepperberg et al. (1997). For the present study, this filter model, slightly modified, will be fit to the recordings from human rod outer segments in vitro of Kraft et al. (1993) to determine the best form of the model.
In this study we shall describe in turn 1) the modified filter model for the rod outer-segment photocurrent generator, 2) the electric circuit comprising rods and the retinal layers in which they lie, 3) simulations of the trans-retinal voltages that are generated by the extracellular flow of the current from rod outer segments and 4) the ability of our model to explain the waveform of published ERGs recorded from both normal and abnormal individuals of various species and 5) a simple quantitative approach to assessing photoreceptor sensitivity from ERG recordings.
2. Photocurrent of rods in vitro
Recordings of the photocurrent of the outer segment of single rods are made by sucking the outer segment of an individual rod into a micropipette that then surrounds this portion of the rod and effectively electrically isolates it from the rod’s inner segment and axon. Fig. 2A shows recordings made in this way by Kraft et al. (1993) of the outer segment current of a human rod in response to flashes of light with a wide range of luminous energies. Similar recordings of the photocurrents of macaque rods (Baylor et al., 1984, see Fig. 1B) and of other mammalian (e.g. Nakatani et al., 1991) and amphibian rods (e.g. Baylor et al., 1979) all show that in response to a flash of light the photocurrent rises monotonically at a rate that increases with stimulus strength to a peak (after about 150–190 ms in isolated human rods) or, if the stimulus is strong enough, to a well-defined plateau which is then maintained for some time (that can be as long as several hundred milliseconds) before it declines to its pre-stimulus level. While the response of rod outer segments is often described in this way, it is now well established that the response of the rod outer segment to light is in reality a reduction in the continuous current that flows in the dark into the outer segment from the surrounding extracellular space. Thus, since the “dark” current can only fall to zero, as it does with strong stimuli, there is never any sign in rod outer-segment current recordings of an overshoot in the photocurrent prior to it reaching its saturated plateau (zero current) level.
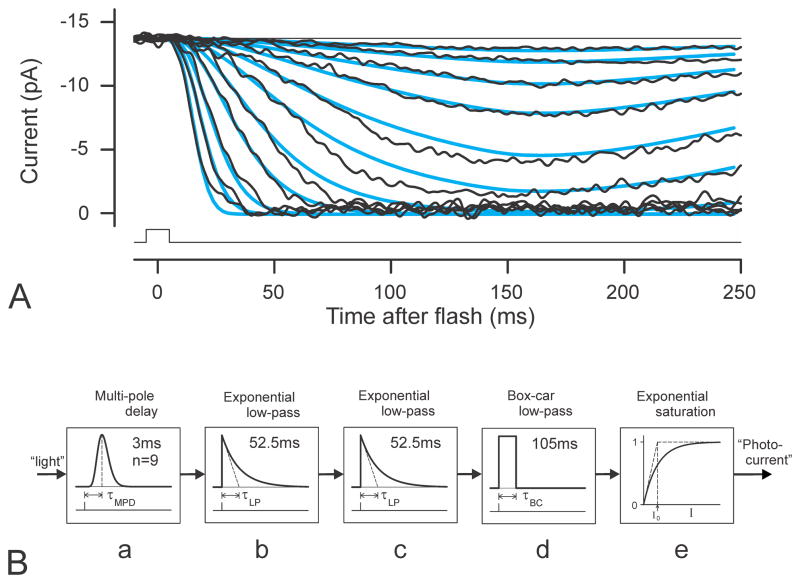
Fig. 2.
Photocurrents and model of photocurrent generator. A) Suction electrode recordings (bandwidth 0 – 100Hz; stimuli ranging from approximately 12 – 5200 R*/rod) of outer-segment photocurrent of a human rod shown in Fig. 1 of Kraft et al. (1993). Blue lines show simulations of the 3-stage filter model described in the text and shown below. Values for dark current, fractional sensitivity and delay time were obtained by ensemble fitting of the model to the recorded data using values for stimulus energy that were very kindly provided by the authors. B) Block diagram of the 3-stage filter model for photocurrent generation used to describe photocurrents of single human rod shown in A) and as input to the model of the electrical circuit of rods and retina (Fig. 5B) for simulations of trans-retinal voltage.
2.1 Modelling the rod outer-segment photocurrent
We have chosen to use Kraft et al.’s (1993) recordings of the outer segment current of a human rod as the basis for the photocurrent component of our simulations of the trans-retinal voltage because of the particular significance of using the ERG to objectively assess photoreceptor function in humans. Kraft et al. showed that the rising edge of the human rod photocurrent responses to a range of moderately strong stimuli (their Fig. 4) was well fitted by a delayed Gaussian function whose time constant was inversely proportional to the square root of flash energy (Lamb and Pugh model). Since the modelling here requires that the whole timecourse of the photocurrent can be simulated, we adopted, as noted above, with slight modification, the model with three simple low-pass stages that Robson et al. (2003) developed to describe the complete timecourse of the response of macaque rods (as derived using the ERG paired-flash technique). A filter with this number of stages, when followed by an exponential saturating nonlinearity, predicts that the response to strong stimuli at early times will have the same Gaussian form as the Lamb and Pugh model and will therefore provide at least as good a description of the rod photocurrent recordings as does that model.
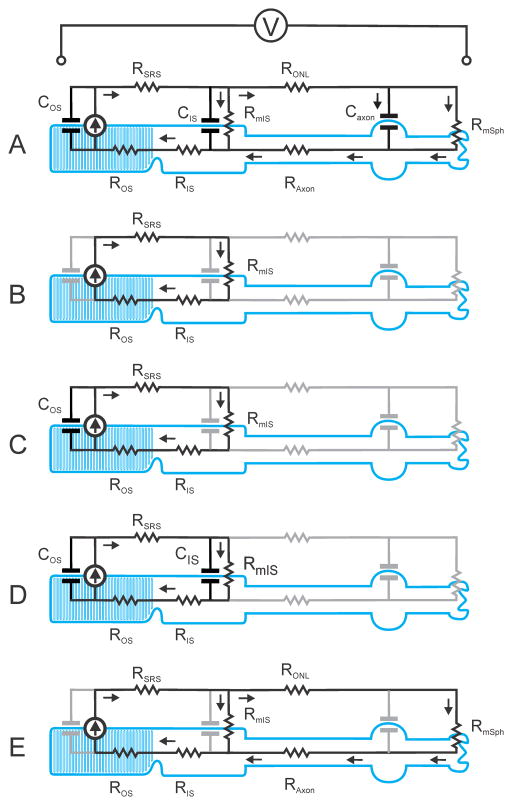
Fig. 4.
Basic lumped-element electrical models of a mammalian rod in situ showing the intracellular and extracellular paths of the photocurrent generated by the current source located in the membrane of the outer segment. The equivalent electrical model of each part of the rod is represented by single components rather than by the distributed arrays that have been used for SPICE simulations (see Fig. 5A). A) Complete (simplified) model as used in the simulations; B) implicit model on which explanation of generation of the ERG is commonly based (e.g. Breton et al., 1994): note that capacitive components in outer and inner segments as well as currents flowing into the rod axon are not considered; C) implicit model including capacitance of outer segment membrane introduced by Smith & Lamb (1997). D) model to take account of membrane capacitance of both outer and inner segments; E) model to explain how voltage-dependent conductance changes in membrane of inner segment could affect the ERG.
2.2 Implementation of a model for rod outer-segment photocurrent generation
In the same way as it was in the model of Robson et al. (2003) the whole transduction process has been realized as a cascade of three linear low-pass filters together with an instantaneous saturating non-linearity at the output. The elements of the present model are shown in Fig. 2B together with the values of the parameters that we believe to be appropriate for human rods in vivo. The input is a signal proportional to the light stimulus which is first delayed (and its waveform shaped) by a “multipole delay” (a multipole low-pass filter labelled “a” in Fig. 2B) that replaces the simple transport delay incorporated in the Lamb and Pugh (1992) model. The impulse response RMPD (t) of a multipole delay with n stages and a response peaking at time τMPD is
| (1) |
A similar modification of the Lamb and Pugh model to include a 3-pole delay was introduced by Cideciyan and Jacobson (1996). However, for all the modelling reported here we have set n to 9 to account for the very rapid rise of the ERG that we have seen at early times after a very strong brief flash (Robson et al., 2003).
Following the initial delay element “b” and “c” are two simple low-pass filter stages each with the same time constant τLP whose impulse response is a decaying exponential
| (2) |
while “d” is a boxcar filter stage with a time constant τBC (where τBC= 2τLP) and whose impulse response is
| (3) |
A “boxcar filter” outputs a “moving average” of its input signal, the average being calculated over an interval equal to the time constant.
These three filter stages together replace the three integrators incorporated in the basic Lamb and Pugh model to approximate the kinetics of the main biochemical stages of the phototransduction cascade. The adoption of low-pass filters with time constants of several tens of milliseconds rather than integrators (low-pass filters with an infinite time constant) results in the model implicitly describing the inactivation and recovery phase as well as the activation phase of the response.
The impulse response, RC(t), of the three-filter cascade combined with the delay element can be obtained by convolving the impulse response functions of all the elements, “a”, “b”, “c” and “d”, together:
| (4) |
These elements are all linear, so that the order in which they are arranged is of no consequence and indeed the several low-pass filter stages of the delay element could also be distributed along the cascade rather than being located at the beginning as implied by the diagram (Fig. 2B). However, this cascade is followed by an instantaneous exponentially saturating non-linearity “e” whose response to an input i is given by
| (5) |
where R̂NL is the maximum value that the response can have and i0 characterises the non-linearity in terms of the level at which R reaches of the maximum.
Putting these together we can calculate the current I (t, P) of a single rod obtained in response to a brief flash of luminous energy producing P isomerisations as
| (6) |
where Î is the maximum current and s is a sensitivity factor that together with the constant k, determines the current produced by a stimulus weak enough for the rod to be operating in the linear range. For convenience, and to simplify comparisons across different species, we have chosen to express the sensitivity as the percentage of the maximum current response of a rod at the time of the peak produced by isomerisation of a single molecule of rhodopsin, 1 R*. This quantity will be referred to as the “fractional sensitivity per isomerisation” (Friedburg et al., 2001), or more briefly as “fractional sensitivity”.
2.3 Simulations of human rod outer-segment photocurrent
The output of the model of Fig. 2B in response to input pulses corresponding to those used by Kraft et al. (1993) to obtain the recordings shown in Fig. 2A were obtained using the behavioural modelling capabilities of SPICE (Simulation Program with Integrated Circuit Emphasis) implemented on a Windows-based personal computer by TINA (v. 9 from DesignSoft, Budapest). As with the fits shown later for ERG recordings, the simulations took into account the duration of the stimulus (10 ms in this case) if this was greater than 1 ms and were filtered in the same way as the original recordings; the blue lines in Fig. 2A show an ensemble fit of the model just described using the stimulus energy values provided by Kraft et al. The photocurrent responses of rods in vitro have often been found to be rather slower than suggested by recordings of ERG in vivo using the paired-flash paradigm. Therefore the time constants of the cascade elements of the model that was used here were adjusted to give a peak at 160 ms (27% longer than for the primate and human ERG records that were subsequently analysed). While not perfect, the quality of the fits over this nearly 1000-fold range of stimulus energies was felt to justify the use of the model for our simulations of trans-retinal voltages for comparison with recorded ERGs. Based on these fits the fractional sensitivity of this human rod in vitro can be estimated as 0.61%, slightly less than the average sensitivity reported by Kraft et al. (0.69%) and considerably less than that derived from human ERGs (about 1% from paired-flash studies of Friedburg et al., 2001, and between 1 and 2% in the present study) or found for macaque rods in vitro (1.5 – 3.5%, Baylor et al., 1984).
2.3.1 Generation of trans-retinal voltage and the ERG by rod photocurrents
There are two reasons why the time course of the direct contribution of the rods to the ERG can be expected to differ from that of the outer-segment photocurrent. Firstly, the ERG, which is a measure of the voltage developed by extracellular currents that flow through the fluid that fills the interstitial spaces in the retina, must to some extent be generated as a result of currents that are effectively driven by the trans-membrane voltage of the inner segment and that flow in and around the axons and cell bodies of the rods in the ONL and the rod terminals in the outer plexiform layer (OPL). Thus, to the extent that the inner-segment voltage response to a strong stimulus manifests a relatively slow partial relaxation towards the resting level following the initial rapid hyperpolarisation (e.g. as observed in macaque rods by Schneeweis and Schnapf, 1995), this will presumably be reflected in the extracellular currents in the ONL and hence, in the ERG. Secondly, it is inevitable that the ERG is partly generated by transient capacitive currents that flow into the proximal regions of the rods in the ONL when the voltage across the inner segment membrane is changing rapidly, an effect that can be expected to become substantial with strong stimuli that result in particularly fast changes of voltage. In order to assess the magnitude of these effects it is necessary to consider which electrical circuit elements are involved and to obtain the best possible estimates of the values of their electrical parameters. We can then define a model of the electrical circuit that is involved in converting the rod photocurrent into a trans-retinal voltage and, by combining this with the photocurrent generator model, use SPICE to obtain simulations of the trans-retinal voltage responses to flashes of light.
We have not, in this study, made any attempt to model voltage-gated changes in membrane conductance, though we shall explain how such changes in the conductance of inner-segment membrane could affect the recorded ERG. We have assumed that voltage-dependent conductance changes are of little significance in shaping the very early part of the ERG because such conductance changes in mammalian rods develop over considerably longer times than the duration of the initial transient nose of a-waves evoked by strong stimuli (<20 ms). Thus, Demontis et al. (1999), recording from guinea pig rods in vitro, illustrated (their Fig. 2B) the membrane current elicited by a membrane voltage step of −35 to −80 mV. This current started to increase as a result of increased membrane conductance after about 50 ms but then continued to rise for at least two seconds before approaching a final value. Even allowing for the fact that these measurements were made at room temperature and this may have slowed down the responses by a factor of 3 or 4, such conductance changes would be too slow to have any significant effect on the rising edge or peak of a-waves obtained with strong stimuli. Kawai et al. (2002) recording from human rods at 37 °C in vitro (with delayed rectifier K+ currents and voltage-gated Ca++ currents both blocked to isolate Ih) reported that the application of hyperpolarizing membrane voltage steps caused the membrane conductance to increase with time as the sum of two exponentials whose time constant depended upon the magnitude of the voltage change. Even with a voltage step to −100 mV (from −60 mV) the time constants were 63 ms and 144 ms. With smaller steps (more likely to be within the physiological range relevant to in vivo ERG recordings the time constants were longer. Their illustrations of currents induced by hyperpolarizing voltage steps also suggest that there may be a delay of at least 10 ms after the voltage changes before the conductance starts to increase.
Although the voltage-gated conductance changes would be expected to have little effect on the rising edge or peak of a-waves evoked by strong stimuli, they might well be of greater significance in shaping the waveform at later times. That conductance changes are of little importance in shaping the very early part of the ERG is confirmed by our simulations in which taking the capacitive currents into account is sufficient to explain the waveform of the rising edge of the a-wave and the existence and timing of both the peak and the initial part of the return of the ERG towards the baseline.
2.4 Electrical properties of rods and their retinal environment
The electrical characteristics of rods are determined by their geometry and the electrical properties of their component materials. While the dimensions of rod outer and inner segments are well known, there is relatively little information available about the exact geometry and dimensions of the more proximal parts of mammalian rods – the axon, the expansion of the axon that houses the nucleus and the spherule that forms the synaptic terminal – and our analysis relies heavily on information relating to rat rods provided by Hagins et al. (1970). Fortunately the rods of mammalian retinas all appear to be quite similar and the rat retina provides a convenient model of mammalian retina both because of its uniformity and because it is has been the subject for classical experimental studies of the sources of the rod component of the ERG (Penn and Hagins, 1969; Hagins et al., 1970; Arden, 1976). As an exception, the axons of rods in the central region of primate retina can be much longer than those of more peripheral rods, and this could well result in the contribution of central rods to the ERG differing from that of the more peripheral rods. However, only a small minority of the rods in the primate retina are located centrally and the large majority of more peripheral rods closely resemble the basic mammalian form. It is these rods that contribute most to the ERG.
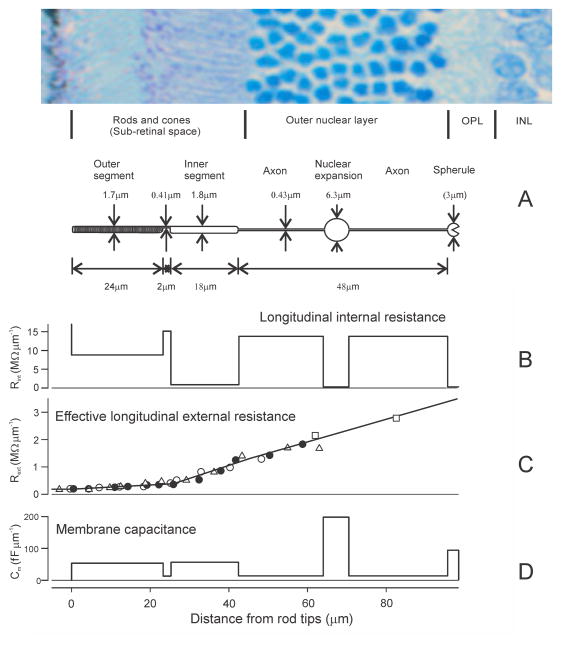
Fig. 3A shows an idealized rod from a rat retina with the average dimensions reported by Hagins et al. (1970) together with a retinal section to show how the different parts of the rod are related to the histologically identified retinal layers. It should be noted that the longest part of each rod is its axon and that this completely spans the outer nuclear layer (ONL) while the spherules at the end of the axons are usually considered to lie just within the outer plexiform layer (OPL). The rod spherules have been arbitrarily represented as spheres with a diameter of 3 μm so as to provide a surface area of 27 μm2 as reported for mouse rod terminals by Zampighi et al. (2011). The nucleus of each rod is contained in a sac-like expansion of the axon that may be located anywhere in the ONL along the length of the axon (not only half way along he axon as shown in the diagram). The diameter of rod nuclei is several times greater than that of rod outer and inner segments so in order to accommodate the nuclei of all the rods in an area of the retina in which the rods are closely packed, the nuclei of adjacent rods are located at different distances along their axons. This makes the ONL appear to be composed of columns of 8 or 9 cells whose nuclei occupy very nearly all the available volume.
Fig. 3.
Dimensions and electrical characteristics of mammalian (rat) rod based on measurements of Hagins et al. (1970). A) Dimensions of the parts of a typical rod and their location within the retinal layers; note that the nuclei of adjacent rods are located at different depths in the outer nuclear layer; B) longitudinal internal resistance of rod cytoplasm (MΩ μm−1) calculated from the dimensions as described in the text; C) effective resistance (MΩ mμ−1) of the extracellular path of the current from each individual rod flowing parallel to the long axis of the rod; symbols other than open squares correspond to those in Fig. 5 of Hagins et al. (1970) for 3 different retinas; open squares plot values derived from Fig. 4 of Hagins et al.; straight lines show the approximation used in simulating the extracellular voltages generated by the flow of photocurrent: D) the capacitance (fF mμ−1)between extracellular space and cytoplasm provided by the plasma membrane of the rod, calculated assuming the specific capacitance of the membrane is 1 μF cm−2. The transverse section of rat retina at the top of the figure was kindly provided by Dr Michael Twa.
Assuming that the rod has a circular cross-section along its length, the longitudinal internal resistance per unit length can be calculated by assuming that the bulk resistivity of the cytoplasm is 200 Ωcm (the value adopted by Hsu et al., 1998) and that the effective cross-sectional areas of the outer and inner segments are 10% and 90% of the total geometric area respectively (as suggested by Hagins et al., 1970). The values for a rod with the dimensions shown in Fig. 3A are shown in Fig. 3B.
To estimate the effective resistance of the path taken by the external current of each rod as it generates the ERG it is necessary to assume that all rods are equally stimulated (as they are assumed to be by a ganzfeld stimulus) and that they all generate the same photocurrent so that the path of the photocurrent will be radial through the retina over very nearly all of its extent. In this case the effective resistance of the external path of the current of a single rod can be obtained from measurements of the resistivity of the interstitial space surrounding the rods by taking into account the number of rods per unit area of the retina. Assuming the density of rat rods is 3.1 × 107 cm−2 (Hagins et al., 1970), the effective resistance per unit length of the external current path of a single rod is ρ × 3.1 × 103 Ωμm−1 where ρ is the measured resistivity in Ωcm. Fig. 3C reproduces the measurements of specific interstitial resistivity of the rat retina (plotted as the resistance per unit length of the external current path) provided by Hagins et al. (1970) and shows the straight-line approximation used in our modelling. Less complete measurements made in whole-mount rat retina by Arden (1976) and in intact eye of macaque monkey (at least as far out as the boundary between inner and outer segments) by Heynen and Van Norren (1985), confirm the steady rise in extracellular resistivity through the retina and are in reasonable agreement with the more detailed observations of Hagins et al. (1970) in rat.
The electrical model of the rod must also incorporate the electrical properties of the cell membrane. Like all normal cell membranes, the rod cell membrane is assumed to have a capacitance of 10 fF μm−2 (equivalent to 1 μF cm−2) and a leakage conductance of 200 fS μm−2 (equivalent to a specific resistance of 50 kΩ cm2). The variation in specific capacitance of the membrane along the length of the rod is shown in Fig. 3D.
2.5 Simplified electrical circuit models of the rod and its retinal environment
Although quantitatively satisfactory simulation of the extra-cellular voltages produced by light stimulation of the rod requires that the rod be represented as a multiple compartment model (e.g. see Hsu et al., 1998) and an electrical circuit model of this kind (Fig. 5A) will be introduced later on, the basic nature of the model that we have used and how it is related to models that have been implicitly invoked in previous studies can be more easily appreciated by inspection of the several simplified circuit models shown in Fig. 4.
Fig. 5.
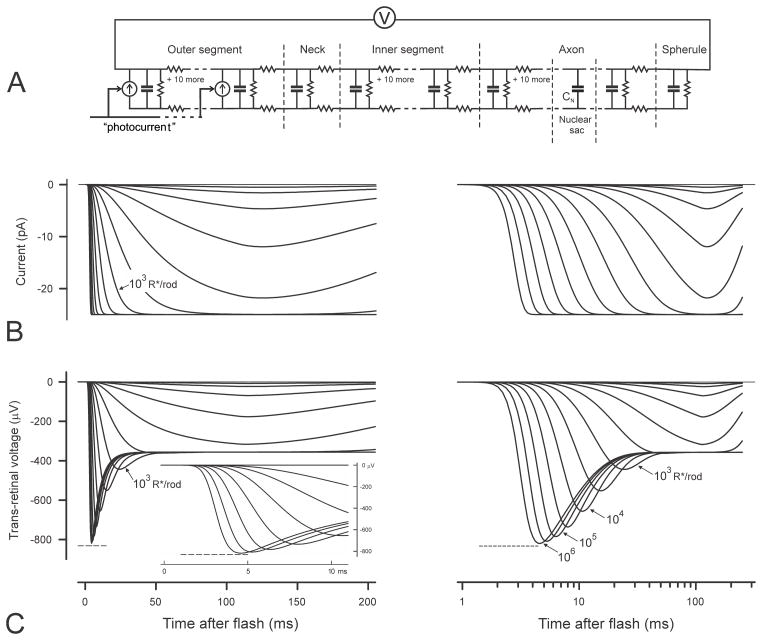
SPICE model of a rod and simulations of photocurrent and trans-retinal voltage. A) Equivalent electrical circuit of a rod with distributed components used for SPICE simulation of trans-retinal voltage responses to outer-segment photocurrent. The photocurrent is provided by 12 signal-controlled current generators located in the membrane of the outer segment. The current-controlling signal is provided by the output of the transduction model shown in Fig. 2B. Outer and inner segments as well as the axon are each assumed to be ladder networks with 12 sections. B and C) Simulations of photocurrent and trans-retinal voltage responses to brief flash stimuli that give rise to 1 – 106 photoisomerizations per rod. B) shows photocurrent simulations while C) shows trans-retinal voltage simulations. The same simulated responses are plotted on both linear and logarithmic time axes to enable all important features to be more clearly seen. All simulations were obtained assuming a fractional sensitivity of 2% per photoisomerisation (i.e. assuming that the amplitude of the photocurrent generated at the time of the peak by a single photoisomerisation in a rod is 2% of the maximum possible) and a dark-current of 25 pA.
Figs. 4A – E all show the basic arrangement of the main elements that comprise the electrical circuit that must be considered in order to interpret the ERG. For a full understanding of the way in which the photocurrent generates a voltage across the retina all elements must be included and are shown in black in the complete simplified model of Fig. 4A. The other panels represent various versions of the model in which different subsets of the elements, indicated by the grey symbols, have been left out of consideration, the effective model circuit being reduced to what remains in black.
The rod appears in the cartoons as having three basic parts, an outer segment, an inner segment and an axon (including the nuclear expansion and terminal spherule). Each part is represented in these simplified diagrams by an internal axial (cytoplasmic) resistor, a longitudinal extracellular resistor and a shunt (membrane) capacitor; the extracellular resistors related to the outer and inner segments have been combined as a single resistor related to the whole subretinal space. For more exact modelling the leakage conductance of the membrane of each part must also be included but is so small that all shunt resistors providing this conductance have been omitted for clarity from the simplified diagrams. On the other hand, the inner segment and axon terminal are shown with shunt resistors to take account of the specialised membrane conductance of these structures while an ideal current generator (shown as the arrowed symbol) is located in the membrane of the outer segment to represent the source of the photocurrent. An ideal current generator is one whose internal slope resistance is very high so that the current that flows from the generator into any circuit to which it is connected is essentially independent of the voltage that is thereby generated; the current-voltage relationship of rods exhibits this kind of characteristic over the range of voltages relevant to normal operation (e.g. see Yau, 1994).
The extracellular pathway in the simplified models is represented by two resistors, one in the subretinal space through which the full outer-segment current flows and a second in the ONL that carries that fraction of the photocurrent that does not return to the outer segment through the membrane of the inner segment but re-enters the cell via the membrane of the rod axon. The trans-retinal voltage is the sum of the voltages developed by the extracellular currents that flow through these two resistors.
In the very simplest version of the model (the model implicitly invoked by Hood and Birch, 1990a, b, c) shown in Fig. 4B, there are no functional capacitors and the only extracellular current that is taken into account is the current that flows through the extracellular fluid in the subretinal space before passing through the membrane of the inner segment to return to the interior of the outer segment via the cytoplasm. It is the passage of this current through the resistance of the extracellular path that has been assumed to give rise to the electrical voltage that can be recorded between electrodes that are effectively connected to the proximal and distal surfaces of the relevant retinal layers. As the extracellular photocurrent flows through the subretinal space it develops a voltage difference across the layer of rod outer and inner segments that is directly proportional to the product of the current and the resistance, RSRS, of the extracellular current path. If the current is provided by a generator with very high internal resistance it will be independent of the resistance of the cytoplasm and that of the membrane of the inner segment. Thus the time course of this current, and hence that of the trans-retinal voltage, would be an exact scaled version of the outer segment photocurrent, as is shown for the filter model prediction in Fig. 1A, and would not be affected by voltage-induced changes in conductance of the inner segment membrane.
However, as first pointed out with respect to cone ERGs by Hood and Birch (1993B, 1995) and with respect to rod ERGs by Cideciyan and Jacobson (1996) and Smith and Lamb (1997), when the stimulus is strong enough for the photocurrent to rise in only a few milliseconds, a significant fraction of the rising current would flow into the capacitance of the outer-segment membrane and the residual current flowing in the subretinal space would be effectively slowed and delayed, an effect that they believed was reflected in some features of the time course of the rising edge of the a-wave. In interpreting the ERG in this way, these authors implicitly assumed the electrical model of Fig. 4C, that is a model that includes the capacitance of the outer segment membrane but takes no account of the capacitance of the inner segment. This model would predict that rod ERGs would have the same waveform as that of the photocurrent but appear to have been filtered by a low-pass filter with a time constant of very approximately 1ms, this being the product of the membrane capacitance of the outer segment and the effective membrane resistance of the inner segment and the internal longitudinal resistance of both segments added together. However, if the capacitance of the membrane of the inner segment were also taken into account (Fig. 4D), the low-pass filtering effect that would be seen in its absence would in fact be partially nullified by the high-pass filtering effect related to its inclusion in the otherwise purely resistive pathway of the external photocurrent.
Most published studies have also not considered the significance of current flow through and around the rod axon in generating the ERG. In the mammalian retina not all the extracellular photocurrent from the rod outer segment passes directly from the sub-retinal space into the inner segment via its membrane. Some small fraction of the current flows through the extracellular space of the ONL and OPL to enter the rod via the membrane of the rod axon and the terminal spherule and thence to return to the inner segment via the cytoplasm of the axon, as illustrated in Fig. 4 A and E. If we assume that the conductance of the axon membrane is negligible and we also neglect, for the moment, the effects of membrane capacitance (as shown in Fig. 4E), then the fraction of the current that is returned to the inner segment via the axon will simply depend upon the relative resistance of the two parallel paths involved, i.e the relative values of RmIS and the sum of RONL, RmSph and RAxon. This implies that the magnitude of the current flowing through RONL, and hence the voltage developed across the ONL, will depend directly upon the membrane resistance of the inner segment, RmIS, and thus will reflect any voltage-dependent changes in the conductance of the inner-segment membrane that occur during the response of the rod. Another way to look at this is to note that the current through RONL is directly dependent upon the voltage across the membrane of the inner-segment and will therefore reflect the timecourse of the inner-segment photovoltage. For this reason it is to be expected that the voltage recorded across the whole retina will contain a component showing the delayed partial relaxation seen by Schneeweis and Schnapf (1995) in the photovoltage of macaque rods in vitro.
The relative magnitude of the voltage (across RONL) whose waveform is similar to the photovoltage compared with the voltage (across RSRS) whose waveform is similar to the photocurrent will depend not only upon how the current from the outer segment divides between the two parallel paths into the inner segment but also on the relative resistance of RONL and RSRS. In this context it may be noted that the effective length of the extracellular current path in the ONL is considerably greater than that in the subretinal space (Fig. 3A) and its specific resistivity is also much higher (Fig. 3C) thus making RONL much greater than RSRS and implying that even a small fraction of the photocurrent flowing through the ONL could give rise to a considerably larger fraction of the trans-retinal voltage. This is consistent with the finding of Penn and Hagins (1969) and Arden (1976) in rat retina in vitro that both the steady voltage difference in the dark and the light-evoked response were larger when measured across the ONL than across the layer of rod outer and inner segments.
Although operation of the circuit of Fig. 4E in which membrane capacitance has not been considered already indicates that the waveform of the trans-retinal voltage might to some extent resemble that of the inner-segment photovoltage, which manifests a relatively slow delayed relaxation towards the resting level, a further modification of the recorded waveform will be provided by current that flows into the inner segment through the capacitance of the axon membrane (Caxon in Fig. 4A) when the voltage across the membrane is changing rapidly (i.e. when the stimulus is particularly strong). The principal path for this current is provided by the capacitance of the membrane of the axon (in total about 2 pF), more than half of which is the capacitance of the membrane surrounding the rod nucleus. Assuming that the rod nuclei are effectively half-way along the axon and the total longitudinal resistance of the cytoplasm is about 1 GΩ, we can calculate the time constant of the series combination of axon capacitance and axon resistance to be about 1 ms. This means that with stimuli that give rise to photocurrents that change by a substantial amount on a timescale of milliseconds a significant fraction of the extracellular photocurrent in the ONL that enters the axons will do so through the capacitance of the axon membrane. This capacitive current will be roughly proportional to the rate of change of inner segment membrane voltage and will therefore primarily be associated with the initial rise of the photocurrent produced by strong flash stimuli. The effect of these capacitive currents will be to add an initial transient nose to the voltage recorded across the retina, an effect associated with the high-pass filtering action of this circuit arrangement.
Although, as noted above, a substantial portion of the total trans-retinal voltage due to rod photocurrents is contributed by currents flowing in the ONL and might therefore be expected to show the effects of voltage-dependent conductance changes in the outer-segment membrane, ERG recordings made in animals in which Ih channels have been blocked (in cats, Kang Derwent et al., 2001) or are nonfunctional (in HCN1 knock-out mice, Knop et al., 2008) indicate that such effects for the Ih channels may be insignificant in vivo at least for the initial “nose” in these species This may be because the voltage levels required to evoke the conductance changes are not reached in vivo or because they are too slow to be evident at the early times that the a-wave nose appears. In any case, we have not attempted to model the effects of such conductance changes but have concentrated on finding out to what extent the time course of the a-wave can be understood in terms of the capacitive currents in the ONL.
3. Simulating the trans-retinal voltage
3.1 Electrical model of rods and retinal environment for SPICE simulations
Quantitative simulations of the voltage that would be recorded across a retina composed of rods having the characteristics that have been described can be obtained using numerical methods once a sufficiently accurate model of the electrical circuits involved has been formulated and after an appropriate photocurrent source has been defined. The simulations that are reported here were performed using SPICE and Fig. 5A shows the equivalent circuit that was used. The three major parts of the rod (outer segment, inner segment and axon) are modelled as ladder networks having 12 sections, each section corresponding to a short length of the rod (1.5 – 4 μm).
For simplicity, the figure shows only the first and last section of each ladder (i.e. there are 10 additional sections in each of the three major ladders that are not shown) together with the single sections representing the neck region between outer and inner segments and the terminal spherule. Apart from the terminal spherule every section has two series resistors to represent the longitudinal extracellular and cytoplasmic resistances and a parallel combination of capacitor and resistor to represent the membrane conductance and capacitance. Each section of the outer segment also has a signal-controlled current generator connected between the internal and external nodes. The signal controlling these current generators is provided by the phototransduction model already described whose output simulates the waveform of the photocurrent that would be recorded from a single rod outer segment (see below). The values of the cytoplasmic resistors and membrane capacitors were constant within each ladder and were set according to the information given in Figs. 3B and 3D. The value of the external resistors varied along the length of the rod according to the straight lines drawn through the data points of Fig. 3C. All resistors representing membrane conductances of the outer segment and the axon were assigned values corresponding to a specific membrane-leakage resistance 50 kΩ cm2. The shunt resistor of the terminal spherule was somewhat arbitrarily set to be 10 GΩ while the 12 shunt resistors of the inner segment were adjusted to give a total input resistance of ~1 GΩ as measured at the inner segment of macaque rods by Schneeweis and Schnapf (1995). The capacitors representing the axon’s nuclear expansion and the terminal spherule were assigned the values 1.25 and 0.3 pF respectively according to the estimates of their surface area.
Although in the much simplified model of Fig. 4A the rod nuclei were all assumed to be located at the same position between the inner segment and the terminal spherule, in the SPICE model it was assumed that the nuclei, and hence the additional membrane capacitor, CNuc could be in any one of five equally-spaced positions along the length of the axon.
3.2 Combining the model photocurrent generator with the electrical model
For the SPICE simulations of the trans-retinal voltage the output signal of the phototransduction model (already described) was applied equally to all the signal-controlled current sources located in the membrane of the rod outer segment. The transconductance of these current sources was set so that a total photocurrent of 25 pA was generated in the outer segment of each rod when the output of the photocurrent simulator was saturated. This value was chosen as a round figure compromise between the Baylor et al. (1984) average value of 34 pA for macaque rods and Kraft et al.’s report of “up to 20 pA” in human rods. The scaling factor relating stimulus luminance to the input to the photocurrent simulator was chosen to provide the desired fractional sensitivity for photocurrent generation i.e. the desired fraction of the maximum peak photocurrent that would be produced by a single isomerisation in each rod.
The time-to-peak of the response of the photocurrent simulator to a brief weak flash is primarily determined by the time constants of the three low-pass filter stages of the model. Based upon paired-flash measurements of the time course of rod response to weak flashes these were set to provide a time-to-peak of 125 ms for simulating primate ERGs (based on the human ERG paired-flash study of Friedburg et al., 2001) and to 100 ms for rodent ERGs (mouse paired-flash study of Hetling and Pepperberg, 1999). It is possible that these times may be a little shorter than would be found if the rods were fully dark-adapted since the paired-flash procedure is likely to result in the rods being slightly light-adapted. However, errors in this parameter will have little effect on the waveform at early times, though it will have a small effect on the estimated sensitivity.
3.3 Simulated photocurrent and trans-retinal voltage waveforms
Fig. 5B shows examples of simulated rod outer-segment photocurrent and Fig. 5C of simulated trans-retinal voltage waveforms generated by the model just described for brief stimuli having a wide range of energies providing from 1 to 106 R*/rod (isomerizations per rod) in steps of 0.5 log units (i.e. 1, 3.16, 10, 31.6,…106). The left hand plots show the simulations on a linear time scale while the right hand plots show the same simulations on a logarithmic time scale. For these examples the parameters of the low pass filters of the photocurrent simulator (n = 9, τMPD = 3ms, τLP = 52.5ms, τBC = 105ms) were set to provide the time-to-peak presumed to be typical of non-saturating responses of dark-adapted human rods, 125 ms, with an initial delay of 3 ms. The parameters of the electrical model of the rods in the retina are those derived for the rat from the observations of Hagins et al. (1970). The dark current of each rod was arbitrarily set to 25 pA and it was assumed that isomerisation of a single rhodopsin molecule would reduce this by 0.5 pA at the peak, i.e that the fractional sensitivity would be 2% per isomerisation a value somewhat higher than the 1% reported by Friedburg et al. (2001) who suggested that the rods in their paired-flash experiments might not have been fully dark adapted.
3.3.1 Simulated photocurrent
Fig. 5B plots the simulated outer-segment photocurrent of an individual rod and reproduces very well the familiar form of the sets of current recordings for stimuli of different strengths made by many authors from mammalian rods (e.g. Fig. 1A of Kraft et al., 1993) showing a waveform that rises monotonically to a peak, or, for stronger stimuli, to a maintained plateau, before relaxing towards the baseline. The plot of photocurrent on a logarithmic time scale (right hand plot of Fig. 5B) displays a feature that is not so clear in plots having a linear time axis that has been scaled to show a substantial part of the complete response, namely the progressive displacement to shorter times of the whole leading edge of the response (including the very earliest rise from the baseline) with increase in stimulus energy. This behaviour is a reflection of the representation in the model of the initial delay of 3 ms by a 9-pole low pass filter as described earlier, rather than by the simple transport delay (equivalent to a multipole filter with an infinite number of stages) used in other models
3.3.2 Simulated trans-retinal voltage
Fig. 5C plots simulations of the voltage across the neural retina that would be generated by the set of photocurrents shown in Fig. 5B. The figure shows that for the weakest stimuli the voltage waveform is quite similar to the photocurrent waveform, rising monotonically to a peak though this is at a slightly earlier time (about 10 ms earlier) than the outer-segment current peak. For somewhat stronger stimuli, the voltage, like the current, also appears to approach a plateau. However, when the stimulus energy is increased further still (to a level between 316 and 1000 R*/rod), the voltage waveform becomes clearly different from that of the photocurrent, developing an initial transient nose whose peak occurs much earlier than the primary peak associated with weaker stimuli. Fig. 5C also shows that the peak of this nose, which first becomes apparent about 30 ms after the stimulus just prior to the establishment of the plateau, gets steadily larger and earlier as the stimulus energy is increased to very high levels and the rising edge of the photocurrent becomes steeper (see inset to Fig. 5C) and earlier. When the energy is raised to give 106 R*/rod, the peak appears less than 5 ms after the stimulus and has an amplitude more than twice that of the later plateau. It should be kept in mind that the stimulus level at which the nose appears as well as the amplitude of the response at early times relative to the final plateau level will depend upon the actual value of the fractional sensitivity of the rods (arbitrarily assumed here to be 2% per isomerisation) while the voltage generated for all stimuli will be scaled proportionately if the dark current differs from the value that has been assumed (25 pA). It can be seen from Fig. 5C that even with a dark current in each rod as low as 25 pA the amplitude of the initial nose of the voltage across the neural retina evoked by strong stimuli can exceed 800 μV.
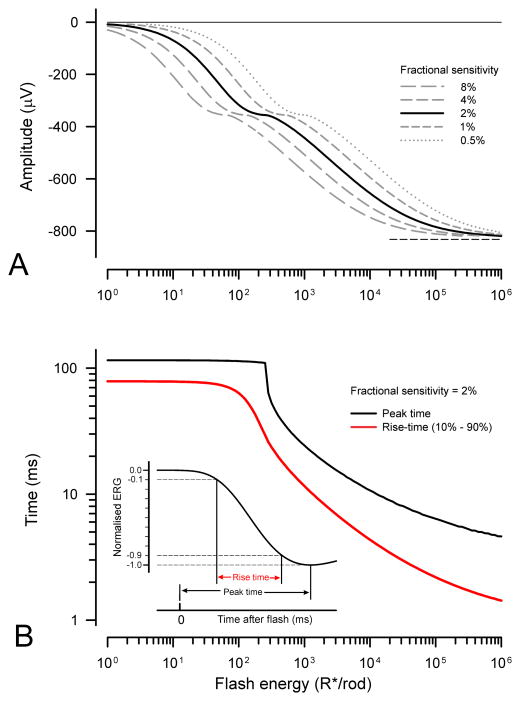
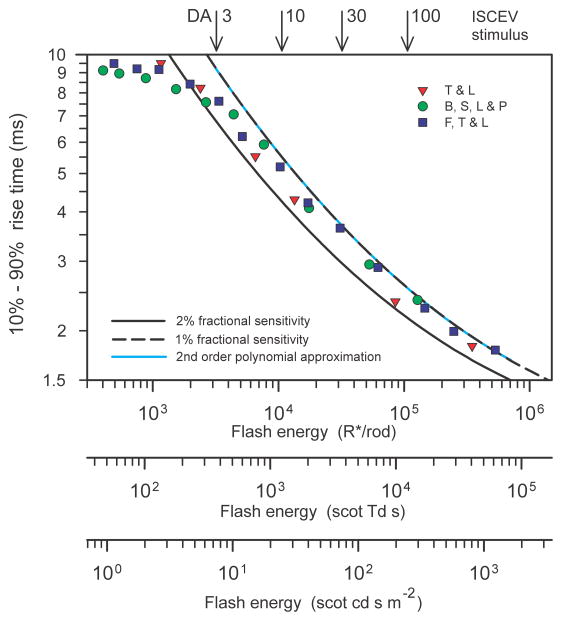
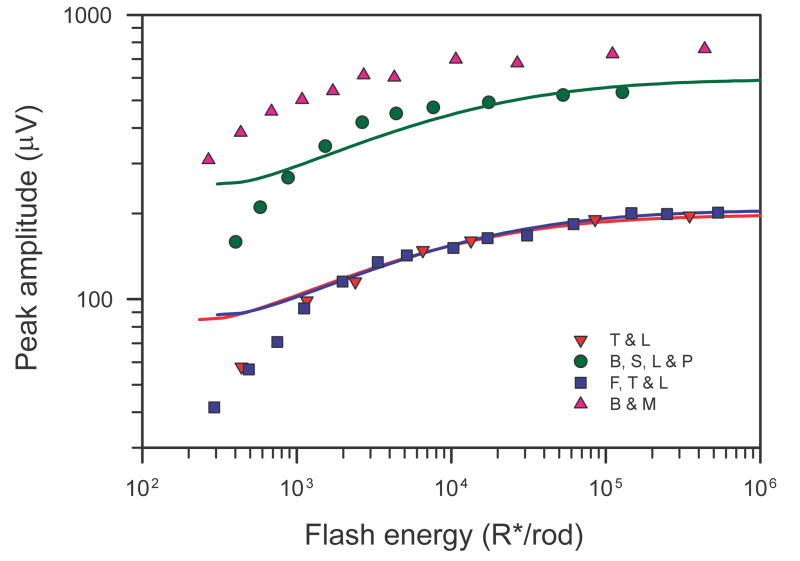
3.4 Amplitude and timing of the peak and rise time of the leading edge of the simulated trans-retinal voltage
Based on the simulations illustrated in Fig. 5 we can predict the way in which the amplitude and time of the peak of the trans-retinal voltage responses grow with stimulus energy. These predictions are shown in Fig. 6A and 6B for a fractional sensitivity of 2% per isomerisation (solid black lines). Fig. 6A also plots the amplitude for other fractional sensitivities (interrupted grey lines); these are the same curve displaced along the logarithmic axis by an amount determined by the factor by which fractional sensitivity differs from 2%. The peak amplitude (Fig. 6A) initially increases linearly with stimulus energy but then clearly begins to level off (at about 360 μV) as the stimulus energy reaches the level at which the photocurrent becomes saturated (i.e. at about 300 R*/rod if the fractional sensitivity is 2%; see Fig. 5B). Over this range of energies the time of the peak (black line in Fig. 6B) remains very nearly constant at 115 ms, the value obtained for 1 R*/rod. However, when the energy is increased further, the peak amplitude starts to rise again as a result of the appearance of an early nose in the trans-retinal voltage that has become obvious by the time the stimulus energy reaches 1000 R*/rod. The amplitude of the nose, and hence that of the whole trans-retinal voltage, continues to grow with increasing stimulus energy until the latter ultimately levels off completely at 832 μV (as indicated by the short dashed lines in the figures) when the stimulus energy exceeds 106 R*/rod. This ultimate value for the peak amplitude is a little more than twice the amplitude that corresponds to the initial saturation of the photocurrent. Corresponding to the secondary rise in amplitude, the time-to-peak (Fig. 6B) steadily shortens to become rather less than 5 ms when the stimulus energy is 106 R*/rod.
Fig. 6.
Amplitude and timing of the simulated trans-retinal voltage. A) Peak amplitude of simulated trans-retinal voltage responses illustrated in Fig. 5C as a function of stimulus energy for different assumed fractional sensitivities. The asymptotic value of the amplitude (832 μV) is shown by the horizontal dashed line. B) Time-to-peak (black line) and 10% – 90% rise time (red line) of simulated trans-retinal voltage responses as a function of stimulus energy for 2% fractional sensitivity. Inset shows definition of peak time and rise time.
Although both amplitude and timing of the trans-retinal voltage depend upon the stimulus energy, it can be presumed that only the amplitude and not the timing of recorded ERGs will also depend upon such arbitrary factors as the the size of the eye or the nature and positioning of the electrodes. Thus it may be supposed that some measure of the timing such as the time of the initial peak for a stimulus of known energy could be used as a direct indication of the fractional sensitivity of the rods. However, although not explicit in the simulations we have illustrated, the time-to-peak will depend not only on the stimulus strength and fractional sensitivity but also upon the duration of the short time delay that is incorporated into the photocurrent generator model (arbitrarily set to 3 ms in these simulations). There is no obvious way in which the duration of this transduction delay in real ERG records can easily be determined but there are measures of the response timing other than the time of the peak that do not depend upon the internal delay. One measure for waveforms such as these which show a clear peak is the time required for the signal to rise from some small fraction to some large fraction of the peak amplitude. The time for a transient signal to rise from 10% to 90% of its maximum value is commonly used to define the speed of step-like electronic signals and this measure (see inset to Fig. 6B) can conveniently be used here to quantify the rise time of the trans-retinal voltage (10%–90% rise time).
The red line in Fig. 6B shows how the 10%–90% rise time (subsequently simply “rise time”) of the simulated trans-retinal voltage responses depends upon stimulus energy. The effect on the rise time of increasing stimulus energy is not very different from the effect on the peak time although, when the stimulus is very strong, the rise time falls rather faster with increasing stimulus energy than the peak time. This potentially makes the rise time a slightly better indicator of the fractional sensitivity than the peak time, quite apart from the benefit relating to the lack of dependence of the rise time upon the brief transduction delay and instrumental delays introduced by the recording system. It should be remembered, however, that the simulations illustrated in Fig. 5, and the derived quantities illustrated in Fig. 6, assume that the stimulus pulse is brief compared with the times after the stimulus at which the amplitude of the response is measured. In practice, this condition will be met if the stimulus duration is less than about 1 ms, a duration that is easily achieved with xenon flash tubes, but may be more difficult to obtain with LED stimulators. For strong stimuli that last longer than this it becomes necessary to recompute the responses of the model to take account of the stimulus duration and waveform.
4. Comparison of trans-retinal voltage simulations with recorded ERGs
In this section we will compare simulations of the trans-retinal voltage with recorded ERGs that have appeared as illustrations in various publications or have been obtained in the authors’ laboratory in the course of studies whose primary results have been published elsewhere. Animal recordings illustrated in Figs. 7–11 were all done in anesthetized preparations following appropriate guidelines for animal research.
Fig. 7.
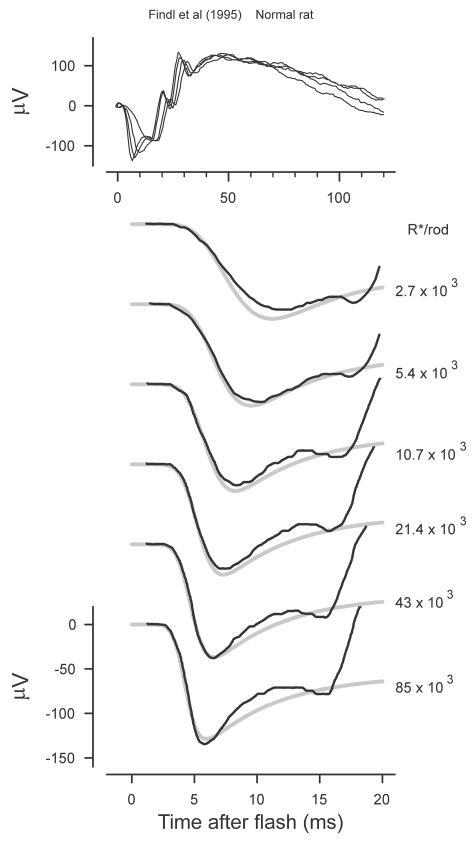
Rat ERG. White light ERGs recorded from a normal rat (redrawn from Findl et al., 1995, Fig. 2A). The grey lines plot simulations with a time delay of 3.8 ms, a time-to-peak of the single photon photocurrent response of 100ms and a fractional sensitivity of 7% per photoisomerisation.
Fig. 11.
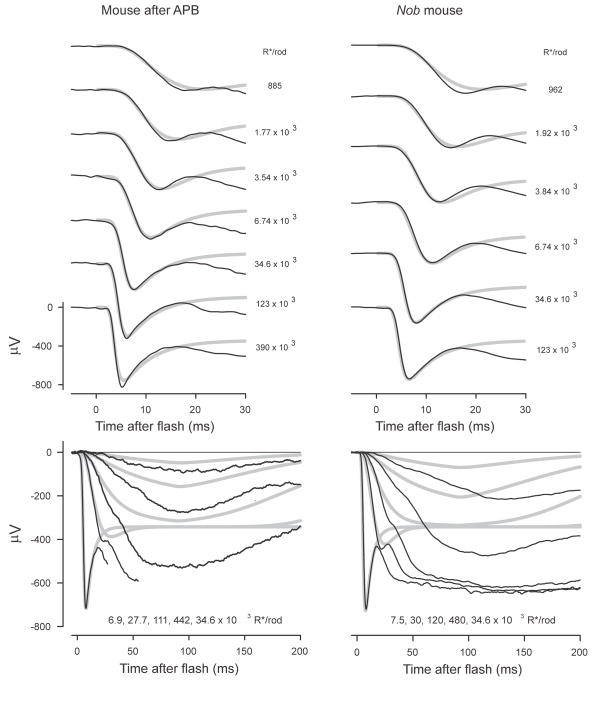
Mouse ERG. ERGs from a normal mouse after intravitreal injection of APB to block On-bipolar cells (left hand column; mm309) and from a nob mouse (right hand column; mm447). Recordings obtained in the course of the study described by Kang Derwent et al. (2007).
4.1 General considerations for these comparisons
Although we do not have a model that we can use to predict the exact relationship between the amplitude of the rod component of the corneal ERG that is generated by a ganzfeld flash in an intact eye and the trans-retinal voltage that gives rise to it, we shall assume, for purposes of comparing recorded ERGs with predictions of our models, that these two signals are identical apart from some unknown amplitude scaling factor. On the basis of this assumption we can expect that, to the extent that our models of the photocurrent generator and the electrical circuit of the retina are realistic, the rod component of the ERG in response to a flash of any given energy will have the same timecourse as the trans-retinal voltage predicted by the models. Thus, given that post-receptoral signals do not reach significant amplitude for some time later than the rod component, we can expect that predictions of our model will match the early part of the ERG although we must expect them to deviate from it at later times.
Of course, in any individual case, we do not know a priori either the exact values of the parameters of the model photocurrent generator (the short time delay, the fractional sensitivity and the time to the peak for weak stimuli) or the scaling factor that relates the corneal ERG to the trans-retinal voltage. Nor in fact can we be certain that the multiple parameters of the electrical model that must be invoked to predict the trans-retinal voltage that a simulated photocurrent will produce will be exactly the same as those based on average measurements made in rat retina. However, we have chosen to limit the parameters that we can arbitrarily adjust to just those three that relate to the photocurrent generator, i.e. the short time delay, the fractional sensitivity and the voltage scaling factor. All other parameters have remained fixed at the values we have reported as the best estimates that are available.
Thus, in presenting comparisons of recorded ERGs with simulations we have adjusted the assumed fractional sensitivity, the initial delay of the photocurrent generator and the voltage scaling factor to provide the best correspondence between the rising edge of the recorded a-waves and the simulations. Because adjusting the initial delay primarily affects the timing of the initial rise from the baseline of the response to the strongest stimuli while adjusting the fractional sensitivity most obviously affects the timing of the negative peak, it is possible to obtain visually satisfactory correspondence with a small number of iterations. In Figs. 7 – 11 that show sets of ERG responses from various species together with simulations, it should be noted that each set of records was treated as an ensemble, that is, the same values of all parameters (including the voltage scaling factor) were used to simulate every record in each set.
Our simulations require the fractional sensitivity of the rods to be expressed in terms of photoisomerizations per rod (R*/rod), and in calculating the fractional sensitivity that provides the best fit of our simulations to published ERGs we have used values of stimulus energy given by the authors when already expressed in this way. Otherwise we have converted luminance values assuming 1 scotopic Td s gives 8.6, 12.5 and 122 R*/rod in human, macaque and mouse with fully dilated pupils respectively. While these are values that have been used in previous publications, (Baylor et al., 1984; Kraft et al., 1993; Saszik et al., 2002) it should be noted that it is generally accepted that there is still some degree of uncertainty in the exact value of these conversion factors. In interpreting the values of fractional sensitivity that provide the best fit it is also worth noting that ganzfeld stimulation does not result in exactly uniform illumination of the retina, the illumination in peripheral regions inevitably being less than that of central regions because of the oblique passage of light through the pupil. Thus, since most of the ways in which the energy of stimuli that are known in photometric units are converted to R*/rod only apply exactly to on-axis stimulation, the fractional sensitivity needed for best fit will probably be somewhat lower than would have been the case if the nonuniformity of retinal illumination had been taken into account.
In summary, we have effectively reduced the fitting of model simulations of trans-retinal voltage to real ERG data to the adjustment of only three parameters (time delay, fractional sensitivity and a voltage-scaling factor), the same number as apply in the fitting of the model proposed by Lamb and Pugh (Breton et al., 1994; Smith and Lamb, 1997) and its variants. Although our model can in theory predict the complete waveform of the component of the ERG directly related to the photocurrent of the rod outer segments, rather than just the leading edge of the a-wave, it should be appreciated that in order to do this it is also necessary to assume some value for the time-to-peak of the outer-segment photocurrent.
4.2 Rat ERG
Since our simulations of the trans-retinal voltage generated by a given photocurrent are based upon the measured electrical constants of the rods and retina of the rat it is most appropriate to compare them with ERGs recorded in this species.
Fig. 7 shows ERGs from a normal rat obtained as control recordings by Findl et al. (1995). The general shape of the rat ERG in response to strong flashes (2,700 – 85,000 R*/rod) can be seen in the upper panel while the lower records show the first 20 ms of responses to flashes of similar energy together with simulations (grey lines). The beginning of the b-wave in the lower records is manifest as the rapid rise that begins slightly later than 15 ms after the stimulus flash.
As with all the other simulations for energy series shown in this paper, the complete ensemble of simulations for the rat ERG records was obtained by taking as given the reported stimulus energies used to generate the series and adjusting only 3 parameters: the initial delay, the fractional sensitivity and a voltage-scaling factor. As explained earlier, the simulations require an assumption about the time-to-peak of the photocurrent response to weak stimuli. In this case (and also for simulations of mouse ERGs to be shown later) the time-to-peak was assumed to be 100 ms, as reported in the paired-flash studies of Hetling and Pepperberg (1999) on mice. The simulations shown in the figure were obtained assuming a delay of 3.8 ms and, based on the authors’ reported stimulus energies, a fractional sensitivity of 7% per isomerization was required to fit the data. This value was considerably higher than we found for recordings from other species. Although the range of stimulus energies in this record set is not much more than 30 to 1, the simulations correspond reasonably well to the reported ERGs over the first 15 ms for all energies.
When evaluating the fit of simulations with recorded ERGs several things should be kept in mind. The initial time delay primarily affects the time of the initial rise from the baseline of the leading edge of the simulated a-wave while the time of the initial peak that is seen when the stimulus is strong and the capacitive currents are largest, is primarily dependent upon the fractional sensitivity. Because of this we chose to adjust these parameters to optimize the fit of the simulations to the leading edge and initial peak of the responses to the strongest two or three stimuli. As a result of this approach the fit for weaker stimuli may have been somewhat less good than it would have been if the parameters had been adjusted to provide the best overall correspondence.
While the a-waves generated by strong stimuli are most likely to show the contribution related to capacitive currents, these responses may also be more likely to include components related to cone photocurrent as well as to rod- and/or cone-driven responses of post-receptoral cells. However, the response of rat cones peaks at a much later time than the dark-adapted a-wave and therefore is likely to be of insignificant amplitude at the time of the a-wave peak (<5 μV at 10 ms, Bui and Fortune, 2006). An early negative post-receptoral component in the rat ERG that could be removed by pharmacologically blocking ionotropic glutamate receptors has been reported by Dang et al. (2011), but extrapolation of their measurements suggests that the amplitude of this post-receptoral signal would be little more than 1/10 of the peak amplitude of the a-wave generated by our strongest stimuli.
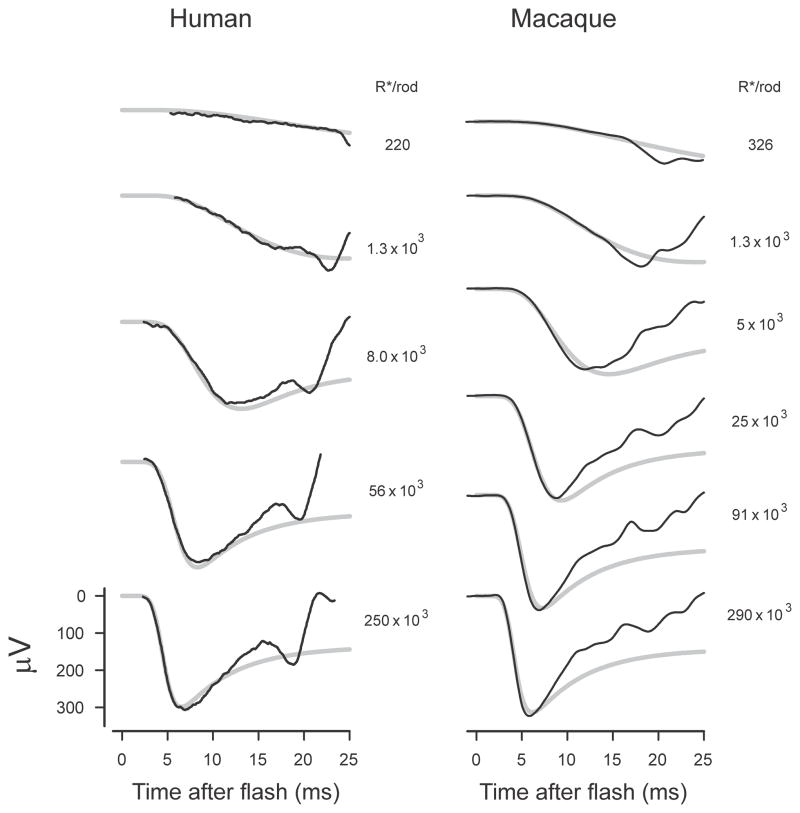
4.3 Human and macaque rod-isolated ERG
Although the direct contribution of cone photocurrents to the rat ERG is probably small enough to be ignored, the contribution of cone currents to the primate ERG is larger and many published studies have used one of several different techniques to isolate the rod-related component. Fig. 8 shows such rod-isolated ERGs obtained by subtracting cone-isolated ERGs from mixed rod and cone recordings from human (left column, from Smith and Lamb, 1997) and from macaque (right column, data of Robson et al., 2003) for stimuli with a wide range of energies. The method of obtaining the cone-isolated ERGs is explained in the cited publications.
Fig. 8.
Primate ERG. Rod-isolated ERGs from human (left column, redrawn from Smith & Lamb, 1997, Fig. 5) and from macaque (sm451; Robson et al., 2003). Simulations shown by grey lines assume a peak time of 125 ms after the flash for the rod photocurrent response to a single photoisomerisation (Friedburg et al., 2001), initial delays of 3.1 and 2.85 ms and fractional sensitivities of 1.5% and 1.6% per isomerisation for human and macaque respectively.
The simulated trans-retinal voltages (grey lines) were obtained assuming the time to the peak of the single-photon rod photocurrent was 125 ms (Friedburg et al., 2001) with initial delays of 3.1 and 2.85 ms and fractional sensitivities of 1.5% and 1.6% per isomerisation for human and macaque respectively.
It should be noted that in both cases the eyes were normal and the ERGs must therefore be assumed to contain all normal post-receptoral components, both oscillatory and non-oscillatory components being clearly visible in the records at later times. Despite this it can be seen that for stimuli over a large range of energies (about 1000:1) the simulations provide a reasonable fit to the first 10 ms or so of the a-wave including the initial peak when this occurs earlier than 10 ms. The fit of the simulations to the macaque ERGs is somewhat less good than that to the human ERGs although, even for these records, a substantial part of the recovery toward the baseline after the a-wave peak can be ascribed to the existence of the a-wave nose that results from capacitive currents. The brief negative dip of the recorded ERG below the simulation that is seen in the macaque ERGs for the two weakest stimuli and commences with a marked downward inflection of the trace was identified by Robson et al., (2003) as a scotopic threshold response from inner retina.
4.3.1 Primate ERG without PII
As noted in the introduction, the ERG evoked by a strong stimulus can display a transient a-wave even in circumstances in which there is no significant response from post-receptoral cells that generate early positive-going signals. Fig. 9 shows examples of such a-waves (dashed lines) in mixed cone-rod ERGs, together with control records (solid lines) from three patients with complete stationary night blindness (upper records; Nakamura et al., 2010) and in a macaque (lower records; Robson et al., 2003) obtained after pharmacological suppression of both On- and Off -bipolar cell responses with a mixture of APB to block metabotropic glutamatergic transmission (Slaughter and Miller, 1981) and PDA to block ionotropic glutamate receptors (Slaughter and Miller, 1983). In both cases it can be assumed that the ERG contains no positive PII component from On-bipolar cells. Despite this the initial negative voltage recovers from its peak value to little more than one half of this within 20 ms in a way that differs only slightly from that see in the control or normal eye. Also shown in the figure are simulations of the trans-retinal voltage (thicker grey lines) based on the models already described.
Fig. 9.
ERGs lacking On-bipolar cell components. Normalised ERGs from three patients with complete CSNB and a normal control (upper records redrawn from Nakamura et al., 2010, Fig. 5A) and the ERG from a macaque (lower records; Robson et al., 2003, sm295). before and after pharmacological suppression of both On- and Off-bipolar cell responses with a mixture of APB and PDA
The simulations in Fig. 9 provide a good fit to the initial peak of the a-wave as well as its leading edge and thus provide strong support for the hypothesis that the direct contribution of the photoreceptors to the ERG includes an initial transient portion or “nose” resulting from capacitive currents. However, while the stimulations manifest a recovery from the peak towards the baseline of roughly the same amplitude as is observed in the ERG without PII, the time course of the later part of this recovery (later than 12 ms) is a little different. Interpretation of these observations is complicated by the fact that the ERG records include responses generated by the activation of cones as well as rods and, in the case of the CSNB records, the responses of post-receptoral cells of the Off pathway as well.
4.4 Mouse ERG
We have also examined the ERGs of both normal and nob (Nyxnob) mice (Pardue et al., 1998) and the effects of intravitreal injection of pharmacological agents. Fig. 10 shows examples of ERG a-waves from various mice generated in response to a strong xenon flash (144 scotopic cd s m−2 giving 123,000 R*/rod; the records were obtained in the course of the study described by Kang Derwent et al., 2007).
Fig. 10.
Mouse ERG. Mouse ERGs evoked by a stimulus of 123,000 R*/rod obtained in the course of the study described by Kang Derwent et al. (2007). A) compares the ERG of a nob mouse (mm311) with that of a wild-type (mm376). B) compares the ERG of a normal mouse (mm376) with that after obtained after blocking the responses of On-bipolar cells with APB (mouse mm309). C) demonstrates the lack of effect on the ERG of a nob mouse (mm443) of blocking the responses of inner retinal cells in the Off pathway with PDA. D) shows the effect of PDA on the ERG of a normal mouse (mm446). Note that the ERGs are displayed as recorded, i.e. without normalization.
In Fig. 10A the ERG from a normal mouse (solid line) is compared with that from a nob animal (dashed line) while in Fig. 10B the ERG of the same normal mouse is compared with that obtained after APB had been injected into the eye. Similarly, Fig. 10D compares the ERG of a different normal mouse before and after injection of PDA while Fig. 10C shows the same comparison for a nob mouse. Although not shown as direct comparisons, it is clear that the ERG of the nob mouse with deficient transmission from photoreceptors to On-bipolar cells is very similar to that of the normal mouse after blockade of transmission from photoreceptors to On-bipolar cells by APB and also that the waveform of these responses are quite similar to the human CSNB and post-drug macaque records of Fig. 9. It should be noted that the pairs of records shown in the figures have been plotted without any rescaling and that in the case of the drug manipulations a substantial time (hours) may have elapsed between the control and experimental recordings so that minor differences could be the result of small changes in extraneous factors (e.g. temperature, corneal hydration, anaesthetic state).
All the ERGs from both normal and nob mice in Fig. 10 show essentially the same initial rapid return of the trace toward the baseline following the a-wave peak that is seen in the primate ERGs (Fig. 8). Although the ERGs of normal mice and primates are generally quite similar, rodent ERGs typically, as here, have a much more prominent oscillatory component whose onset after about 15 ms gives rise to the deviation of normal records from the simulations seen at times later than this.
An immediately obvious effect of both APB and PDA on the ERG of the normal animals (Fig. 10B and 10D) is to remove the oscillatory component of the response and to give an ERG that looks (for the first 20 ms or so) very like that of the nob animal (Fig. 10A). As well as removing the oscillatory component, APB, but not PDA, also removed the positive-going rise in the record that began at around 20 ms and may be assumed to represent the contribution of the rod-driven On-bipolar cells (PII) that would be manifest at later times as the b-wave of the ERG (compare Figs. 10B and 10D). When PDA was injected into the eye of a nob mouse (Fig. 10C), it had no clear effect on the ERG indicating the absence, or insignificance, of signals from Off-pathway cells in the mouse.
Each of the panels of Fig. 10 shows a simulation of the trans-retinal voltage generated by the model already described (grey lines). As before, the initial brief delay was adjusted to make the a-wave rise from the baseline at the right time, the fractional sensitivity was adjusted to give an initial negative wave peaking at the right time, and the voltage-scaling factor was adjusted to provide the best overall fit to the first 15 ms of the ERG. With these adjustments the simulation provides a good description of this portion of the a-wave in every case.
These experimental observations support the assumption that the first 15 ms of the mouse ERG, including a substantial part of the early recovery from the initial negative peak towards the baseline, is entirely, or nearly entirely, produced by currents from photoreceptors alone and is not the result of antagonism between opposing photoreceptor and On-bipolar cell signals. Moreover, although the exact shape of the early part of the recovery phase may be dependent upon a small contribution from an oscillatory post-receptoral source, the close correspondence between the initial recovery of all ERGs towards the baseline and the recovery predicted by the simulations, provides strong support for believing that that the initial negative peak of the ERG (a-wave) is essentially a reflection of the transient capacitive currents in the retina that are evoked by strong stimuli.
It has already been noted that a characteristic feature of the simulations of trans-retinal voltage (Fig. 5) is the dependence upon stimulus energy of both the timing and amplitude of the initial negative nose of the ERG. We have therefore examined for a wide range of stimulus energies the correspondence between the simulations and ERGs recorded both from normal mice after intravitreal injection of APB as well as from nob mice.
Fig. 11 shows a series of mouse ERGs evoked by stimuli with a range of energies of about 5000 to 1 (from data obtained by Kang Derwent et al., 2007). On the left are recordings from a wild-type mouse after intravitreal injection of APB while the recordings on the right were obtained from a nob mouse. The upper record sets show only the early part of responses to the stronger stimuli while the lower record sets show responses on a much longer time scale over the full range of energies from about 7 R*/rod to the highest levels. As would be expected the records from both mice are quite similar, the functional defect being the same in each case. Given that the three free parameters of the models underlying the simulations were adjusted to make the initial peaks correspond as well as possible to the ERGs evoked by the strongest stimuli, it is satisfying to see that the correspondence was nearly as well maintained at times less than about 15 ms over the whole range of energies. In both cases the fractional sensitivity was 2% per isomerisation while the delay for the mouse after APB and the nob mouse were 2.9 ms and 3.2 ms respectively. It should be emphasized that the simulations for each mouse, as for records for other species, were computed as an ensemble, the same values of the parameters being used for each of the stimulus energies.
We have not attempted to obtain a numerical measure of the goodness of fit of the simulations to the recordings but it looks as though the fits at the higher energies over the first 15 ms in the mouse are slightly better than in the primates whose ERGs are shown in Fig. 8. This might be because the contribution to the ERG of cones and Off-bipolar cells is less in the mouse, or because the ONL of the mouse retina may be rather more similar to that of the rat (on which the simulations are based) than that of the primates or for some other reason (see discussion). It is not possible to tell from examination of these ERG recordings.
Although it was stated earlier that the simulations of the trans-retinal voltage should correspond to the contribution of the photocurrent to the ERG at later as well as early times, it is clear from the lower sets of records in Fig. 11 that there is a considerable discrepancy at later times between the simulations and the ERG recorded from both nob mice and mice in which post-receptoral responses in the On pathway were blocked. In these animals the amplitude of the negative wave of the recorded ERG that peaks around 90 ms with low energy stimuli or forms the negative plateau with high energy stimuli is substantially greater (roughly two times) than the simulated value and in some individuals can be as great as, or even greater than, that of the early nose. Although the voltage at later times may indeed become about as negative as that of the earlier negative peak, the initial recovery of the voltage toward the baseline after this peak remains a prominent feature of the mouse ERG with even the strongest stimuli.
The existence of a plateau voltage that is substantially more negative than that recorded 20–30 ms after the nose is seen neither in humans with CSNB (Fig. 9A) nor in macaques with total post-receptoral blockade (Fig. 9B and see Jamison et al., 2001). The additional late negativity in the mouse ERG may be due to the existence in this species (as well as in rats; unpublished observations) of a peculiarly large slow PIII component.
5. Discussion
We have found that simulations that take account of the electrical characteristics of rods based on measurements in rats (Hagins et al., 1970), predict that the direct contribution of the rods’ photocurrent to the ERG will have a time course that is significantly different from that of the outer-segment photocurrent. In particular, the simulated trans-retinal voltage response to a flashed stimulus that is more than minimally strong enough to saturate the photocurrent of the rods (i.e. to shut off completely the circulating dark current) shows a clear initial negative transient or “nose: whose amplitude increases and time-to-peak decreases with increasing stimulus energy.
Comparison of such simulations with available recordings of the ERGs of rats, humans, macaques and mice has indicated that for flashed stimuli that are strong enough to generate an a-wave whose peak is earlier than about 12 ms, the waveform of the early part of the ERG (including the recovery towards the baseline following the initial peak) can be explained as mainly being a direct reflection of the capacitive currents that flow extracellularly in the ONL of the retina around the soma and axon of the rods. Because the early negative wave of the ERG is directly generated by the extracellular flow of the rod photocurrent through the retina it is not surprising that ERGs from eyes in which transmission from photoreceptors to On-bipolar cells is defective or suppressed continue to manifest the same timecourse of the early negative a-wave as normal eyes.
Our new explanation for the existence of an early negative a-wave peak in the ERG is at variance with the historically offered explanation that envisages the contribution of the rods as a monotonically rising negative voltage generated by a monotonically falling circulating current from the outer segments that is cut into by a slightly delayed positive-going signal (PII) from On-bipolar cells. As a corollary of the explanation offered here it follows that the negative voltage that is directly contributed to the ERG by strongly-stimulated rods at later times when the circulating current is completely suppressed (i.e. more than about 40 ms after the flash) will be substantially less than the voltage of the nose. Thus, since the peak of the ERG b-wave always occurs significantly later than 40 ms, its amplitude, measured in the conventional way as the height of its positive peak above the most negative point on the a-wave, will inevitably exceed the amplitude of the positive post-receptoral component that is responsible for generating it. It therefore seems probable that it is the nose on the a-wave which is induced by capacitive currents that is responsible for the secondary rise in the b-wave amplitude in the stimulus response function that is seen when the stimulus energy is increased beyond that initially required to saturate the post-receptoral response (e.g. see Peachey et al., 1989).
5.1 The fit of our new model and comparison with previous models
5.1.1 The leading edge of the a-wave
Although our simulations predict that the direct contribution made to the ERG by rods at later times after a flash will be very different from that predicted by the currently popular models, they provide, as far as we can tell, no less satisfactory a description of the rising edge of ERG a-waves. While it is, perhaps, a little surprising that ERGs that have been satisfactorily fitted by models based on Lamb and Pugh’s approximation can also be adequately fitted by simulations based on our rather different interpretation of the mechanism of generation of the ERG, this certainly seems to be the case. For example, the human records shown in Fig. 8A which are quite well fitted by our simulations over the first 10 ms or so (including in some cases the peak of the a-wave), were used as illustrations by Smith and Lamb (1997) of the applicability of their slightly modified version of the Lamb and Pugh formulation to describing the rising edge of the a-wave.
A more direct illustration of similarities and differences between the Lamb and Pugh models and our own is provided by Fig. 12. This plots a selection of the rod-isolated ERG recordings (black lines) illustrated in Fig. 1E of Friedburg et al. (2001) together with those authors’ fits of a Lamb and Pugh model incorporating a membrane time constant of 0.7 ms (dashed magenta lines). Also shown (cyan lines) are simulations provided by the model that we have just described that takes into account the electrical circuit of the rods and the retina (both models fitted to the data as an ensemble).
Fig. 12.
Human ERG. Human rod-isolated ERGs redrawn from Friedburg et al. (2001, Fig. 1E) with the authors’ predictions (dashed magenta lines) of the Lamb and Pugh model of phototransduction convolved with an exponential decay (time constant 0.7 ms) “representing the rod’s membrane time constant”. The cyan lines represent the predictions of the model described in the text that takes account of the membrane capacitance of the rod axon, nuclear sac and terminal spherule as well as the membrane capacitance of inner and outer segments.
There is no obvious difference between the goodness of the fits of these two models as far as the leading edge of the a-wave is concerned but their predictions start to diverge just before the time of the peak and the difference becomes much greater at later times.
5.1.2 The model at times after the peak of the a-wave
Our analysis of the effect of the electrical circuits around the rods predicts that strong stimuli will give rise to a trans-retinal voltage with an additional initial negative transient as a result of capacitive currents, provides a reasonably accurate prediction of the time of the resulting a wave peak and explains why the voltage after the peak recovers a substantial way towards the baseline. It does not, however, provide an exact prediction of the form that the ERG can take at times after the peak. It is obvious in Fig. 12 and in earlier illustrations of human and macaque ERGs (Figs. 1C, 8 and 9) that the recovery towards the baseline usually proceeds further and a little more rapidly than predicted by the model even at times prior to the intrusion of responses from post-receptoral cells. While this could simply result from the electrical parameters appropriate to the primate retina being different from those of the rat from which the values we used for the simulations were derived, it is also possible that deviations from our model predictions reflect the relatively slowly increasing conductance of the inner-segment membrane that is triggered by hyperpolarization, i.e. via (HCN) Ih channels. Studies of this hyperpolarisation-induced conductance change (in guinea pig rods in vitro by DeMontis et al., 1999 and in mouse rods by Della Santina et al., 2012) indicate that it is responsible for the decline in the inner-segment photovoltage that is seen to develop over many tens (or even hundreds) of milliseconds following the initial rapid response to a strong flash stimulus. This effect, a mechanism for which was not included in our model, would certainly operate in addition to the capacitance effect on which we have focused and would be expected to result in the ultimate level of the voltage plateau being less negative than predicted by our model. It is interesting to note that the ERG of a patient with CSNB (Fig. 1C) shows a time for return of the ERG to near the baseline of about 100 ms, a time very similar to that taken by for the membrane voltage of an isolated human rod to fall to the ultimate plateau level (Schneeweis and Schnapf, 1995, their Fig. 1B.)
While the most obvious deviation of human and macaque ERGs from the simulations of our model relate to the model underestimating the extent and speed of the return of the ERG towards the baseline at times after the a-wave peak, the more obvious discrepancy in ERGs evoked by strong stimuli in mice lacking PII (see the bottom panels in Fig. 11) is the failure of the ERG after the a-wave to return as far towards the baseline as our simulations predict. In fact following an initial return towards the baseline the ERG can once more become more negative and then level off at a considerably more negative voltage than the plateau level predicted by our model, and presumably a level very much more negative than the plateau level that would be expected if the voltage-dependent membrane conductance effect were operating as well. We have speculated that this negative-going wave is slow PIII, a wave that is created by K+ spatial buffer currents in Müller glia cells, set up when photoreceptors hyperpolarize strongly and stop leaking K+ into the subretinal space. At later times the negative wave is cancelled by the positive trans-epithelial c-wave from pigment epithelium that is triggered by the same photoreceptor-dependent reduction of [K+] in the subretinal space (e.g. Steinberg et al., 1991). Supporting this speculation, a negative-going wave of similar timecourse recorded trans-retinally in isolated mouse retina was eliminated by BaCl2, a blocker of the inward rectifying K+ channels found in the glial cells (Heikkinen et al., 2012). Although the primate ERGs show no such dramatically large slow PIII component, there presumably is at least a small slow PIII component that will affect the rod-related voltage at later times and make the voltage deviate even further from the prediction of our model.
Taken together, the existence of these later components of the rod and rod-related response that have yet to be fully understood or satisfactorily modelled, currently make it inappropriate to use any of the available models of rod photocurrent whose parameters are derived by analysis of the early part of the a-wave to predict what should be subtracted from the whole ERG to reveal the contribution of post-receptoral components at later times.
5.2 Estimation of rod parameters: a proposed simple method
As noted earlier, many studies of normal and abnormal rod function that rely on recordings of the ERG have obtained estimates of the basic parameters of the Lamb and Pugh model of rod response by fitting model curves (i.e. model-based simulations for a given set of parameters) to the recorded ERGs using computer-based methods of error minimization that involve iterative computation of model responses for many different sets of parameters. While a similar approach could be taken to estimating the parameters of the alternative model we have now described, the much greater complexity of this model and the much longer time required for the SPICE computation of each simulated response waveform, makes this less practicable. A more practical approach might be to compare recorded ERGs with previously computed arrays of the simulated responses of models with a range of parameters. However, while this could be incorporated into a formal computer-based error-minimization procedure, comparison of the simulations provided by our model and recorded ERGs suggest that an altogether simpler approach may be adequate.
5.2.1 Rod sensitivity and the rise time of the a-wave
We have shown that simulations provided by our model can, when its three main parameters — the fractional sensitivity, the brief delay and a voltage scaling factor — have all been appropriately adjusted, reproduce the waveform of the leading edge and initial peak of a-waves generated by strong stimuli and that the model can effectively predict the way in which these features of the waveform change with changes in stimulus energy. This implies that it should be possible to estimate all three parameters from the response to a single flash of appropriate high energy. There are various ways in which this might be done using no more than three measurements of the amplitude at different times after the flash, but on heuristic grounds we have chosen to examine one simple way that involves measuring the amplitude of the a-wave peak together with measurements of the times at which the leading edge of the a-wave reaches 10% and then 90% of this amplitude. These measurements can be used to calculate the time for the leading edge to rise from 10% to 90% of its ultimate maximum value, a quantity that may be called the “10% – 90% rise time” of the ERG, or (where there is no ambiguity) simply the “rise time” of the ERG. The rise time so defined is uniquely related to the fractional sensitivity of the underlying model of the generation of the rod component of the a-wave in a way that can easily be derived from our simulations of the trans-retinal voltage.
Justification for this choice is provided by examining how well our model can predict the relationship between the rise time of the ERG and stimulus energy. Fig. 13A shows measurements of ERG rise time from three different normal human subjects whose ERGs are illustrated in three published studies (Breton et al., 1994; Thomas and Lamb, 1999; Friedburg et al., 2001). In all three cases it can be seen that over a range of rise times from about 2 to 6 ms obtained with flash energies from about 10,000 to 300,000 R*/rod, the relationship predicted by our model for a fractional sensitivity in the range 1% to 2% per photoisomerisation could provide a satisfactory fit to the experimental data (dashed and solid black lines show predictions for 1% and 2% fractional sensitivity). Of course, in any individual case where data such as this is available, the best estimate of fractional sensitivity would be obtained by fitting all the data in the appropriate range with the function predicted by the model, but it looks as though estimates that would be sufficiently good for most purposes could be obtained with a single well-chosen stimulus energy. Again, on heuristic grounds, it may be supposed that a stimulus giving about 10,000 or 30,000 R*/rod (or some intermediate energy) would be a good choice. This would be a strong enough stimulus (for normal subjects though maybe not in pathological cases) to be reasonably certain that the model we have described will apply but not so strong as to require a very long interval between successive flashes to allow for retinal recovery. It is interesting to note that these stimuli corresponds closely to the two stronger white light stimuli (DA 10 and DA 30 – specified in photopic cd s m−2) that have been proposed by the International Society for the Clinical Electrophysiology of Vision (ISCEV) as additional standard stimuli for full-field electroretinography (Marmor et al., 2009). The ISCEV standard stimulus values are indicated above the plot.
Fig. 13.
Estimation of rod parameters. Measurements of the 10% – 90% rise time of the ERG a-waves of three normal subjects measured from illustrations in Breton et al. (1994, Fig. 3A), Thomas and Lamb, (1999, Fig. 1B), and Friedburg et al., (2001, Fig. 1E) shown as a function of stimulus energy. The black lines are the predictions made by the model described in the text for this relationship when the fractional sensitivity is 1% or 2% per photoisomerisation (dashed and solid lines respectively). The energy scales on the abscissa assume that the pupil diameter is 8mm and that 1 scotopic Td s gives 8.6 photoisomerisations in each rod. The arrows above the graph indicate the energies of several white light stimuli to be used for stimulating the dark-adapted eye defined by ISCEV and designated by their luminous energy in photopic units (cd s m−2) (Marmor et al., 2009).
The cyan line is a 2nd order polynomial approximation to the model rise time (in milliseconds) for a fractional sensitivity of 1% per photoisomerisation. It is defined by: log10(rise time) = 3.3077 – 0.8817 log10(E) + 0.0607 log10(E)2 where E is the energy of the stimulus in R*/rod.
While the functional capacity of the rods in an individual subject could be assessed by directly comparing the rise time of the a-wave elicited by a standard strong stimulus with the range of values elicited by the same stimulus in a normal population, the same measurement could also be interpreted in terms of the model we have described to provide a procedure-independent estimate of rod sensitivity. Based on the definition of sensitivity that we have so far employed – a definition adopted to aid comparison across species – this estimate would be expressed as the fraction of the maximum possible rod photocurrent that is generated as a result of activating a single molecule of rhodopsin in each rod. However, in circumstances in which it could be supposed that changes in overall sensitivity might be the result of something other than a reduction in the effectiveness of single photoisomerisations in a photoreceptor (e.g. a reduction in light transmission in the eye or a reduction in the amount of photopigment in each rod) it might be more appropriate to express the sensitivity in terms of the fractional effect of a given amount of light measured externally to the eye. Thus, human rod sensitivity could, for example, be expressed as the fraction of the photocurrent suppressed by a ganzfeld stimulus having an energy of 1 scotopic Td s. This value would be obtained by multiplying the fractional sensitivity for a single photoisomerisation by the factor 8.6, the value that has been assumed for the number of molecules that are activated in each rod of a normal human eye by a 1 scotopic Td s stimulus; similar calculations using the appropriate conversion factors would provide estimates of rod sensitivity in other species.
5.2.2 A-wave amplitude
Although the rise time of the a-wave is uniquely related to the sensitivity of the rods and independent of those external factors that determine what proportion of the trans-retinal voltage is recorded (electrode type and positioning, eye size, etc), the recorded amplitude of the a-wave is very dependent upon these factors in a way that we have not tried to model. Despite this, it is to be expected that in any given recording situation, the amplitude of the a-wave will vary with stimulus energy and rod sensitivity in the way predicted by the model of trans-retinal voltage generation that we have described.
Fig. 14 shows how stimulus energy affected the a-wave amplitude of the ERGs whose a-wave rise time is plotted in Fig. 13; also plotted in this figure are a-wave amplitude measurements reported by Breton and Montzka (1992). Although the amplitude of the a-waves recorded with unipolar contact lens electrodes (the upper two sets) are much larger than those recorded with DTL silvered-nylon fibre electrodes (Dawson et al., 1979; lower two sets), the way in which the amplitude varies with stimulus energy is quite similar in all cases. For the data for which we had estimates of rod sensitivity based on the rise-time measurements illustrated in Fig. 13, we have plotted the predictions of our model for that sensitivity. Given that we have already taken account of the rod sensitivity, there remains only one degree of freedom. This allows arbitrary vertical placement of the log-log plots and the model curves have been positioned to describe the amplitude of responses to the strongest stimuli. As would be expected, the model clearly fails to describe the data when the stimulus energy is so low (below about 1000 R*/rod) that it is reduced by intrusion of postreceptoral responses. However, at the higher stimulus energies at which the a-wave nose becomes prominent, the model provides a relatively good fit to the measurements. The fit is particularly good for the two sets of data obtained from recordings made with DTL fibres, but this probably relates more to the greater number of recordings that were averaged to obtain the data than the nature of the electrodes.
Fig. 14.
A-wave amplitude. The amplitude of the a-waves of four normal subjects are shown as a function of stimulus energy. All but the top data set were obtained by measuring the records illustrated by Breton et al. (1994, Fig. 3A), Thomas and Lamb, (1999, Fig. 1B), and Friedburg et al., (2001, Fig. 1E). These are indicated by the same symbols as used to plot rise times in Fig. 13. The top data set is from Breton & Montzka (1992). The smooth curves represent the predictions of our model for the fractional sensitivities estimated from the rise-time data shown in Fig. 13.
Assuming that the ability of our model to describe the effect of stimulus energy (and hence of rod sensitivity) is substantiated by further studies, the model could be used to extrapolate measurements of a-wave amplitude to obtain an estimate of the maximum possible amplitude or some other procedure-independent amplitude measure.
5.3 Future directions
We have shown that the waveform of the recorded ERG can be substantially different from that of the rod photocurrent, especially when the stimulus is of high energy. When the nature of the electrical circuits in the retina in which the photocurrent flows is taken into account, it is possible to understand how the amplitude and timing of the initial peak of the a-wave as well as the form of its leading edge are related to the rod photocurrent, and do not depend upon the activation of any post-receptoral retinal components.
On the basis of our new understanding of the way in which the ERG is generated we have proposed a simple method of analysing the ERG evoked by a single moderately strong stimulus to determine both the sensitivity and maximum response of the rods. This may make it easier to estimate these parameters in both human and animal subjects than the methods presently in vogue. We have suggested that a good choice of stimulus energy would be one that gives rise to between about 10,000 and 30,000 photoisomerizations in each rod, a considerably weaker stimulus than is commonly recommended (e.g. Hood and Birch, 2006). In a normal human subject this level of activation can be provided by a stimulus of between about 1100 and 3300 scotopic Td s which, with a dilated pupil, can be achieved with a stimulus of between 22 and 66 scotopic cd s m−2, or, if a white light is used, a flash of roughly 10 – 30 (photopic) cd s m−2, as suggested in the ISCEV Standard (Marmor et al., 2009). These are stimulus energies that can easily be provided by most modern clinical and other electrophysiological equipment so that the method we propose could easily be implemented in both laboratories and clinics.
Our proposal is mainly based upon theoretical considerations and animal experimentation, though the information about human ERGs that we have found in published literature has provided strong support for its applicability in humans. It is clear that more basic work will be necessary to confirm the practicality and usefulness of the method in research and clinical settings, but this is a most opportune time to be considering a simple non-invasive method for assessing rod function given the current interest in new therapies for countering the various degenerative and other disorders that can affect retinal photoreceptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arden GB. Voltage gradients across the receptor layer of the isolated rat retina. J Physiol. 1976;256:333–360. doi: 10.1113/jphysiol.1976.sp011328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden GB. Three components of the photocurrent generated in the receptor layer of the rat retina. In: Barlow HB, Fatt P, editors. Vertebrate Photoreception. Academic Press; London: 1977. pp. 140–167. [Google Scholar]
- Audo IM, Robson AG, Holder GE, Moore AT. The negative ERG: clinical phenotypes and disease mechanisms of inner retinal dysfunction. Survey of Ophthalmology. 2008;53:16–40. doi: 10.1016/j.survophthal.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. The membrane current of single rod outer segments. J Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton ME, Montzka DP. Empiric limits of rod photocurrent component underlying a-wave response in the electroretinogram. Doc Ophthalmol. 1992;79:337–361. doi: 10.1007/BF00160948. [DOI] [PubMed] [Google Scholar]
- Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- Brown KT. The electroretinogram: its components and their origins. Vision Res. 1968;8:633–677. doi: 10.1016/0042-6989(68)90041-2. [DOI] [PubMed] [Google Scholar]
- Bui BV, Fortune B. Origin of electroretinogram amplitude growth during light adaptation in pigmented rats. Vis Neurosci. 2006;23:155–167. doi: 10.1017/S0952523806232024. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vision Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Dang TM, Tsai TI, Vingrys AJ, Bui BV. Post-receptoral contributions to the rat scotopic electroretinogram a-wave. Doc Ophthalmol. 2011;122:149–156. doi: 10.1007/s10633-011-9269-y. [DOI] [PubMed] [Google Scholar]
- Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18:988–991. [PubMed] [Google Scholar]
- Della Santina L, Bouly M, Asta A, Demontis GC, Cervetto L, Gargini C. Effect of HCN channel inhibition on retinal morphology and function in normal and dystrophic rodents. Invest Ophthalmol Vis Sci. 2010;51:1016–1023. doi: 10.1167/iovs.09-3680. [DOI] [PubMed] [Google Scholar]
- Demontis GC, Longoni BCB, Barcaro U, Cervetto L. Properties and functional roles of hyperpolarization-gated currents in guinea-pig retinal rods. J Physiol. 1999;515:813–828. doi: 10.1111/j.1469-7793.1999.813ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einthoven W, Jolly WA. The form and magnitude of the electrical response of the eye to stimulation by light at various intensities. Quart J Exp Physiol. 1908;1:373–416. [Google Scholar]
- Findl O, Hansen RM, Fulton AB. The effects of acetazolamide on the electroretinographic responses in rats. Invest Ophthalmol Vis Sci. 1995;36:1019–1026. [PubMed] [Google Scholar]
- Friedburg C, Thomas MM, Lamb TD. Time course of the flash response of dark- and light-adapted human rod photoreceptors derived from the electroretinogram. J Physiol. 2001;534:217–242. doi: 10.1111/j.1469-7793.2001.t01-1-00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, Rushton WA. The human rod ERG: correlation with psychophysical responses in light and dark adaptation. Vision Res. 1978;18:793–800. doi: 10.1016/0042-6989(78)90119-0. [DOI] [PubMed] [Google Scholar]
- Granit R. Isolation of components in the retinal action potential of the decerebrate dark-adapted cat. J Physiol. 1932;76 (suppl):1P–2P. [Google Scholar]
- Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933;77:207–239. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. Sensory Mechanisms in the Retina with an Appendix on Electroretinography. Oxford University Press; London: 1947. [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline JK. The electric response to illumination in the eye in intact animals, including the human subject and in decerebrate preparations. Am J Physiol. 1925;73:600–612. [Google Scholar]
- Heikkinen H, Vinberg F, Pitkanen M, Kommonen B, Koskelainen A. Flash responses of mouse rod photoreceptors in the isolated retina and corneal electroretinogram: comparison of gain and kinetics. Invest Ophthalmol Vis Sci. 2012;53:5653–5664. doi: 10.1167/iovs.12-9678. [DOI] [PubMed] [Google Scholar]
- Hetling JR, Pepperberg DR. Sensitivity and kinetics of mouse rod flash responses determined in vivo from paired-flash electroretinograms. J Physiol. 1999;516:593–609. doi: 10.1111/j.1469-7793.1999.0593v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen H, van Norren D. Origin of the electroretinogram in the intact monkey eye – II. Current source density analysis. Vision Res. 1985;25:709–715. doi: 10.1016/0042-6989(85)90177-4. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. The relationship between models of receptor activity and the a-wave of the human ERG. Clin Vis Sci. 1990a;5:293–297. [Google Scholar]
- Hood DC, Birch DG. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci. 1990b;5:379–387. doi: 10.1017/s0952523800000468. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. The a-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci. 1990c;31:2070–2081. [PubMed] [Google Scholar]
- Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8:107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Light adaptation of human rod receptors: The leading edge of the human a-wave and models of rod receptor activity. Vision Res. 1993a;33:1605–1618. doi: 10.1016/0042-6989(93)90027-t. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Human cone receptor activity: the leading edge of the a-wave and models of receptor activity. Vis Neurosci. 1993b;10:857–871. doi: 10.1017/s0952523800006076. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Phototransduction in human cones measured using the a-wave of the ERG. Vision Res. 1995;3520:2801–2810. doi: 10.1016/0042-6989(95)00034-w. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. Measuring the health of the human photoreceptor with the leading edge of the a-wave. In: Heckenlively JR, Arden GB, editors. Principles and Practice of the Clinical Electrophysiology of Vision. MIT Press; Cambridge, MA: 1996. pp. 487–502. [Google Scholar]
- Hsu A, Tsukamoto Y, Smith RG, Sterling P. Functional architecture of primate cone and rod axons. Vision Res. 1998;38:2539–2549. doi: 10.1016/s0042-6989(97)00370-2. [DOI] [PubMed] [Google Scholar]
- Jamison JA, Bush RA, Lei B, Sieving PA. Characterization of the rod photoresponse isolated from the dark-adapted primate ERG. Vis Neurosci. 2001;18:445–455. doi: 10.1017/s0952523801183112. [DOI] [PubMed] [Google Scholar]
- Kang Derwent JJ, Linsenmeier RA. Intraretinal analysis of the a-wave of the electroretinogram (ERG) in dark-adapted intact cat retina. Vis Neurosci. 2001;18:353–363. doi: 10.1017/s0952523801183021. [DOI] [PubMed] [Google Scholar]
- Kang Derwent JJ, Saszik SM, Maeda H, Little DM, Pardue MT, Frishman LJ, Pepperberg DR. Test of the paired-flash electroretinographic method in mice lacking b-waves. Vis Neurosci. 2007;24:141–149. doi: 10.1017/S0952523807070162. [DOI] [PubMed] [Google Scholar]
- Kawai F, Horiguchi M, Suzuki H, Miyachi E. Modulation by hyperpolarization-activated cationic currents of voltage responses in human rods. Brain Res. 2002;943:48–55. doi: 10.1016/s0006-8993(02)02531-3. [DOI] [PubMed] [Google Scholar]
- Knop GC, Seeliger MW, Thiel F, Mataruga A, Kaupp UB, Friedburg C, Tanimoto N, Müller F. Light responses in the mouse retina are prolonged upon targeted deletion of the HCN1 channel gene. Eur J Neurosci. 2008;28:2221–2230. doi: 10.1111/j.1460-9568.2008.06512.x. [DOI] [PubMed] [Google Scholar]
- Kraft TW, Schneeweis DM, Schnapf JL. Visual transduction in human rod photoreceptors. J Physiol. 1993;464:747–765. doi: 10.1113/jphysiol.1993.sp019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M, et al. ISCEV standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Horiguchi M, Terasaki H, Kondo M. Scotopic threshold response in complete and incomplete types of congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1994;35:3770–3775. [PubMed] [Google Scholar]
- Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–1020. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sanuki R, Yasuma TR, Onishi A, Nishiguchi KM, Koike C, Kadowaki M, Kondo M, Miyake Y, Furukawa T. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis. 2010;16:425–437. [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Tamura T, Yau KW. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991;97:413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998;39:2443–2449. [PubMed] [Google Scholar]
- Peachey NS, Alexander KR, Fishman GA. The luminance-response function of the dark-adapted human electroretinogram. Vision Res. 1989;29:263–270. doi: 10.1016/0042-6989(89)90075-8. [DOI] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Birch DG, Hood DC. Photoresponses of human rods in vivo derived from paired-flash electroretinograms. Vis Neurosci. 1997;14:73–82. doi: 10.1017/s0952523800008774. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–530. doi: 10.1113/jphysiol.2002.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol. 2002;543:899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. Photovoltage of rods and cones in the macaque retina. Science. 1995;268:1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid, a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983;219:1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Smith NP, Lamb TD. The a-wave of the human electroretinogram recorded with a minimally invasive technique. Vision Res. 1997;3721:2943–2952. doi: 10.1016/s0042-6989(97)00094-1. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Frishman LJ, Sieving PA. Negative components of the electroretinogram from proximal retina and photoreceptor. In: Osborne NN, Chader GJ, editors. Progress in Retinal Research. Vol. 10. Oxford, UK: Pergamon; 1991. pp. 121–160. [Google Scholar]
- Thomas MM, Lamb TD. Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol. 1999;518:479–496. doi: 10.1111/j.1469-7793.1999.0479p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren D, Valeton JM. The human rod ERG: the dark-adapted a-wave response function. Vision Res. 1979;19:1433–1434. doi: 10.1016/0042-6989(79)90219-0. [DOI] [PubMed] [Google Scholar]
- Waller AD. On the double nature of the photo-electrical response of the frog’s retina. Quart J Exp Physiol. 1909;1:169–185. [Google Scholar]
- Weleber RG, Francis PJ. Differential Diagnosis of Electronegative Electroretinogram. In: Heckenlively JR, Arden GB, editors. Principles and Practice of the Clinical Electrophysiology of Vision. MIT Press; Cambridge, MA: 2006. pp. 809–822. [Google Scholar]
- Yau KW. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- Zampighi GA, Schietroma C, Zampighi LM, Woodruff M, Wright EM, Brecha NC. Conical tomography of a ribbon synapse: structural evidence for vesicle fusion. PLoS One. 2011;6:1–12. e16944. doi: 10.1371/journal.pone.0016944. [DOI] [PMC free article] [PubMed] [Google Scholar]