Abstract
Background
Safety of fibreoptic bronchoscopy (FOB) in nonintubated critically ill patients with acute respiratory failure have not been extensively evaluated. We aimed to measure the incidence of intubation and need to increase ventilatory support following FOB and to identify predictive factors of this event.
Methods
A prospective multicenter observational study was carried out in 8 French adult intensive care units. 169 FOB performed in patients with a PaO2/FiO2 ratio equal or less than 300 were analyzed. Our main end point was intubation rate. The secondary end point was rate of increased ventilatory support defined as greater than a 50% increase in oxygen requirement, the need to start non invasive-positive pressure ventilation (NI-PPV) or increase NI-PPV support.
Results
Within 24 hours, an increase in ventilatory support was required following 59 (35%) bronchoscopies, of which 25 (15%) led to endotracheal intubation. The existence of chronic obstructive pulmonary disease (COPD) (OR:5.2 [1.6–17.8], p=0.007) or immunosuppression (OR : 5.4 [1.7–17.2], p=0.004) were significantly associated with the need for intubation in multivariable analysis. None of the baseline physiological parameters including the PaO2/FiO2 ratio was associated with intubation.
Conclusion
Bronchoscopy is often followed by an increase in ventilatory support in hypoxemic critically ill patients, but less frequently by the need for intubation. COPD, immunosuppression are associated with a need for invasive ventilation in the following 24 hours.
Keywords: Acute Disease; Adult; Aged; Aged, 80 and over; Anoxia; therapy; Bronchoscopy; Critical Illness; Female; Humans; Male; Middle Aged; Positive-Pressure Respiration; Prospective Studies; Respiration, Artificial; Respiratory Insufficiency; therapy
INTRODUCTION
Fiberoptic bronchoscopy (FOB) is widely used in the intensive care units (ICUs) (1–3), as a diagnostic or therapeutic procedure (4, 5) and sometimes as an aid to perform intubation (6). Studies of bronchoscopy performed in mechanically ventilated patients suggest an acceptable safety profile, except the occurrence of hypoxemia as the main adverse event (7–11). Data are lacking in hypoxemic critically ill patients breathing spontaneously, except in hematology and oncology patients. In this situation, some authors recommend performing FOB, for accurately diagnosing the cause of ARF, despite the suppposed high risks associated with bronchoscopy-induced respiratory deterioration leading to endotracheal intubation. The administration of a continuous positive airway pressure (CPAP) (12) or a noninvasive positive-pressure ventilation (NI-PPV) (13) has been suggested to improve the tolerance of FOB with bronchoalveolar lavage (FOB-BAL). However, these were single-center studies with small series of patients, and the degree of hypoxemia during the procedure was the main evaluation criterion. Thus, the safety of FOB remains unclear in critically ill nonintubated patients with hypoxemic ARF, who may probably constitute the largest population of patients managed with bronchoscopy in the ICU. Furthermore, no data are available on the safety of bronchoscopy in ICU patients who are recovering from acute organ insufficiency or who have chronic cardiac or respiratory diseases.
We designed this prospective multicenter observational study to evaluate the safety of FOB in critically ill nonintubated patients with hypoxemic ARF. Our objectives were to determine the subset of patients in whom intubation or need to increase ventilatory support were necessary 24 hours after performing FOB, and to identify factors predicting those changes.
PATIENTS AND METHODS
Study design and ethical consideration
This prospective, observational, multicenter study was approved by the Ethic Committee of the Francophone Society for Critical Care. Each participant was informed orally and via a written document. Patients cannot be included twice if they underwent several FOB procedures.
Study population
We screened all the consecutive patients admitted to 8 university-hospital ICUs between June 2005 and July 2006, in whom a FOB was indicated. Patients were eligible if they met all the following criteria: age ≥ 18 years, oxygen supplementation ≥ 8 L/min or NI-PPV, with a PaO2/FiO2 ratio (P/F) equal or below 300. Exclusion criteria were age< 18 years, pregnancy, unstable angina and recent (less than one week) myocardial infarction, cranial hypertension, platelets levels less than 40. 109/L, treatment-limitation decisions (including modification of ventilator support). Before FOB, oxygen supplementation was increased to obtain a SpO2 higher than 94 %. NIV was allowed during bronchoscopy if oxygenation or respiratoy rate was not considered safe for the physicians in charge (generally SpO2 < 90 % or respiratory rate > 30/min). These changes of oxygen supplementation or ventilatory support during and in the 30 minutes following FOB were not considered as a need of increase ventilatory support in the study.
For each patient, the following data were recorded: demographics, comorbid conditions and underlying diseases, treatments with anticoagulants or anti-platelet agents, severity scores [Simplified Acute Physiological Score II (SAPS II) and Organ Dysfunction and INfection score (ODIN)], and baseline physiological variables, including respiratory rate (RR), heart rate (HR), and systolic blood pressure (SBP). Baseline blood gases, i.e. P/F ratio and arterial CO2 partial pressure (PaCO2), prothrombin time (PT), platelets, blood urea and serum creatinin were recorded. In spontaneously breathing patients, the inspired fraction of oxygen is estimated by the oxygen flow into the high FiO2 mask [FiO2 = 0.21 + (0.03 x oxygen flow L/min)] (14).
Radiological patterns were described as unilateral or bilateral, alveolar or interstitial infiltrates, possibly associated with pleural involvement.
Evaluation criteria
The primary evaluation criterion (end point) is defined as the need of intubation with invasive mechanical ventilation within 24 hours following FOB, whether patients had or not NI-PPV at baseline. The secondary evaluation criterion was designed to assess the need of increase ventilatory support within 24 hours after bronchoscopy, defined as following (i) need of invasive mechanical ventilation (ii) increase in oxygen delivery greater than 50% in patients breathing spontaneously with no pressure support; (iii) increase in levels of inspiratory or expiratory pressures greater than 20% in patients breathing spontaneously with NI-PPV; or increase in levels of FiO2 greater than 20% in patients breathing spontaneously with NI-PPV; or increase in daily duration of pressure support in patients breathing spontaneously with NI-PPV; (iiii) initiation of NI-PPV in patients breathing spontaneously with no pressure support.
Initiation of NI-PPV was performed as recommended by standard guidelines (15) and predefined criteria were used for initiating invasive ventilation (16).
Other possible bronchoscopy related-complications as death, cardiac arrest, cardiac arythmia, pneumothorax or hemoptysis were recorded within 24 hours following FOB.
Statistical analysis
Results are expressed as median and minimal and maximal values for continuous variables and percentages for categorical variables.
Comparisons between groups were performed using the Mann-Whitney and χ2 tests for continuous and categorical variables, respectively, in the univariable analysis. The alpha error was set at 0.05. p value was two-tailed.
An increase of at least 15% of patients requiring an increase of ventilatory support was expected, on the basis of previous studies comparing noninvasive support and oxygen in hypoxemic patients (12,13).
Multivariable analysis aimed to provide evidence for the variables that predicted intubation. A logistic forward LR regression, with a Hosmer-Lemeshow goodness of fit test, was performed. Six categorical variables potentially impacting the initiation of invasive ventilation and showing a significant difference between the two groups, with p < 0.10 were included in models of logistic regression : COPD, immunosuppression, NI-PPV support before FOB, RR (cut-off was < 30/min and increased defined as ≥ 30/min), HR (cut-off was < 100/min and increased defined as ≥ 100/min). These cut-off values were chosen because they were close to the median and clinically significant. Regression coefficients were considered significant with a p value < 0.05. Cumulative-event curves were assessed by a Kaplan-Meier method.
Statistical analyses were performed using SPSS 13 software (SPSS Incorporation, Chicago, IL).
RESULTS
Overall, 181 consecutive FOB were performed in 169 patients during the 14-month study period, among them 169 first FOB were included in this study. Demographic data are reported in the Table 1. Reasons (multiple in some cases) for performing FOB were immunodeficiency (n=62, 37%), atelectasis (n = 49; 29%), hospital-acquired pneumonia (n = 46; 27%), acute diffuse infiltrative pneumonia (n = 45; 27%), community-acquired pneumonia (n = 20; 12%), hemoptysis (n = 5; 3%), suspected malignancy (n = 5; 3%), and chronic diffuse infiltrative pneumonia (n = 1; 1%).
Table 1.
Demographics, radiological, and physiological and biological data at baseline
| Total | Initiation of invasive ventilation | |||
|---|---|---|---|---|
|
| ||||
| Number of FOB procedures | 169 | No | Yes | p |
|
| ||||
| 144 | 25 | |||
| Age (years) | 59 [23–91] | 59 [23–88] | 64.5 [35–91] | 0.723 |
| Gender (% males) | 115 (68) | 98 (68) | 17 (68) | 0.996 |
| BMI (kg/m2) | 23 [14–38] | 24 [14–38] | 22 [17–35] | 0.198 |
|
| ||||
| Interstitial opacities (%) | 43 (26) | 36 (26) | 7 (28) | 0.749 |
| Bilateral opacities (%) | 63 (38) | 51 (36) | 12 (48) | 0.451 |
| Pleural syndrome (%) | 28 (17) | 24 (17) | 4 (16) | 0.888 |
|
| ||||
| SAPS II | 38 [6–99] | 37 [6–81] | 43 [19–99] | 0.107 |
| ODIN | 2 [0–8] | 2 [0–8] | 2 [1–6] | 0.118 |
|
| ||||
| RR (breaths/min) | 26 [14–52] | 26 [14–52] | 30 [18–50] | 0.043* |
| HR (beats/min) | 98 [47–155] | 97 [47–155] | 112 [74–132] | 0.014* |
| SBP (mmHg) | 127 [69–191] | 127 [69–191] | 125 [92–172] | 0.689 |
| PaO2/FiO2 (mmHg) | 194 [61–300] | 196 [61–300] | 184 [92–293] | 0.819 |
| PaO2 (mmHg) | 72 [39–167] | 70 [39–167] | 78 [44–132] | 0.492 |
| FiO2 (%) | 53 [25–100] | 50 [25–100] | 60 [25–100] | 0.197 |
| PaCO2 (mmHg) | 40 [24–91] | 40 [24–91] | 37 [24–80] | 0.245 |
| PT (%) | 76 [14–100] | 77 [14–100] | 71 [37–100] | 0.276 |
| Platelets (giga/ml) | 253 [6–740] | 258 [6–740] | 236 [41–663] | 0.378 |
| Blood Urea (mmol/l) | 7 [1.5–47] | 7 [1.5–47] | 10.9 [2.8–39.2] | 0.198 |
| Serum Creatinin (μmol/l) | 79 [30–664] | 78 [30–601] | 93 [32–664] | 0.760 |
Results are given either as number (percentages) or median (minimal and maximal values).
Abbreviations: FOB, fibreoptic bronchoscopy; BMI, body mass index; SAPS II, Simplified Acute Physiologic Score II; ODIN, Organ Dysfunction and Infection score; PaO2/FiO2, ratio of partial oxygen pressure in arterial blood over inspired fraction of oxygen; PaCO2, partial carbon dioxide pressure in arterial blood; RR, respiratory rate; HR, heart rate; SBP, systolic blood pressure; PT, prothrombin time.
p<0.05.
Bronchoscopy provided the diagnosis in 100 (59%) procedures, and the results led to the introduction or discontinuation of a treatment in 86 (51%) procedures.
Need for intubation and invasive mechanical ventilation was recorded in 25 (15%) cases during the 24 hours following FOB (figure 1).
Figure 1. Changes in modalities of oxygenation delivery in acutely ill hypoxemic patients undergoing fiberoptic bronchoscopy.
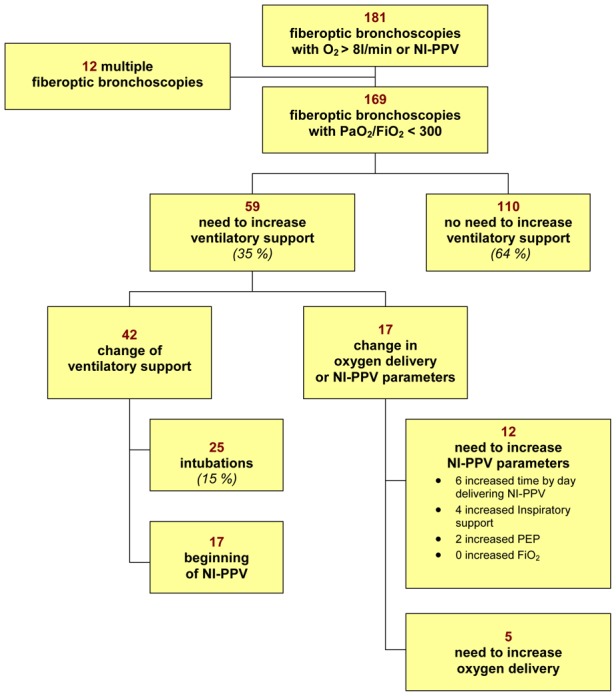
The percentage is relative to the whole population. Abbreviations: NI-PPV, noninvasive positive-pressure ventilation; PEEP, positive end-expiratory pressure; FiO2, inspired fraction of oxygen.
Altogether, need to increase ventilatory support were recorded after 24 hours in 59 (35%) FOB. NI-PPV was started in 17 (10 %) cases. In the 17 remaining cases, oxygen delivery was increased by more than 50% (n=5) or NI-PPV support was increased (n=12).
Median time to need to increase ventilatory support amounted to 3.75 hours (25–75 IQR: 3.45–8.79) (Figure 2). Following 20 (12%) bronchoscopies, ventilatory support was increased (included 7 intubations with invasive mechanical ventilation) within 2 hours of bronchoscopy.
Figure 2.
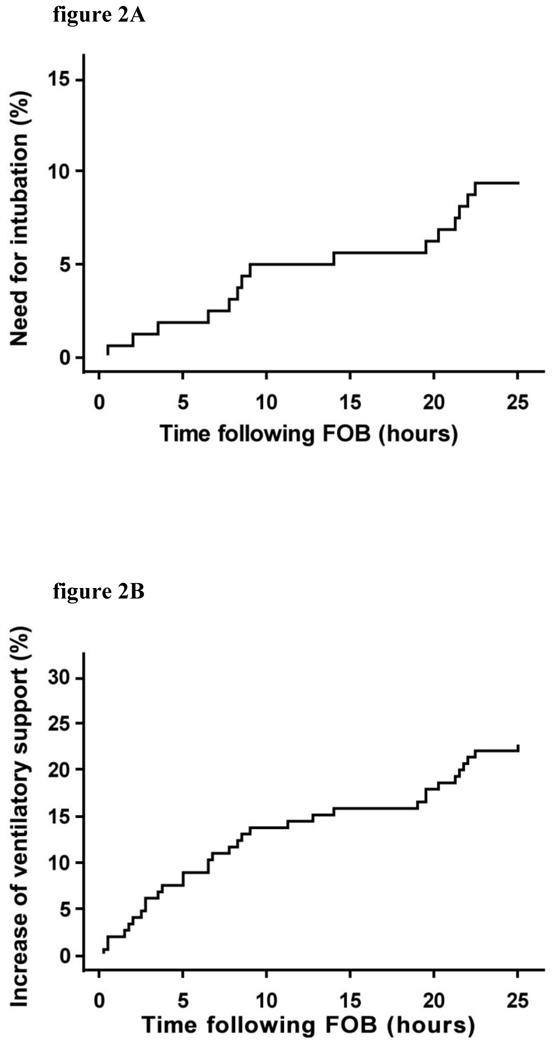
Cumulative incidence of bronchoscopy followed by a need for intubation (figure 2A) Or increased ventilatory support (figure 2B) within 24 hours after bronchoscopy. Time is in hours (abscissas) and the cumulative incidence (ordinates) is given as a probability.
Altogether, 11 patients had other post-fiberoptic bronchoscopy events after 24 hours. Cardiac arrest occurred in 4 cases, cardiac arrhythmia in 9 cases, and pneumothorax in 2 cases. One cardiac arrest occurred during the procedure which was stopped and the patient died 5 hours later, whereas the other three occurred between 17 and 23 hours afterwards. Those cardiac arrests complicated multiorgan failure for two of them, and a massive hemoptysis for the last one. This hemoptysis was the original indication for FOB, and it recurred the following day. Two patients developed pneumothoraces, one 2 and the other 11 hours after bronchoscopy. Both patients had undergone BAL without bronchial biopsies. One of the pneumothoraces occurred after intubation and mechanical ventilation in a patient with an acute exacerbation of idiopathic pulmonary fibrosis. It has to be noted that, although 18 % of patients were on anticoagulant therapy, no bleeding event was reported during/after fiberoptic bronchoscopy.
Altogether, 36 patients (21%) died in the ICU. Median time from bronchoscopy to death was 12 days [1–92]. Three patients died within 24 hours after bronchoscopy.
Factors predicting initiation of invasive ventilation after bronchoscopy
Initiation of invasive mechanical ventilation (endotracheal intubation) was associated with HR (p=0.014), RR (p=0.043) (Tables 1, 2 and 3; univariate analysis), immunosuppression (p=0,036) and hematological malignancy (p=0,023), and administration of NI-PPV before FOB (p=0.043). Blood gas and level of hypoxemia were not associated with need to increase ventilatory support (Table 1). Neither were the characteristics of FOB (Table 3).
Table 2.
Underlying diseases and comorbidities
| Total | Initiation of invasive ventilation | |||
|---|---|---|---|---|
|
| ||||
| Number of FOB procedures | 169 | No | Yes | p |
|
| ||||
| 144 | 25 | |||
| Cardiovascular disease (%) | 65 (38) | 54 (38) | 11 (44) | 0.537 |
| Coronary artery disease | 29 (17) | 26 (18) | 3 (12) | 0.459 |
| COPD (%) | 26 (15) | 19 (13) | 7 (28) | 0.058 |
| CRPD (%) | 15 (9) | 12 (8) | 3 (12) | 0.552 |
| Immunosuppression (%) | 89 (53) | 71 (49) | 18 (72) | 0.036* |
| Hematological malignancy | 34 (20) | 25 (17) | 9 (36) | 0.023* |
| HIV | 21 (12) | 20 (14) | 1 (4) | 0.166 |
| Solid organ transplantation | 4 (2) | 3 (2) | 1 (4) | 0.561 |
| Solid cancer | 15 (9) | 13 (9) | 2 (8) | 0.975 |
| Corticosteroid therapy | 39 (23) | 31 (22) | 8 (32) | 0.251 |
| Immunosuppressive drugs | 25 (15) | 20 (14) | 5 (20) | 0.427 |
| Diabetes (%) | 24 (14) | 19 (13) | 5 (20) | 0.322 |
| Chronic renal failure (%) | 21 (15) | 16 (11) | 5 (20) | 0.182 |
| Neurological, swallowing impairment (%) | 20 (12) | 18 (13) | 2 (8) | 0.520 |
| Tobacco use (%) | 68 (40) | 55 (38) | 13 (52) | 0.194 |
| Anticoagulant therapy (%) | 31 (18) | 28 (19) | 3 (12) | 0.375 |
| Anti-platelet therapy (%) | 20 (12) | 17 (12) | 3 (12) | 0.978 |
Results are given either as number (percentages) or median (minimal and maximal values).
Abbreviations: FOB, fiberoptic bronchoscopy, HIV, positive serology for the human immunodeficiency virus., COPD: chronic obstructive pulmonary disease, CRPD, chronic restrictive pulmonary disease
p<0.05.
Table 3.
Characteristics of the 169 bronchoscopy procedures
| Total | Initiation of invasive ventilation | |||
|---|---|---|---|---|
|
| ||||
| Number of FOB procedures | 169 | No | Yes | p |
|
| ||||
| 144 | 25 | |||
| Number of BAL performed (%) | 102 (60) | 86 (60) | 16 (64) | 0.686 |
| Volume of fluid injected (ml) | 150 [20–200] | 150 [20–200] | 150 [80–200] | 0.191 |
| Δ Volume (injected – recovered) (ml) | 81 [14–195] | 85 [14–195] | 70 [40–170] | 0.366 |
| Ventilatory support | ||||
| NI-PPV before bronchoscopy (%) | 64 (38) | 50 (35) | 14 (56) | 0.043* |
| NI-PPV during bronchoscopy (%) | 54 (32) | 44 (31) | 10 (40) | 0.350 |
| Duration of FOB [min] | 11 [3–60] | 15 [3–40] | 11 [3–60] | 0.373 |
Results are given either as number (percentages) or median (minimal and maximal values).
NI-PPV during bronchoscopy represent the number of patients for them NI-PPV was used to improve FOB tolerance. Abbreviations: BAL, bronchoalveolar lavage; NI-PPV, noninvasive positive-pressure ventilation.
p<0.05.
Finally, factors associated with invasive ventilation in the multivariate analysis were chronic obstructive pulmonary disease (COPD) (p=0.007) and immunosuppression (p=0.004). (Table 4).
Table 4.
Factors associated with initiation of invasive ventilation within 24 hours after bronchoscopy in multivariable analysis
| Initiation of invasive ventilation | |||
|---|---|---|---|
|
| |||
| OR | [95% CI] | p value | |
| COPD | 5.3 | [1.6 17.8] | 0.007 |
| Immunosuppression | 5.4 | [1.7 17.2] | 0.004 |
Abbreviations: COPD, chronic obstructive pulmonary disease; RR; OR, odds ratio; 95%CI, 95% confidence interval. The Hosmer-Lemeshow chi-square test for the final model yields a p-value of 0.787 suggesting a model with good predictive value.
DISCUSSION
In this study, one third of the procedures of fiberoptic bronchoscopy performed in hypoxemic patients breathing spontaneously were complicated by an increase in ventilatory support. Endotracheal intubation was required in 15% of the cases overall but this complication occurred 2 hours after bronchoscopy in only 4% of the cases (Figure 2). Other complications such cardiac arrhythmias and hemoptysis were infrequentlty observed. Factors independently associated with a need of invasive ventilatory support were chronic obstructive pulmonary disease and immunosuppression, in multivariable analysis. Despite high respiratory and heart rates were associated with a need of invasive support in univariate analysis, none of the physiological parameters before FOB was independently associated with a need of invasive support in the multivariate analysis. These results may be partially explained by a relative small number of events and a lack of statistical power.
To our knowledge, this is the first study of the safety of bronchoscopy in patients with acute respiratory failure. Fiberoptic bronchoscopy is well known to be associated with gas exchanges alterations. In hypoxemic intubated patients, FOB is reputed to induce a drop in PaO2 up to 30%, with a return to baseline within 2 hours (11). As a result, FOB is traditionally considered hazardous in hypoxemic patients. Although, acute hypoxemia is not listed as a contraindication to bronchoscopy in international guidelines (7), there is general agreement that a pulse oximetry value greater than 90% or a PaO2 value greater than 8 kPa are necessary to perform bronchoscopy with safety. In our series, 34% of the bronchoscopies are followed by an increased ventilatory support and 15% by a need for endotracheal intubation. However, whether or not bronchoscopy was a separate causative factor is unclear. The SAPSII score (38±15) indicated severe physiological impairment, and oxygenation was severely altered before bronchoscopy; as underlined by the P/F ratio and mean RR of our patients. Although increased ventilatory support was required in 59 cases, the increase was needed within 2 hours after bronchoscopy in only 20 cases. As shown in Figure 2, a fixed number of patients worsened each hour after bronchoscopy, suggesting gradual deterioration of the respiratory status. We suggest that respiratory status deterioration was more likely to represent the natural progression of the underlying disease. Support for this hypothesis can be found in studies of Acute Lung Injury (ALI) outcomes, in which the intubation rate is close to that seen in our study (18–20).
The use of CPAP or NI-PPV during bronchoscopy has been suggested to improve safety in patients with acute hypoxemia. Both methods improved the tolerance of the procedure. However, they were evaluated only in single-center studies, each involving fewer than 40 patients and using physiological evaluation criteria (12, 13, 21). In our study, the 64 patients who had NI-PPV before and/or during FOB are more severe than the 105 patients without NI-PPV with particularly a lower P/F ratio and a higher PaCO2 (data not shown). The lack of standardized procedures among the ICU centers to perform FOB with concomitant use of NIPPV does not permit us to draw a firm conclusion about the beneficial use of NIPPV in this setting.
The yield of bronchoscopy and BAL in immunocompromised patients, most notably those with hematological malignancies, remains controversial. It was estimated to be about 33% in studies with large percentages of neutropenic patients, most of whom were receiving empiric antibiotic therapy (22). This low diagnostic yield and the high mortality rate in patients with hematological malignancies who require endotracheal intubation underline the need for carefully evaluating the risk of intubation related to bronchoscopy. In a multicenter study of 148 cancer patients, of whom 45 were not intubated, respiratory status deteriorated after bronchoscopy in 49% of patients; a change in ventilatory support was required in 35% of patients and endotracheal ventilation in 27% (23). BAL was associated with invasive ventilation but not with higher mortality (22). Thus, in cancer patients, bronchoscopy with BAL generally has a low yield but does not seem to increase mortality. In our study of nonintubated patients, 89 patients had immunosupression, including 34 with hematological malignancies. By multivariate analysis, only immunosuppression was associated with change of ventilatory support after bronchoscopy in our study and confirms the risk of respiratory deterioration in these patients. However, a recent study conducted by Azoulay and coworkers compared the incidence of respiratory failure in oncology patients following a non invasive and an invasive testing including FOB lacks to show any difference in both groups, which argue for a worsening of respiratory failure unrelated to FOB (24).
Among underlying diseases, COPD was independently associated with intubation after FOB. Little is known about tolerance of FOB in patient with chronic respiratory failure (25) but one can imagine precipitating hypercapnic ventilatory failure with an increased functional respiratory capacity (FRC), as shown during FOB (17). Currently, no prospective study had focus on FOB tolerance and safety in this group of patients despite this procedure is often warranted for cancer or infectious diagnosis. The small number of COPD patients (N=26) in our study does not allowed us to see if NI-PPV, in this setting of hypoxemic respiratory failure in COPD, increase the safety of FOB and this may warrant further study.
Our study has several limitations. First, four ICUs were in respiratory departments, and their senior physicians performed large numbers of bronchoscopies, most notably in patients with acute respiratory failure. Although no center effect was found in our study (data not shown), tolerance and safety of bronchoscopy may improve with physician experience. Second, most of the bronchoscopies in our study were performed to evaluate infections. The high proportion of infections may have spuriously increased the diagnostic yield of bronchoscopy (59% of bronchoscopies). Third, the bronchoscopic procedure was not standardized. However, the need to perform intubation was not influenced by bronchoscopy duration, BAL, injected BAL volume, or recovered BAL volume. Surprisingly, the injected BAL volume was about 150 ml, i.e., the amount generally used in stable patients. Finally, The FIO2 ratio has been only estimated in non-ventilated patients who may question about the lack of relation between baseline PaO2/FiO2 ratio and post FOB intubation.
In this observational study, we looked for factors that predicted a need for increased ventilatory support after bronchoscopy. By multivariate analysis, a need for invasive ventilatory support was not associated with the extent of radiological opacities, PaO2/FiO2 ratio, BAL, or injected BAL volume. COPD and immunosuppression are the only factors associated with a risk of intubation. The time course between FOB and these events suggest that the respiratory status deterioration might have been related to the natural course of the acute respiratory failure rather than bronchoscopy.
Acknowledgments
Sources of support: None
Christophe CRACCO, Alexandre DEMOULE and Bernard MAITRE participated in the study design. Christophe CRACCO and Bernard MAITRE supervised the study. Christophe CRACCO, Muriel FARTOUKH, Helene PRODANOVIC, Elie AZOULAY, Christine LORUT, Gaetan BEDUNEAU, Hoang Nam BUI and Camille TAILLE collected the data. Cécile CHENIVESSE, Christophe CRACCO and Bernard MAITRE analysed the data. Cécile CHENIVESSE provided statistical expertise. Christophe CRACCO, Alexandre DEMOULE and Bernard MAITRE drafted the report, and the report was revised for important intellectual content by Muriel FARTOUKH, Elie AZOULAY, Christine LORUT, Gaetan BEDUNEAU, Hoang Nam BUI and Laurent BROCHARD. We thank Dr Joanna DORSETT (Medical Intensive Care Unit, Pulmonology Department, Pitié-Salpêtrière Teaching Hospital, Paris, France) for the English reading.
Appendix: FOBREA study group
Antoine RABBAT (MD; Medical Intensive Care Unit, Pulmonology Department, Hôtel Dieu Hospital, Paris, France), Aurélie LEFEBVRE (MD; Medical Intensive Care Unit, Pulmonology Department, Hôtel Dieu Hospital, Paris, France), Antoine PARROT (MD; Medical Intensive Care Unit, Pulmonology Department, Tenon Hospital, Paris, France), Christophe GIRAULT (MD; Medical Intensive Care Unit, Charles Nicolle Hospital, Rouen, France), Jérôme DEVAQUET (MD; Medical Intensive Care Unit, Henri Mondor Hospital, Créteil, France), Pablo ALVAREZ (MD; Medical Intensive Care Unit, Henri Mondor Hospital, Créteil, France).
Footnotes
Conflict of interest: None
References
- 1.Jolliet P, Chevrolet JC. Bronchoscopy in the intensive care unit. Intensive Care Med. 1992;18:160–169. doi: 10.1007/BF01709240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med. 2001;22:241–261. vii. doi: 10.1016/s0272-5231(05)70041-1. [DOI] [PubMed] [Google Scholar]
- 3.Tobin MJ, D’Alonzo GE. Bronchoscopy in intensive care. Appl Cardiopulm Pathophysiol. 1991;3:319–325. [PubMed] [Google Scholar]
- 4.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 5.Gruson D, Hilbert G, Valentino R, et al. Utility of fiberoptic bronchoscopy in neutropenic patients admitted to the intensive care unit with pulmonary infiltrates. Crit Care Med. 2000;28:2224–2230. doi: 10.1097/00003246-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ovassapian A. The flexible bronchoscope. A tool for anesthesiologists. Clin Chest Med. 2001;22:281–299. doi: 10.1016/s0272-5231(05)70043-5. [DOI] [PubMed] [Google Scholar]
- 7.British thoracic society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56 (Suppl 1):i1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the american college of chest physicians. Chest. 2003;123:1693–1717. doi: 10.1378/chest.123.5.1693. [DOI] [PubMed] [Google Scholar]
- 9.Surrat D, Smiddy J, Grubert B. Deaths and complications associated with fiberoptic bronchoscopy. Chest. 1976;69:747–751. doi: 10.1378/chest.69.6.747. [DOI] [PubMed] [Google Scholar]
- 10.Lindholm CE, Ollman B, Snyder JV, et al. Cardiorespiratory effects of flexible fiberoptic bronchoscopy in critically ill patients. Chest. 1978;74:362–368. doi: 10.1378/chest.74.4.362. [DOI] [PubMed] [Google Scholar]
- 11.Trouillet JL, Guiguet M, Gibert C, et al. Fiberoptic bronchoscopy in ventilated patients. Evaluation of cardiopulmonary risk under midazolam sedation. Chest. 1990;97:927–933. doi: 10.1378/chest.97.4.927. [DOI] [PubMed] [Google Scholar]
- 12.Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med. 2000;162:1063–1067. doi: 10.1164/ajrccm.162.3.9910117. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs. Conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest. 2002;121:1149–1154. doi: 10.1378/chest.121.4.1149. [DOI] [PubMed] [Google Scholar]
- 14.Société de Pneumologie de Langue Française. Recommandations pour la prise en charge des BPCO. Rev Mal Resp. 1997;14:S22–30. [PubMed] [Google Scholar]
- 15.Non invasive ventilation during acute respiratory failure. Consensus conference of the French Society of Intensive Care. 2006. http://www.srlf.org/Data/upload/Files/2006_10_12_conference_de_consensus_commune_ventilation_non_invasive_resume.pdf.
- 16.Antonelli M, Conti G, Bufi M, et al. A comparison of non-invasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 17.Matsushima Y, Jones RL, King EG, et al. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984;86:184–188. doi: 10.1378/chest.86.2.184. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001;27:1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 19.Confalonieri M, Potena A, Carbone G, et al. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–1591. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- 20.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA. 2000;284:2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 21.Da Conceicao M, Genco G, Favier JC, et al. fiberoptic bronchoscopy during noninvasive positive-pressure ventilation in patients with chronic obstructive lung disease with hypoxemia and hypercapnia. Ann Fr Anesth Reanim. 2000;19:231–236. doi: 10.1016/s0750-7658(00)00213-6. [DOI] [PubMed] [Google Scholar]
- 22.White P, Bonacum JT, Miller CB. Utility of fiberoptic bronchoscopy in bone marrow transplant patients. Bone Marrow Transplant. 1997;20:681–687. doi: 10.1038/sj.bmt.1700957. [DOI] [PubMed] [Google Scholar]
- 23.Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: Prospective multicenter data. Crit Care Med. 2008;36:100–107. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 24.Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–1046. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 25.Hattatuwa K, Gamble EA, O’Shaughnessy T, et al. Safety of bronchoscopy, biopsy, and BAL in research patients with COPD. Chest. 2002;122:1909–1912. doi: 10.1378/chest.122.6.1909. [DOI] [PubMed] [Google Scholar]


