Abstract
Maternal care has been shown to affect the development of behavioral and endocrine systems. In rats, periodic maternal deprivation (PMD) serves as an early life stressor that directly influences maternal care by promoting more pup-directed behaviors in stressed dams. To further assess the qualities of PMD that may ameliorate long-term anxiety effects in trait anxiety animals, we coded behaviors across lactation (postnatal day (PND) 5, 16, 21) in dams phenotyped as high (HAn) and low-anxiety (LAn). We assessed anxiety-like behavior in male offspring using the elevated plus maze (EPM), focusing on percent open arm (%OA) time and latency to enter OA (OA LAT) as a measure of anxiety-like behavior. Finally, we examined the brains of representative male pups to determine if the stress-related protein brain-derived neurotrophic factor (BDNF) might show persistent changes in the amygdala. Dams phenotyped as HAn had lower %OA time and longer OA LAT relative to dams designated as LAn. During PMD, HAn dams had higher incidences of lick-grooming (L/G) and more pup-directed behaviors on PND 5 and 16 compared to LAn dams. Further, as adults, HAn male offspring exhibited less anxiety traits than their maternal line with greater %OA time and %OA entries relative to LAn. HAn offspring showed markedly more BDNF immunoreacted cells in the amygdala than LAn. The combination of these findings suggests that the mild stressor, PMD alters anxiety-like behavior in offspring likely by influencing HAn dams’ L/G activity and altering stress related proteins in the amygdala.
Keywords: maternal separation, lick/groom, elevated plus maze, BDNF, high anxiety
1. Introduction
The early environment has received much attention for the critical role it plays in the development of the neural, behavioral and stress reactivity of infants. Early adverse experiences can have a negative impact on the development of the hypothalamic pituitary adrenal (HPA) axis, emotional behavior and stress reactivity of affected offspring (Roth and Sweatt, 2011). In clinical populations that have suffered childhood verbal, physical and sexual abuse and neglect, there is a correlation of greater instances of emotional distress in later life (Heim and Nemoroff, 2001) and hyperactivity of the HPA axis (Wismer Fries, Shirtcliff and Pollak, 2008).
Long periods of maternal deprivation models in rodents and primates are used to mimic human-infant early life stress given that it leads to similar impacts on the neural, hormonal and behavioral systems described in clinical populations (Kikusui and Mori 2009; Macrì, Zoratto and Laviola, 2011). In contrast, short-term socio-environmental interventions can help to improve HPA axis functioning in compromised animals through sustained alterations in proteins implicated in plasticity and stress adaptability (Branchi, 2009; Ravenelle et al., 2013). For instance, in periodic maternal separation models in inbred trait anxiety animals (e.g., animals that show high anxiety-like behavior), adult offspring experience recovery of the HPA axis such that in response to anxiogenic stimuli they show a blunted adrenocorticotropic response (Neumann et al., 2005). Moreover, in an animal model of activity-based anorexia, if rodents experience long maternal separation (LMS) (i.e., daily separation for 180 min during the pre-weaning period), all offspring experience greater weight gain and females have markedly increased survival time.
Brain derived neurotrophic factor (BDNF) is a neurotrophin implicated in brain plasticity and stress adaptability and is a likely mechanism of change associated with social enrichment paradigms (Branchi, 2009). Indeed, increased levels of brain derived neurotrophin (BDNF) gene and protein levels are often correlated with adaptive neural and behavioral changes found in populations that received early insult and later life socio-environmental intervention (Roth and Sweatt, 2011). In adults with mood disorders and in adult animal models of emotional decline following early life stressors there is an associated decrease in overall BDNF functioning (for review, see Calabrese et al., 2009). Since BDNF appears to be at the center of stress adaptation at critical moments in development and into adulthood, and in extreme trait or state anxiety animals appear to show uncharacteristic abilities to adapt, changes in BDNF were investigated in a periodic maternal deprivation (PMD) model using dams and their litters. We also focused on the BDNF levels in the basolateral amygdala since this region is implicated in fear memory storage (Schafe et al., 2001) and new synthesis of BDNF parallels emotional memory formation (Schafe and LeDoux, 2000). In the current study, dams were divided along high (HAn) and low anxiety (LAn) lines using the elevated plus maze, and after birth pups experienced PMD across the lactation period. We measured anxiety-like behavior in male offspring at adulthood as well as post-mortem immunoreactivity for the BDNF protein in the basolateral amygdala.
2. Methods
2.1 Animals and housing
Sixteen adult Long Evans females (240–300 g) were purchased timed-pregnant from Charles River Breeding Labs (Wilmington, MA). Prenatal housing for the animals occurred in separate Plexiglas cages with ad libitum access to food and water in a temperature-controlled environment (21±1 °C) with relative humidity (60±10 %). Lights were on from 0700 to 1900 h and dams delivered between gestation days 19–21 with an average of 16 pups per litter. Litter sizes averaged 6.1 male pups for the high anxiety dams and 6.6 male pups for the low anxiety dams. On the day of delivery, dams were monitored at a distance and litters were counted but were not adjusted for overall number, only sex (i.e., all females were removed from the litter). Post-delivery dams were housed together with their male offspring until weaning at PND 21. All procedures were approved by the University of Massachusetts Boston Institutional Animal Care and Use Committee and adhered to both state and federal guidelines for humane care of animal research subjects.
2.2 Elevated Plus Maze
2.2.1 Equipment
The EPM consisted of two opposing open arms (measuring 50 × 10 cm) and two opposing closed arms (measuring 50 × 10 × 40 cm) constructed of black Plexiglas (Med Associates, St. Alban, VT). The center of the four arms was a 10 × 10 cm square that was elevated 70 cm above the floor. All sessions were video-recorded for 5 min using a digital recorder with stand elevated above the testing apparatus and testing occurred during the light phase (between 0900 and 1200 h).
2.2.2 Procedure
Dams were tested on the EPM apparatus after a brief handling and acclimation period for 5 days post arrival to the University of Massachusetts animal vivarium. On a testing day, animals were habituated to the testing environment for 15 min then placed in the center platform and video-recorded for 5 min. We used percent open arm (%OA) time and latency to enter OA (OA LAT) as dependent measures, doing a median split separating low %OA time and high %OA time, taking care to choose animals from the lower quartile (low %OA) for the HAn group and the upper quartile (high %OA) for LAn. Male offspring were tested on the EPM at young adulthood (PND 95) using the same procedures as described above.
2.2.3 Behavioral Assessment
Raters blind to the experimental condition showing a high level of inter-rater reliability (Pearson R = 0.99, p<0.E+0) coded 5-min video recordings of EPM trials for each subject. Raters recorded the times of full body entries into the closed arms (CA) or open arms (OA), beginning with placement into the center platform. From these, we calculated percent time on OA (OA/(OA+CA)*100) and latency (in seconds) to enter OA (OA LAT).
2.3 Periodic Maternal Deprivation
2.3.1 Procedure
Pups were removed from their dams on postnatal days 5, 16, and 21 with each period of maternal deprivation (PMD) lasting 30 min. Reunions of dam and offspring were videotaped for 5 min from the moment of re-introduction.
2.3.2 Periodic maternal deprivation
Maternal behavior was scored using an adapted method of assessment described previously (Caldji, 1998). Three raters (Cohen’s κ = 0.613) coded 5-minute reunion videos for each of the sixteen litters on postnatal days 5, 16 and 21 according to the predominant behavior observed within 10-sec intervals. Thirty 10-sec blocks were coded for each video. We hoped to collect data at postnatal day 10 as well but lost data due to mechanical error. While the quality of the reunion behavior changed between postnatal days 5 and 16, a diminution of reunion behaviors was further observed at postnatal day 21.
The following dam behaviors were scored: no observed behavior/self-cleaning, exploring/rearing behaviors, nesting behaviors, pup retrieval, passive/side nursing, blanket nursing, arched-back nursing (ABN)/licking and grooming (L/G), and licking and grooming (L/G). For analysis purposes, we divided these behaviors into three behavioral categories, no contact, non-pup-directed, and pup-directed behaviors. For the pup-directed behaviors, we scored the number of ABN/L/G and L/G.
2.4 Brain-derived neurotrophin factor (BDNF) immunocytochemistry
At the termination of the behavioral monitoring, 4–5 representative male offspring were overdosed with sodium pentobarbital (1 ml, 50 mg/kg) and transcardially perfused with isotonic saline (50 ml) followed by 4% paraformaldehyde (200–250 ml). Brains were extracted and cryoprotected in sucrose-formaldehyde (10% followed by 20% for 48 h each). Serial cross-sections (30 m) were cut on a cryostat and immunocytochemistry processing was done on free-floating sections. In order to detect BDNF protein in the basolateral amygdala of representative male offspring, we ran free-floating 30 μm reactions using the avidin –biotin system method (Vectastain ABC System, Vector Laboratories, Inc., Burlingame, CA, USA). Sections were rinsed in Tris-buffered saline and then incubated in H2O2 and 10% methanol to block endogenous peroxidase activity followed by 48 h incubation at 4 °C with anti-BDNF (1:1,000) diluted in 0.01 M PBS (pH 7.4), containing 0.5% Triton X-100 and normal goat serum. For the avidin–biotin system, sections were incubated with the appropriate biotinylated secondary antibodies for 1 h (1/250, Vector Labs) followed by the avidin–biotin–peroxidase complex (1:200; Vector) and developed in a solution of 0.015% 3,3-diaminobenzidine, 0.0024% H2O2 in 0.05 M Tris–HCl (pH 7.6). After adhesion on gelatin-coated slides, sections were dehydrated and coverslipped with permount. Digital images (Spot Camera/software) of the basolateral amygdala were captured under light microscopy. Images were later analyzed using ImageJ software by researchers blinded to the experimental conditions of the representative animals. A 250 μm2 reticule was placed over the region of interest from antero-posterior −2.80mm to 3.80mm from Bregma according to the atlas of Paxinos and Watson (1998). All BDNF-positive cells were counted within the reticule for 3 corresponding bilateral sections (1.0mm) for representative animals (n=4) in each experimental group. The cell density was expressed as the number of immuoreactive somata within the 250 μm2 reticule.
2.5 Data analysis
We ran matrix correlation analyses on EPM data for dams, pups, and periodic maternal deprivation data to determine the relatedness of the behavioral and cellular variables. To examine the behavioral responses on the EPM for the dams and pups, we ran separate unpaired Student’s t-tests comparing animals from the lower and upper quartiles. Dam data passed the test for normality while the pup data did not and so Student’s t-test and the Mann-Whitney U tests were used, respectively. Data on tissue volume were tested for normality using a Kolmogorov--Smirnov (KS) test and since results met the criterion of a normal distribution they were compared using a Student’s t-test (two-tailed, unpaired, 95% CI). To determine any differences between dams (LAn v. HAn) on behaviors (e.g., L/G, ABN, pup-directed and non pup-directed behaviors), we ran an MANOVA. For graphing, all data are represented as group means ± SEM. Differences were considered to be statistically significant at p ≤ 0.05.
3. Results
3.1 Elevated Plus Maze
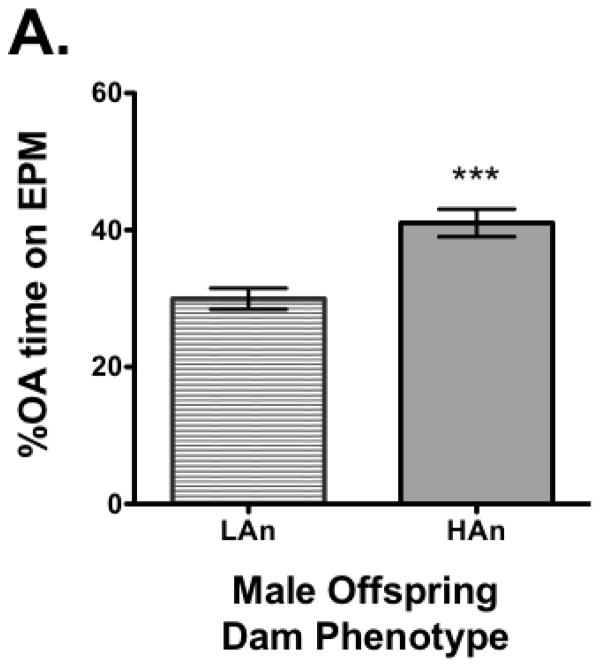
All dams were tested for response on the elevated plus maze measuring %OA time and latency to enter OA (OA LAT). A median split was made for %OA time and all females that were in the lower quartile (lowest %OA time) were designated high anxiety (HAn) while those in the upper quartile (highest %OA time), low anxiety (LAn). Nine of the dams were in the lower quartile (HAn) and seven were in the upper quartile (LAn). Once separated based on this distinction and assured the data were normally distributed (KS test), a Student’s t-test was run. The analysis revealed a significant difference between groups with LAn dams having more %OA time (t=4.841, df=16, p<0.001, two-tailed) and shorter OA LAT (t=5.218, df=16, p<0.0001) (Fig. 1A–B). The EPM data for the pups did not pass the test of normality and thus the Mann Whitney U test was used for analysis. Data indicated there were significant differences between groups, but in the opposite direction, pups from LAn parental lines had less %OA time and longer OA LAT than HAn pups (respectively, p<0.001 and p<0.001, two-tailed) (Fig. 2A–B). The correlation matrix for EPM data for dams and pups also supported these findings with the HAn dams’ %OA time being significantly positively correlated with LAn pups’ %OA time (Pearson R=.782, p<0.05, two-tailed).
Fig. 1.
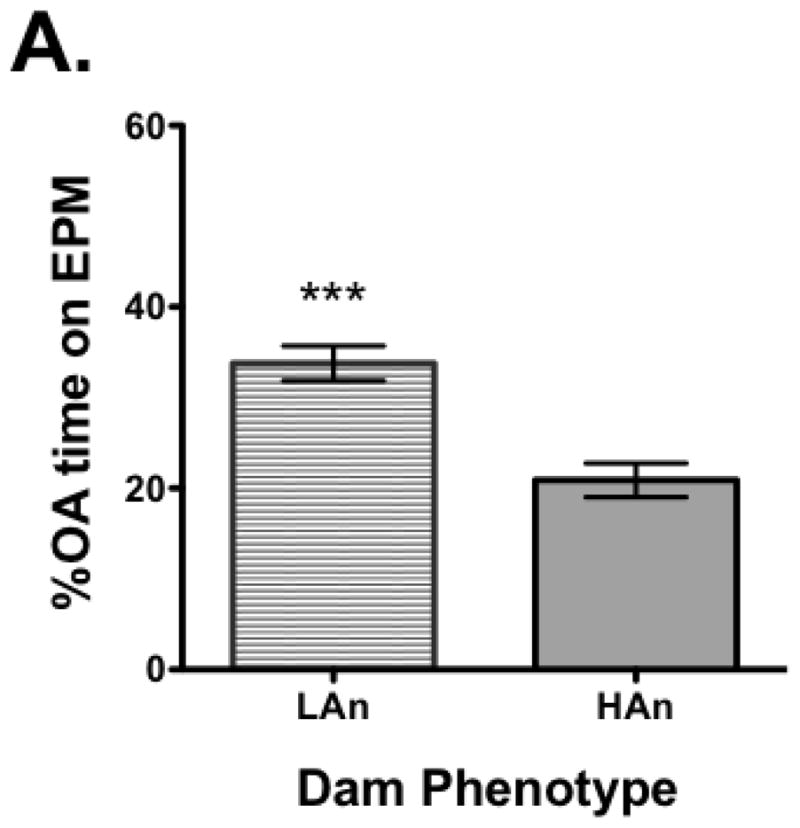
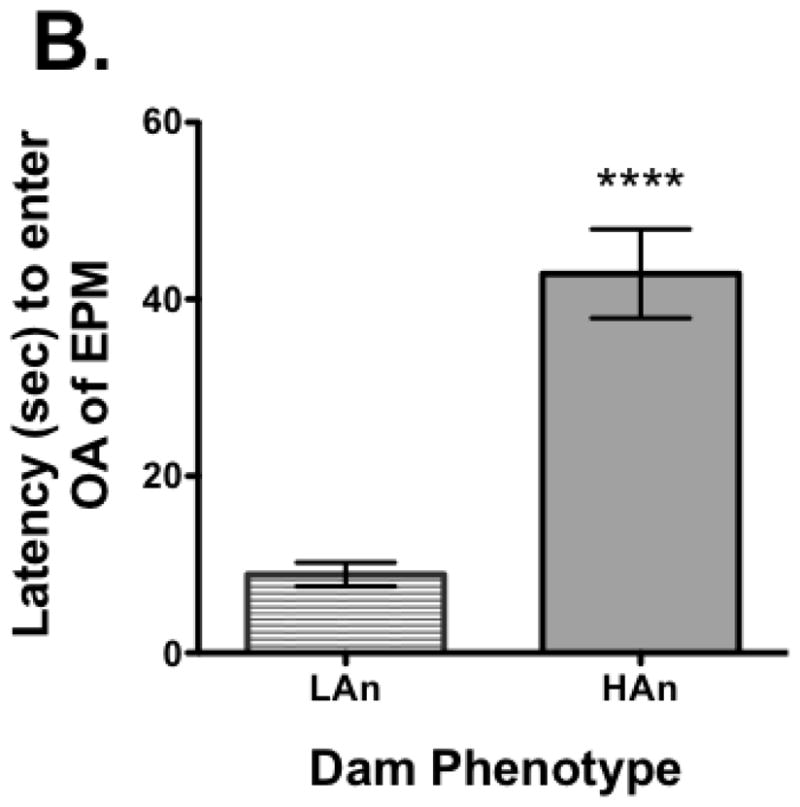
Fig. 1A–B. Anxiety-like behavior on the elevated plus maze (EPM) in dams with those (A) spending more percent time on open arm (OA) designated as low anxiety (LAn, n=9), left bar, and those spending less time on OA designated as high anxiety (HAn, n=7), right bar. (B) LAn dams also had shorter OA latencies, entering the OA of the EPM maze more quickly than HAn dams. Data are presented as group means ± SEM. ***p<0.001, ****p<0.0001.
Fig. 2.
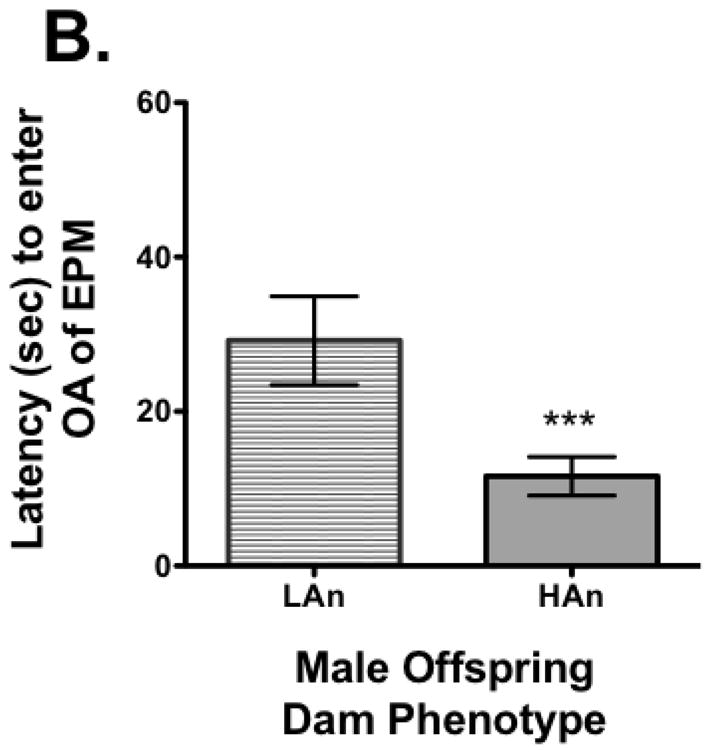
Fig. 2A–B. Adult male offspring showed a reversal in anxiety-like behavior on the EPM from their maternal line after experiencing periodic maternal deprivation. (A) Male offspring of dams showing low anxiety-like behavior spent less percent time (M=29.96±1.55) on the OA while male offspring of dams displaying high anxiety-like behavior spent more percent time (41.05±2.01) on the OA as adults following brief periodic maternal deprivation during pre-weaning. (B) Male offspring from low anxiety dams showed greater percent OA latencies compared to male offspring from high anxiety dams. ***p<0.001.
3.2 Periodic maternal deprivation
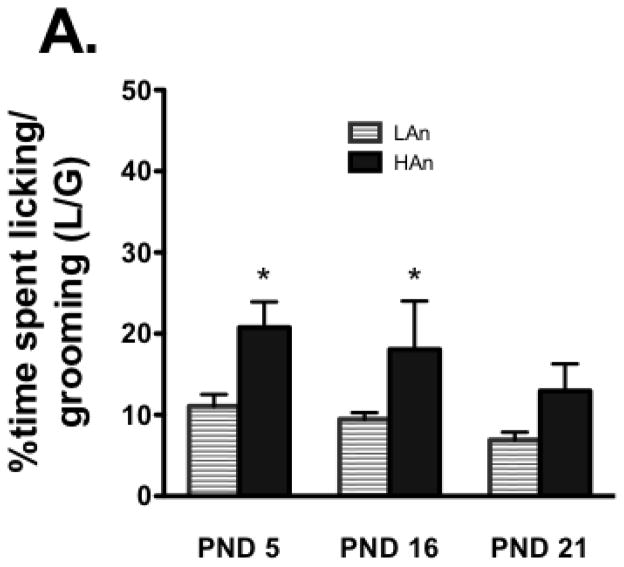
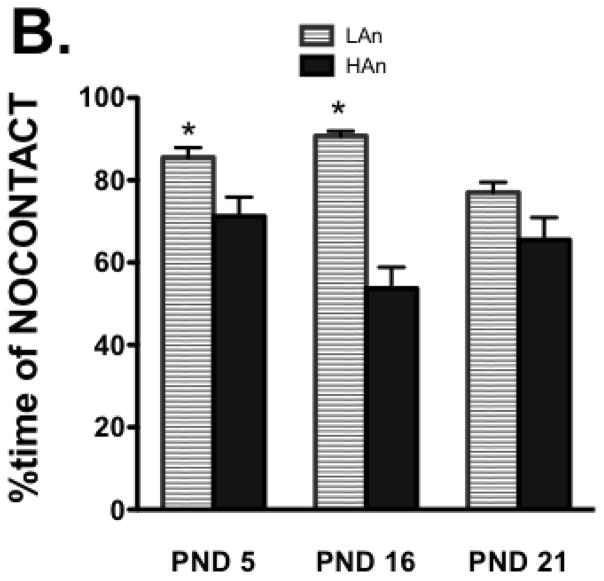
MANOVA on PMD behaviors comparing HAn and LAn animals during the 5-min reunion phase across the lactation period indicated that the percent time of NOCONTACT [F(1,14)=3.063, p<0.05] was greatest in the LAn dams and L/G and L/GABN were greatest in HAn dams [F(1,14)=4.667, p<0.01], particularly on PND 5 and 16 (p<0.05, compared to PND 21) (Fig. 3A–B). Multivariate correlation analyses revealed that in fact, the dam’s closed arm activity (an index of general activity) was significantly negatively correlated with %contact time (%CONTM) on postnatal day (PND) 5 (r=−0.184, p<0.05) and significantly positively correlated with %no contact time (%NCONTM) on PND 5 (r=.294, p<0.001).
Fig. 3.
Fig. 3A–B. Following periodic maternal deprivation, reunion behavior was recorded for a 5-min period. (A) The percent time spent licking/grooming was greater for dams phenotyped as showing high anxiety-like behavior relative to those showing low anxiety-like behavior, and it declined across the 3-week lactation period. *p<0.05, relative to PND 21, same trait. (B) Dams phenotyped as showing low anxiety-like behavior were more likely to have NOCONTACT behavior during the reunion; this, too, declined across the lactation period. #p<0.05, within-group effects, relative to PND 21.
3.3 BDNF-IR in the basolateral amygdala
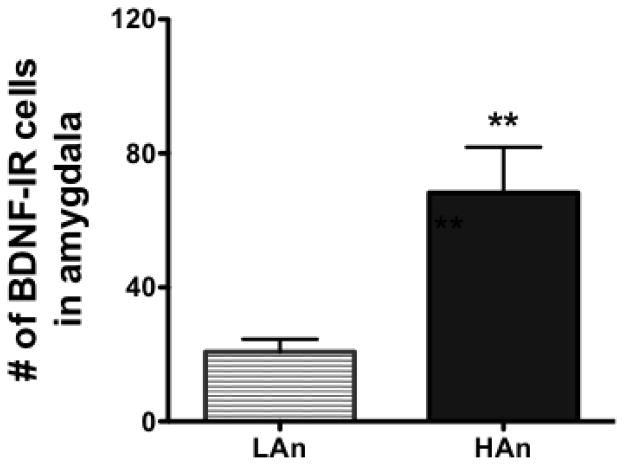
BDNF-IR in the basolateral amygdala of adult male offspring was markedly increased for those from parental HAn lines, with a mean of 68.25 (±13.59) compared to the LAn lines that showed a mean 20.75 (±3.78). Since the data passed the test of normality, a Student’s t-test was run, (t=3.367, df=14, p≤0.01 two-tailed) indicating a significant difference between groups (Fig. 4).
Fig. 4.
Brain-derived neurotrophin (BDNF) immunoreactive somata in the basolateral amygdala of representative male offspring of dams phenotyped as showing high anxiety (HAn)- and low anxiety (LAn)-like behavior. BDNF-IR was significantly increased in the basolateral amygdala of male offspring of HAn dams following brief periodic maternal deprivation compared to males of LAn dams. **p<0.01
4. Discussion
Periodic maternal deprivation had distinct effects on dam-pup interactions in trait anxiety dams, with dams from high anxiety (HAn) lines showing more pup-directed behaviors during the reunion. We found HAn dams with low %OA time and longer OA LAT on the EPM engaged in more pup-directed behaviors following the brief PMD. We examined the behavioral responses of the adult male offspring on the EPM following the early exposure to PMD. The HAn male offspring showed greater %OA time and shorter OA LAT on the EPM and had greater BDNF-IR in the basolateral amygdala. The findings may suggest a protective role of low levels of PMD during the early postnatal period in interacting with stress profiles of mature offspring from HAn lines, and neuroplasticity in the basolateral amygdala parallels this stress adaptability. Although in our current design all pups were submitted to PMD and we did not have HAn and LAn dams that did not experience PMD, we suspect this manipulation modulated the offspring behavioral outcomes. In a separate study, we reported same trait intergenerational transmission of anxiety-like behavior in rats phenotyped as HAn/LAn on the elevated plus maze (Ravenelle et al., 2013).
We found that dams characterized as showing high anxiety-like behavior on the elevated plus maze engaged in much more L/G and greater %CONTM in response to periodic maternal deprivation. These pup-directed behaviors (%CONTM), namely licking and grooming, have been shown to mediate stress in offspring (Champagne and Meaney, 2007). Further, maternal deprivation and handling during the early developmental life of rodents can promote growth (Carrera et al., 2009; Cirulli et al., 2003), neurogenesis (Lemaire et al., 2006) and protect against insult (for review, see Pryce and Feldon, 2003).
Interestingly, that PMD can serve as a mild stressor to mitigate poor maternal care in animals showing high anxiety-like behavior has been demonstrated previously (Neumann et al., 2005). Researchers found that with daily PMD for 3 h during the first two weeks of the lactation period, inbred strains of rats showing high anxiety-like behavior and low anxiety-like behavior were differentially affected by PMD. High anxiety dams engaged in more pup-directed behaviors and the reverse was true for low anxiety dams; moreover, high anxiety adult offspring show a profile more closely resembling low anxiety dams with less anxiety-like behavior and a lower adrenocortical response (Neumann et al., 2005).
In the present design, we used a brief PMD schedule with two intervening dam-pup separations during the first two weeks and one in the culminating week of lactation. Each PMD lasted for only 30 min and we recorded activities during a 5 min reunion period. As adults, the offspring of the dams characterized as HAn that experienced PMD and more L/G showed less anxiety-like behaviors on the EPM, making many more entries into the OA and having shorter OA LAT relative to offspring from LAn dams. Interestingly, pup EPM %OA time was negatively correlated to dam (parental line) %OA time but instead was positively correlated with the pup-directed behaviors during PMD, particularly L/G behaviors. This supports the notion that activities during the life of the neonates can be manipulated to improve the outcomes of the developing offspring, and this can be found even in compromised dams (Carrera et al., 2009). In an activity-based anorexia (ABN) paradigm, Carrera and colleagues (2009) found that an extended periodic maternal deprivation paradigm (180 min, daily from PND 1–20) was able to protect against fatalities in the ABN female rats. Resiliency has also been observed, and more consistently in males following minor early life challenges (Macri, Zorrato and Laviola, 2011; Roth and Sweatt, 2011). In a similar fashion, by introducing a brief maternal deprivation paradigm in the present work, we were able to improve the quality of care by the HAn dams by promoting more L/G during the reunion. Increased L/G, in turn, impacted the developmental life of the offspring and protected them against the HAn phenotype of their parental line. Timing of the intervention is critical given that pre-weaning interventions that fail to promote mother-infant interactions are unsuccessful at impacting neurogenesis (Kohl et al., 2002).
Several mechanisms have been identified in mediating the effects of postnatal stimulation on reversing early developmental stress, including neurogenesis and cell proliferation (Koo et al., 2003), as well as synaptic plasticity and adaptive changes in brain areas implicated in stress and stress adaptation (Ravenelle et al., 2013). Earlier work suggests that postnatal enrichment reverses early insult as a result of adaptive learning at the behavioral and cerebral levels (Ip et al., 2002). Across development, the trophic factors have been implicated in promoting neuronal development, endurance and plasticity, in particular in pups that experienced high levels of L/G and ABN (Meaney, 2001). To determine whether changes in the neurotrophin brain-derived neurotrophic factor (BDNF) would correlate with the adaptive behavioral changes observed in the adult offspring exposed to PMD currently, we measured BDNF-IR in the basolateral amygdala, a region critical for fear/anxiety. In fact, BDNF-IR was more pronounced in offspring from HAn lines and this corresponded to reduced responses to anxiogenic stimuli. Thus, our findings implicate BDNF as a mechanism underlying the reversal in HAn phenotype in offspring exposed to brief PMD. Further work is necessary to determine whether any other instances of neuroplasticity occur and if these adaptations hold across the biological sexes.
Highlights.
Pregnant Long-Evans rats showed distinct anxiety-like behavior in the EPM.
High anxiety in dams was correlated with maternal care after maternal deprivation.
Adult males that received more licking/grooming showed low anxiety-like behavior.
Amygdala brain-derived neurotrophin factor was associated with offspring anxiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branchi I. The mouse communal nest: Investigating the epigenetic influences of early social environment on brain and behavior development. Neurosi Biobehav R. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology. 2009;34S:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera O, Cerrato M, Sanchez A, Gutierrez E. Long maternal separation has protective effects in rats exposed to activity-based anorexia. Dev Psychobiol. 2009;51:616–624. doi: 10.1002/dev.20396. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral responses to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: Effects on brain plasticity and implications for psychopathology. Neurosci Biobehav R. 2003;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff C. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Ip EY, Giza CC, Griesbach GS, Hovda DA. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J Neurotrauma. 2002;19:573–585. doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. J Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Kuhn HG, Cooper-Kuhn CM, Winkler JAL, Kempermann G. Preweaning enrichment has no lasting effects on adult hippocampal neurogenesis in four-month-old mice. Genes Brain Behav. 2002;1:46–54. doi: 10.1046/j.1601-1848.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, et al. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J. 2003;17:1556–1558. doi: 10.1096/fj.02-1032fje. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Macri S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neuroscience Biobehav Rev. 2011;35:1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Neuroscience. 2001;24:1161–192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Krömer S, Frank E, Landgraf R, Bosch OJ. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience. 2005;132(3):867–877. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1998. [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci Biobehav R. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Ravenelle R, Byrnes EM, Byrnes JJ, McInnis C, Park JH, Donaldson ST. Environmental enrichment effects on the neurobehavioral profile of selective outbred trait anxiety rats. Behav Brain Res. 2013;252:49–57. doi: 10.1016/j.bbr.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experiences. Horm Behav. 2011;59:315–320. doi: 10.1016/j.yhbeh.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96, 1–5. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early deprivation in children. Dev Psychiobiol. 2008;50(6):588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]