Abstract
Objective
To evaluate the efficacy and safety of combination bevacizumab/pemetrexed for the treatment of recurrent epithelial ovarian cancer (EOC).
Methods
Platinum-sensitive or -resistant patients with recurrent or persistent EOC were eligible if they had received up to 2 prior chemotherapy regimens, including a platinum/taxane regimen without prior bevacizumab. Pemetrexed 500 mg/m2 IV and bevacizumab 15 mg/kg IV were administered every 3 weeks. The primary endpoint was 6-month progression-free survival (PFS); other endpoints included toxicities, PFS and overall survival (OS).
Results
Thirty-four patients received a median of 7 treatment cycles (range, 2–26). Median follow-up was 25.7 months (range, 3.0–47.2). Six month progression-free survival (PFS) was 56% (95%CI: 38–71). The following response rates were documented (%; 95%CI): 0 complete response, 14 partial responses (41%; 25–59), 18 stable disease (53%; 35–70) and 2 progressive disease (6%; 1–20). Median PFS was 7.9 months (95%CI, 4.6–10.9), with a median OS of 25.7 months (95% CI, 15.4–29.8). Twenty-two patients (64.7%) had a platinum-free interval (PFI) of >6 months prior to enrollment. Grade 3–4 hematologic toxicities included neutropenia (50%), leukopenia (26%), thrombocytopenia (12%) and anemia (9%). Non-hematologic grade 3–4 toxicities included metabolic (29%), constitutional (18%), pain (18%) and gastrointestinal (15%). Two patients developed hematologic malignancies within one year of treatment.
Conclusions
Combination bevacizumab/pemetrexed is an active option for both platinum-sensitive and -resistant recurrent EOC. Further investigation of cost and novel toxicities associated with this regimen may be warranted.
INTRODUCTION
Ovarian cancer remains the most lethal gynecologic malignancy(1). While 70–80% of women achieve complete responses to upfront therapy with surgery and chemotherapy, the majority will experience recurrence and have incurable disease. Goals for second-line therapy are to improve disease-free intervals and quality of life. While incidence has been relatively stable since the 1990s, death rates for ovarian cancer have decreased by 2% per year from 2005–2009(2). Combinations of targeted and cytotoxic therapies that improve efficacy while minimizing toxicity are necessary for continued progress in lengthening progression-free intervals.
Vascular endothelial growth factor (VEGF) and other markers of angiogenesis appear to correlate with prognosis in ovarian cancer. Bevacizumab, a monoclonal antibody targeting VEGF, is now a well-established component of treatment programs for recurrent ovarian cancer(3). Maintenance bevacizumab increases progression-free survival when given after adjuvant chemotherapy in the upfront(4, 5) and recurrent platinum-sensitive(6) and platinum-resistant(7) settings.
Pemetrexed (Alimta, Eli Lilly, Indianapolis, IN) is a multi-targeted anti-folate agent that inhibits several enzymes required for DNA synthesis including thymidylate synthase, dihydrofolate reductase and glycinamide ribonucleotide formyl transferase(8). Its multiple targets may help to achieve a broader spectrum of anti-tumor efficacy compared to other antimetabolites. Pemetrexed has demonstrated activity in non-small-cell lung cancer, mesothelioma, breast, colorectal, pancreas, bladder and head and neck cancers(9). Its activity in platinum-resistant ovarian cancer was demonstrated in a Gynecologic Oncology Group trial of single-agent pemetrexed in 51 women with recurrent ovarian cancer that demonstrated a response rate of 19%, including one (2%) complete response, and disease stabilization in 35% of patients(10). Two phase II trials of combination pemetrexed/carboplatin in platinum-sensitive patients with recurrent ovarian cancer have been reported, demonstrating overall response rates of 51%(11) and 33%(12) with minimal toxicity.
With continued interest in bevacizumab combinations, evidence of pemetrexed’s activity in ovarian cancer, and the need for efficacious treatments for recurrent ovarian cancer with minimal toxicity, we studied the combination of bevacizumab/pemetrexed in patients with recurrent ovarian cancer.
METHODS
Eligibility Criteria
Patients with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer at Washington University School of Medicine were deemed eligible for this study if they were ≥18 years of age, had a Gynecologic Oncology Group performance status of 0 or 1, histologic confirmation of the primary tumor, and measurable disease with at least one target lesion to assess response by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0) criteria.
Patients were required to have had one prior platinum-based chemotherapeutic regimen for management of primary disease along with one prior regimen containing a taxane compound. First-line treatment could have been administered either intravenously or intraperitoneally. Patients were not permitted to have received prior therapy with pemetrexed or bevacizumab. No more than 2 prior cytotoxic chemotherapy regimens (adjuvant therapy plus one additional regimen) were allowed. Consolidation or extended therapy as part of first-line treatment was considered a single regimen. There was no minimum platinum-free interval required for eligibility.
Patients were required to have adequate bone marrow function, with an absolute neutrophil count (ANC) greater than or equal to 1,500/μl and platelets ≥ 100,000/μl. Patients were also required to have a creatinine clearance >45 ml/min, bilirubin ≤ 1.5x upper limit of normal (ULN) and aspartate aminotransferase and alkaline phosphatase ≤ 2.5x ULN. Adequate coagulation status was required, defined as an international normalized ratio ≤ 1.5 (or 2–3 for patients on stable doses of therapeutic warfarin). Sensory and motor neuropathy was required to be ≤ grade 1 by common toxicity criteria (version 3.0). Due to the known ability of NSAIDs to prolong renal excretion of antifolates(13, 14), patients were required to stop NSAID use prior to treatment on study. Various comorbidities were also listed as exclusion factors (see Supplemental Material).
The protocol was approved by the Institutional Review Board of Washington University School of Medicine, and each patient gave informed consent prior to enrollment. The study was registered on ClinicalTrials.gov (identifier: NCT00868192). This was an investigator-initiated study sponsored and partially supported by Genentech, who provided bevacizumab, and Eli Lilly, who provided pemetrexed, free of charge.
Treatment and Dose Modifications
Treatment was given on day 1 of a 21-day cycle. Pemetrexed was administered intravenously (IV) at a dose of 500 mg/m2 IV over 10 minutes, followed by IV bevacizumab at a dose of 15 mg/kg. Patients with prior radiation therapy for any cancer were required to initiate pemetrexed at one dose level reduction, then escalated if no toxicity > grade 1 ensued. A maximum body surface area of 2.0 m2 was used for dose calculations according to the GOG Chemotherapy Procedures Manual (2003) at the time of study.
Folic acid (350–1000 micrograms) was provided by the study and given daily beginning 5–7 days prior to the first dose of pemetrexed and continued until 3 weeks after the last dose of study therapy. Vitamin B12 (1000 micrograms) was also provided and administered intramuscularly 1–2 weeks prior to the first dose of pemetrexed and repeated every 9 weeks until 3 weeks after the last dose of study therapy. NSAIDs were required to be discontinued 2 days before (5 days for long-acting NSAIDs) pemetrexed administration and could not be restarted until 2 days after pemetrexed. Dexamethasone 4 mg orally was given twice daily for three days surrounding each dose of pemetrexed for rash prophylaxis.
Patients with treatment delays for toxicity were evaluated on a weekly basis until adequate parameters were met. Delays of greater than two weeks resulted in removal from the study. No bevacizumab dose modification was allowed during the study. A level-1 dose reduction for pemetrexed was planned as 375 mg/m2, and 250 mg/m2 for a level-2 dose reduction.
Patients did not receive prophylactic or routine use of white blood cell growth factors (filgrastim, sargramostim or pegfilgrastim) unless they experienced recurrent neutropenic complications after treatment modifications. Growth factors were only allowed for ANC < 1500/μl, neutropenic fever or documented infections while neutropenic. Use of erythropoiesis-stimulating agents was not allowed. Patients were continued on study treatment until disease progression or intolerable toxicity.
Study End Points
This was a Phase II study without randomization. The primary endpoint was 6-month progression-free survival. Secondary endpoints included toxicity and objective clinical response by RECIST and CA-125 criteria. A CA-125 response was defined as at least a 50% reduction in CA-125 levels from a pretreatment sample following guidelines described by the Gynecological Cancer Intergroup(15).
Statistical Analysis
The sample size for this study was determined based on Fleming’s single-stage Phase II design. We assumed that a new agent with 6-month PFS of 15% or less would be considered of little clinical significance and an agent with PFS 40% or higher would be of interest for further investigation. With 90% power and at a 2-sided 0.05 significance level, at least 32 evaluable patients were required to detect the above difference.
Demographic and clinical characteristics, as well as toxicity by grade were summarized using descriptive statistics. Response rates were calculated and an exact binomial 95% confidence interval was computed for these estimates. Overall survival (OS) was defined as time from patient registration to death for any reason. Patients remaining alive were censored at the last clinical contact. Progression-free survival (PFS) was defined as the time from patient registration to documented progression or death, whichever occurred first. Patients without progression were censored at the last clinical contact. A Kaplan-Meier product limit estimator was utilized to graphically describe PFS and OS, and to estimate the 6-month PFS, 12-month OS, and median PFS and OS. Patients were also stratified into 3 groups based on platinum-free intervals (<6 months, 6–12 months, >12 months) prior to registration for this study. The between-group differences in PFS and OS were described using Kaplan-Meier product limit methods and compared by log-rank tests. Hazard ratios (HR) for PFS were estimated using a univariate Cox regression model. All statistical analyses were performed using SAS version 9.2 (SAS Institutes, Cary, NC).
RESULTS
Patient Characteristics
Between May 2009 and November 2010, 39 patients were enrolled, and 34 eligible patients were treated. 5 patients were enrolled but deemed ineligible prior to receiving any treatment. Treatment on trial was completed for all patients by June of 2011. Demographic and baseline patient characteristics are reported in Table 1. 33 (97%) patients were white and one was black. Most patients had stage IIIC papillary serous cancer. All 34 patients had received prior platinum and taxane-based therapy, with 20 patients (59%) receiving treatment on this protocol for their first recurrence. Platinum-free intervals (PFI) from prior treatment varied (range, 1–44 months); 12 patients (35.2%) had a PFI < 6 months, 11 (32.4%) had a PFI of 6–12 months, and 11 (32.4%) had a PFI >12 months.
Table 1.
Patient Demographics
| n (%) | |
|---|---|
| Age | |
| Mean (min-max) | 61.5 (31–86) |
|
| |
| Stage | |
| I/II | 1 (2.9) |
| III | 27 (79.4) |
| IV | 6 (17.6) |
|
| |
| Histology | |
| Serous | 24 (70.6) |
| Endometrioid | 2 (5.9) |
| Mixed | 3 (8.8) |
| Other | 5 (14.7) |
|
| |
| Tumor Grade | |
| 1 | 3 (8.8) |
| 2 | 1 (2.9) |
| 3 | 30 (88.2) |
|
| |
| Pathologic Diagnosis | |
| Ovarian | 27 (79.4) |
| Fallopian tube | 1 (2.9) |
| Primary peritoneal | 6 (17.6) |
|
| |
| Performance Status | |
| 0 | 22 (64.7) |
| 1 | 12 (35.3) |
| Prior # Chemotherapy Regimens | |
| 1 | 20 (58.8) |
| 2 | 14 (41.2) |
|
| |
| Platinum-free Interval | |
| <6 months | 12 (35.2) |
| 6–12 months | 11 (32.4) |
| >12 months | 11 (32.4) |
All patients received at least one cycle of study treatment. In total, 316 cycles were administered with a median of 7 cycles per patient (range 2–26). 18 patients were discontinued from the study due to objective progression by RECIST criteria, while 11 patients were electively discontinued, mainly for reasons of fatigue. Five patients were discontinued for grade 3 or greater toxicity that included one case each of a high-grade bowel obstruction, severe proteinuria, renal failure, an acute embolic cerebral vascular accident and severe neutropenia. Median follow-up time was 25.7 months. At last contact, 27 patients had died of disease (79.4%) and 7 patients were alive with disease (20.6%).
Efficacy
All patients were RECIST-evaluable, with a median follow-up of 25.7 months (range, 3.0–47.2 months). Six-month PFS was 56% (95% CI, 38–71) with median PFS of 7.9 months (95%CI, 4.6–10.9). 12-month OS was 79% (95% CI, 62–90) and median OS was 25.7 months (95% CI, 15.4–29.8), as shown in Figure 1.
Figure 1.
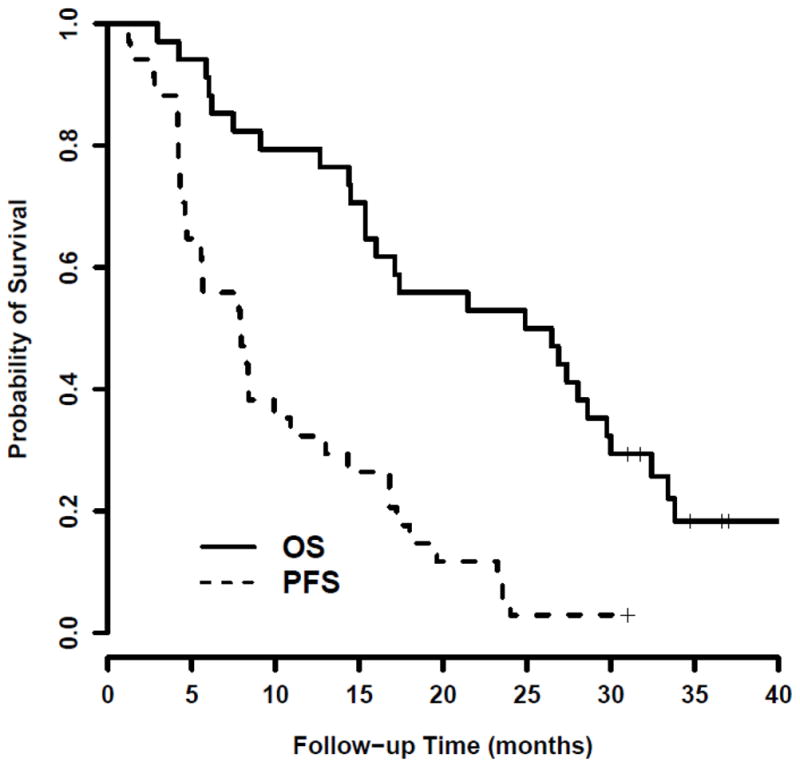
Kaplan-Meier analysis of progression-free (PFS) and overall survival (OS), where the vertical bars represent the times of censoring.
The overall response rate was 41% (95% CI, 25–59). All responses were partial responses; there were no complete responses. 18 patients had stable disease (53%; 35–70) and 2 had progressive disease (6%; 1–20). The disease control rate was 94%. Twenty-seven patients were evaluable by CA-125 criteria; a 50% CA-125 response was documented in 17 patients (62%; 44–79), and a 75% CA-125 response in 8 patients (30%; 16–49).
After stratification by PFI prior to study treatment, median PFS for those with a PFI < 6 months was 6.7 months (95% CI, 4.1–9.9), compared to 8.4 months for those with a PFI ≥ 6 months (p = 0.097). Median PFS was 4.7 months (95% CI 2.8–8.4) for those with a PFI 6–12 months, and 16.8 months (95% CI 4.6 – 23.2 mo.) for those with a PFI >12 months (p=0.045). Compared to patients with a PFI < 6 months, those treated with bevacizumab/pemetrexed after a PFI > 12 months have a 64% decreased risk of progression (HR=0.36, 95% CI 0.15–0.88; p=0.024). Median OS (95% CI) for patients with PFI <6 months was 16.7 months, compared to 26.5 months for those with PFI ≥ 6 months (p = 0.52). Median OS was 24.9 months (6.2–28.6) for those with a PFI 6–12 months, and 28 months (95% CI not estimable), for those with a PFI > 12 months (p=0.609). These results are shown in Table 2 and Figure 2.
Table 2.
Progression-free and Overall Survival by Platinum-Free Interval.
| Platinum-Free Interval | Median PFS (months, 95% CI) | Median OS (months, 95% CI) |
|---|---|---|
| 0–6 months (n=12) | 6.7 (4.1–9.9) | 16.7 (7.5–33.4) |
|
| ||
| >6 months (n=22) | 8.4 | 26.5 |
| 6–12 mo. (n=11) | 4.7 (2.8–8.4) | 24.9 (6.2–28.6) |
| >12 mo. (n=11) | 16.8 (4.6–23.2) | 28 (N/E) |
Figure 2.
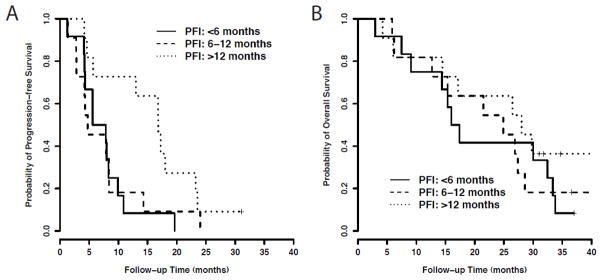
Kaplan-Meier analysis of A) progression-free survival and B) overall survival based on platinum-free interval prior to study enrollment.
Safety and Tolerability
All patients were evaluated for toxicity at the beginning of each cycle. Grade 3–4 hematologic toxicities occurred in 53% of patients (neutropenia 50%, leukopenia 26%, thrombocytopenia 12%, anemia 9%). One patient was removed from the study due to prolonged neutropenia, and eventually developed acute myeloid leukemia (AML) as described further below. No other neutropenic dose delays were >2 weeks, and there were no dose reductions used throughout the study. There were no febrile neutropenic events. One patient developed a septic pulmonary embolus requiring hospitalization, anticoagulation and intravenous antibiotics, but no dose delay was necessary.
Fatigue was the most common non-hematologic toxicity, experienced by 94% of patients, but was predominantly low-grade (76% grade 1–2). Using a strict cut-off for creatinine clearance (CrCl) of 45 ml/min, only 6% of patients experienced grade 3 renal toxicity; similarly, strict monitoring for proteinuria was enforced. One patient’s bevacizumab was held completely for a cycle due to proteinuria but recovered for subsequent cycles. Gastrointestinal side effects were common, experienced by 91% of patients, and predominantly low-grade (79% of GI toxicities were grade 1–2). There were no documented bowel perforations. A summary of all toxicities is found in Table 3.
Table 3.
Hematologic and Non-hematologic Toxicities
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Anemia | 18 | 82 | 5 | 15 | 5 | 15 | 0 | 0 | ||
| Leukopenia | 11 | 32 | 8 | 24 | 5 | 15 | 4 | 12 | 0 | |
| Thrombocytopenia | 11 | 32 | 0 | 1 | 3 | 3 | 9 | 0 | ||
| Neutropenia | 13 | 38 | 4 | 12 | 6 | 18 | 10 | 29 | 0 | |
| Allergy | 10 | 29 | 1 | 3 | 0 | 0 | 0 | |||
| Auditory | 1 | 3 | 0 | 0 | 0 | 0 | ||||
| Cardiovascular | 5 | 15 | 5 | 15 | 1 | 3 | 0 | 0 | ||
| Impaired coagulation | 4 | 12 | 0 | 2 | 6 | 0 | 0 | |||
| Constitutional | 9 | 26 | 17 | 50 | 5 | 15 | 0 | 1 | 3 | |
| Rash | 12 | 35 | 11 | 32 | 0 | 0 | 0 | |||
| Endocrine | 0 | 1 | 3 | 0 | 0 | 0 | ||||
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | |||||
| Gastrointestinal | 12 | 35 | 15 | 44 | 4 | 12 | 0 | 0 | ||
| Genitourinary | 1 | 3 | 3 | 9 | 2 | 6 | 0 | 0 | ||
| Hemorrhage | 3 | 9 | 0 | 1 | 3 | 0 | 0 | |||
| Infection/Febrile Neutropenia | 3 | 9 | 10 | 29 | 0 | 1 | 3 | 0 | ||
| Lymphatics | 0 | 0 | 1 | 3 | 0 | 0 | 0 | |||
| Metabolic | 12 | 35 | 11 | 32 | 7 | 21 | 1 | 3 | 0 | |
| Musculoskeletal | 1 | 3 | 0 | 1 | 3 | 0 | 0 | |||
| Neurologic | 8 | 24 | 12 | 6 | 1 | 3 | 0 | 0 | ||
| Ocular | 6 | 18 | 0 | 0 | 0 | 0 | ||||
| Pain | 5 | 15 | 17 | 50 | 6 | 18 | 1 | 3 | 0 | |
| Pulmonary | 8 | 24 | 8 | 24 | 0 | 0 | 0 | |||
| Vascular | 0 | 1 | 3 | 2 | 6 | 0 | 0 | |||
Two patients in our study have subsequently developed hematologic malignancies. One, a 46-year-old who initially received IV/IP platinum/taxane therapy with a complete response, upon recurrence (PFI 13 months) had an optimal secondary debulking including splenectomy and distal pancreatectomy, followed by treatment with pemetrexed/bevacizumab on study. She was taken off study after 7 cycles for severe neutropenia and was diagnosed with acute myeloid leukemia (AML) 4 months later. She received treatment with fludarabine, high-dose cytosine arabinoside, idarubicin and G-CSF followed by an allogeneic stem cell transplant. At the time of last follow-up, she had stable disease treated with single-agent bevacizumab.
The second patient to develop a hematologic malignancy was a 60-year-old previously treated with IV platinum/taxane with a complete response and disease-free interval of 8 months. She was then treated with 25 cycles of single-agent weekly topotecan at recurrence, followed by 10 cycles of pemetrexed/bevacizumab on study. She was taken off study due to extreme fatigue, continued to have persistent fatigue and anemia, and 6 months later underwent a bone marrow biopsy showing hypercellular marrow with trilineage dysplasia consistent with myelodysplastic syndrome (MDS). She did not receive further chemotherapy for ovarian cancer and has since died.
DISCUSSION
In this cohort of patients with recurrent ovarian, fallopian tube and primary peritoneal cancer, the combination of pemetrexed and bevacizumab demonstrated favorable efficacy with a 6-month PFS of 56% and an overall response rate of 41%. This is a novel combination of cytotoxic and biologic agents for recurrent ovarian cancer showing response rates comparable to those seen in published platinum doublets for both platinum-sensitive and -resistant recurrent disease.
No dose reductions were required throughout the entire trial, and only 5 patients (14.7%) were discontinued from study due to grade 3 or greater toxicities. Grade 1–2 fatigue was the predominant symptom noted, but was not dose limiting. Routine use of corticosteroids, vitamin B12 and folic acid seemed to effectively control rash and mucositis, toxicities that have been reported in prior trials(16). Grade 3 hematologic toxicities did occur at a high rate, with 50% of patients experiencing at least grade 3 neutropenia, but short dose delays were enough for counts to recover quickly.
Therapy-related myeloid neoplasms (t-MN)(17), defined as AML and MDS occurring after cytotoxic chemotherapy or radiation, have been reported to develop in 0.17% of ovarian cancer patients, with a median latency of 4 years(18). A wide range of cytotoxic therapies have been associated with t-MN, including alkylating agents, topoisomerase II inhibitors, antimetabolites and anti-tubulin agents, with a latency period ranging from one to 10 years(19). Two out of 34 patients (5.8%) in our study did eventually develop hematologic malignancies after completion of treatment, with a latency range of only 4–6 months following treatment. One of our affected patients had received 25 cycles of weekly topotecan, but this therapy would be expected to cause abrupt AML, not MDS; the other had only received standard platinum/taxane therapy. To our knowledge, neither pemetrexed nor bevacizumab has previously been associated with t-MN, but the antimetabolite actions of pemetrexed could conceivably predispose to a higher risk of t-MN. Therefore, this may represent a new subgroup within t-MN, although our small sample size prohibits any definitive attribution to this particular regimen. Long-term follow up and monitoring of blood counts after treatment with bevacizumab and pemetrexed is certainly recommended.
Table 4 provides a summary comparing reported response rates to bevacizumab- and platinum-containing regimens for recurrent ovarian cancer. 35% of our study patients were platinum-resistant, with a median PFS of 5.6 months and a median OS of 16.7 months. For these platinum-resistant patients, our results show a modest improvement compared to historical controls of bevacizumab alone (3, 20), and comparable survival data with Phase II studies of bevaciuzmab plus oral cyclophosphamide (21) or topotecan (22). Phase III data provide the most meaningful statistical bar by which to judge proposed new chemotherapy regimens; in a platinum-resistant population, the AURELIA trial demonstrated an improvement in PFS by 3.3 months with the addition of bevacizumab to chemotherapy alone(7). Our results add further support for the role of bevacizumab in combination with cytotoxic chemotherapy in this population.
Table 4.
Comparison of response rates for platinum- and bevacizumab-containing Phase II regimens for recurrent ovarian cancer.
| Authors | Agents | n | %PS | %PR | OR | 6-month PFS | Platinum- Sensitive | Platinum- Resistant | Combined Population | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| PFS | OS | PFS | OS | PFS | OS | |||||||
| Matulonis 2008 | Carbo + bev | 44 | 100 | - | 51 | NR | 7.6 | 20.3 | - | - | - | - |
| Sehouli 2011 | Carbo + pem | 61 | 100 | - | 33 | NR | 9.4 | NR | - | - | - | - |
| Burger 2007 | Bev | 62 | 58 | 42 | 21 | NR | - | - | - | - | 4.7 | 17 |
| Cannistra 2007 | Bev | 44 | - | 84 | 16 | NR | - | - | 4.4 | 10.7 | - | - |
| Garcia 2008 | Bev + oral cyclophos | 70 | 60 | 40 | 33 | 56 | 10 | 20 | 5 | 12 | 7.2 | 16.9 |
| McGonigle 2011 | Bev + topotecan | 40 | - | 100 | 25 | 55 | - | - | 7.8 | 16.6 | - | - |
| Kudoh 2011 | Bev + PLD | 30 | 4 | 96 | 33 | 47 | - | - | 6 | NR | - | - |
| del Carmen 2012 | Bev + PLD | 54 | 100 | - | 72 | NR | 14 | NR | - | - | - | - |
| Current Study | Bev + pem | 34 | 65 | 35 | 41 | 56 | 8.4 | 26.5 | 5.6 | 16.7 | 7.9 | 25.7 |
PS = platinum-sensitive; PR = platinum-resistant; OR = overall response; 6-month PFS = progression-free survival; PFS = median progression-free survival in months; OS = median overall survival in months; Bev = bevacizumab; pem = pemetrexed; cyclophos = cyclophosphamide; NR= not reported.
Our platinum-sensitive patients showed a greater response than previously shown in phase II trials of bevacizumab alone (3), with a median PFS of 8.4 months and a median OS of 26.5 months for those with a PFI > 6 months. Our results are comparable to other Phase II doublets containing either bevacizumab or pemetrexed (11, 12, 21, 23) and furthermore concur with the OCEANS trial, the first Phase III trial to demonstrate an improved PFS with the addition of bevacizumab to treatment with combination gemcitabine and carboplatin (12.4 months with bevacizumab versus 8.4 months without)(6) in a recurrent, platinum-sensitive population. Our platinum-sensitive cohort data compare favorably to the 32.8% response rate and 9.4-month PFS seen in platinum-sensitive patients treated with carboplatinum/pemetrexed in a phase II study by Sehouli et al (12), but notably with half the incidence of thrombocytopenia (12% in the current study versus 24%). While platinum-based regimens would typically be considered in this patient population(24, 25), these studies support the hypothesis that bevacizumab can be added to a variety of cytotoxic agents to improve PFS and minimize delays due to thrombocytopenia. Since our study lacks a comparator arm, we acknowledge that assessing relative contributions from either drug is difficult. Furthermore, it is possible that the disease response gained with this regimen, particularly in the platinum-sensitive population, simply reflects the stability of a relatively indolent disease.
Our study is limited by the absence of any cost evaluation. Our patients received both study drugs free of charge, rendering any economic evaluation of this combination inapplicable and beyond the scope of this study. However, cost analyses have shown that while bevacizumab may improve PFS, treatment is associated with significant increased direct and indirect costs(26), and the addition of pemetrexed will only add to these.
In summary, this Phase II trial utilizing bevacizumab and pemetrexed demonstrates an active combination regimen for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer, particularly for those with longer platinum-free intervals, with lower risk of thrombocytopenia than seen in platinum-containing doublets. However, the 5% rate of t-MN in this series is cause for concern. If future studies involving this combination in platinum-sensitive patients are carried out, they must carefully monitor for t-MN.
Supplementary Material
Research Highlights.
This phase II study of bevacizumab/pemetrexed in recurrent ovarian cancer showed a response rate of 41%.
Median OS was 16.7 months for platinum-resistant and 26.5 months for platinum-sensitive patients.
Two cases of therapy-related myeloid neoplasms were noted as a potential new toxicity.
Acknowledgments
Support: This investigator-initiated trial has been supported by research grants from Eli Lilly and Genentech. This work was also supported by the Alvin J. Siteman Cancer Center’s Biostatistics Core. The Siteman Cancer Center is supported by NCI Cancer Center Support Grant P30 CA91842.
Footnotes
This study was presented at a poster discussion session at the 48th ASCO Annual Meeting, June 1–4, 2012, Chicago, IL.
Conflict of Interest Statement
Andrea R. Hagemann, Akiva P. Novetsky, Feng Gao, L. Stewart Massad, Premal H. Thaker, Matthew A. Powell and David G. Mutch have no conflicts of interest. Israel Zighelboim and Jason D. Wright have each received research funding and support from Genentech for clinical trials evaluating bevacizumab in other gynecologic cancer populations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures. Atlanta: 2013. [Google Scholar]
- 3.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007 Nov 20;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 4.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine. 2011 Dec 29;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 5.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine. 2011 Dec 29;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 6.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jun 10;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine EHF, Weber B, Reuss A, Poveda A, Kristensen G. AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC) J Clin Oncol. 2012;30(Supplementary):Abstract LBA5002. [Google Scholar]
- 8.Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997 Mar 15;57(6):1116–23. [PubMed] [Google Scholar]
- 9.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005 Feb 1;23(4):792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 10.Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, et al. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Jun 1;27(16):2686–91. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matulonis UA, Horowitz NS, Campos SM, Lee H, Lee J, Krasner CN, et al. Phase II study of carboplatin and pemetrexed for the treatment of platinum-sensitive recurrent ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Dec 10;26(35):5761–6. doi: 10.1200/JCO.2008.17.0282. [DOI] [PubMed] [Google Scholar]
- 12.Sehouli J, Alvarez AM, Manouchehrpour S, Ghatage P, Szczylik C, Zimmermann A, et al. A phase II trial of pemetrexed in combination with carboplatin in patients with recurrent ovarian or primary peritoneal cancer. Gynecol Oncol. 2012 Feb;124(2):205–9. doi: 10.1016/j.ygyno.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney CJ, Takimoto CH, Latz JE, Baker SD, Murry DJ, Krull JH, et al. Two drug interaction studies evaluating the pharmacokinetics and toxicity of pemetrexed when coadministered with aspirin or Ibuprofen in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006 Jan 15;12(2):536–42. doi: 10.1158/1078-0432.CCR-05-1834. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldi DA, Kuhn JG, Burris HA, Dorr FA, Rodriguez G, Eckhardt SG, et al. A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer chemotherapy and pharmacology. 1999;44(5):372–80. doi: 10.1007/s002800050992. [DOI] [PubMed] [Google Scholar]
- 15.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011 Feb;21(2):419–23. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 16.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 May 1;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 17.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012 Jan 5;119(1):34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vay A, Kumar S, Seward S, Semaan A, Schiffer CA, Munkarah AR, et al. Therapy-related myeloid leukemia after treatment for epithelial ovarian carcinoma: an epidemiological analysis. Gynecologic oncology. 2011 Dec;123(3):456–60. doi: 10.1016/j.ygyno.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 19.Godley LA, Larson RA. Therapy-related myeloid leukemia. Seminars in oncology. 2008 Aug;35(4):418–29. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007 Nov 20;25(33):5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 21.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008 Jan 1;26(1):76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 22.McGonigle KF, Muntz HG, Vuky J, Paley PJ, Veljovich DS, Greer BE, et al. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: results of a phase 2 study. Cancer. 2011 Aug 15;117(16):3731–40. doi: 10.1002/cncr.25967. [DOI] [PubMed] [Google Scholar]
- 23.del Carmen MG, Micha J, Small L, Street DG, Londhe A, McGowan T. A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol. 2012 Sep;126(3):369–74. doi: 10.1016/j.ygyno.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003 Jun 21;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 25.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Oct 10;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 26.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Apr 1;29(10):1247–51. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


