Abstract
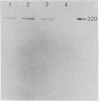
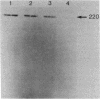
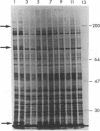
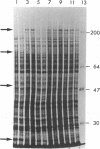
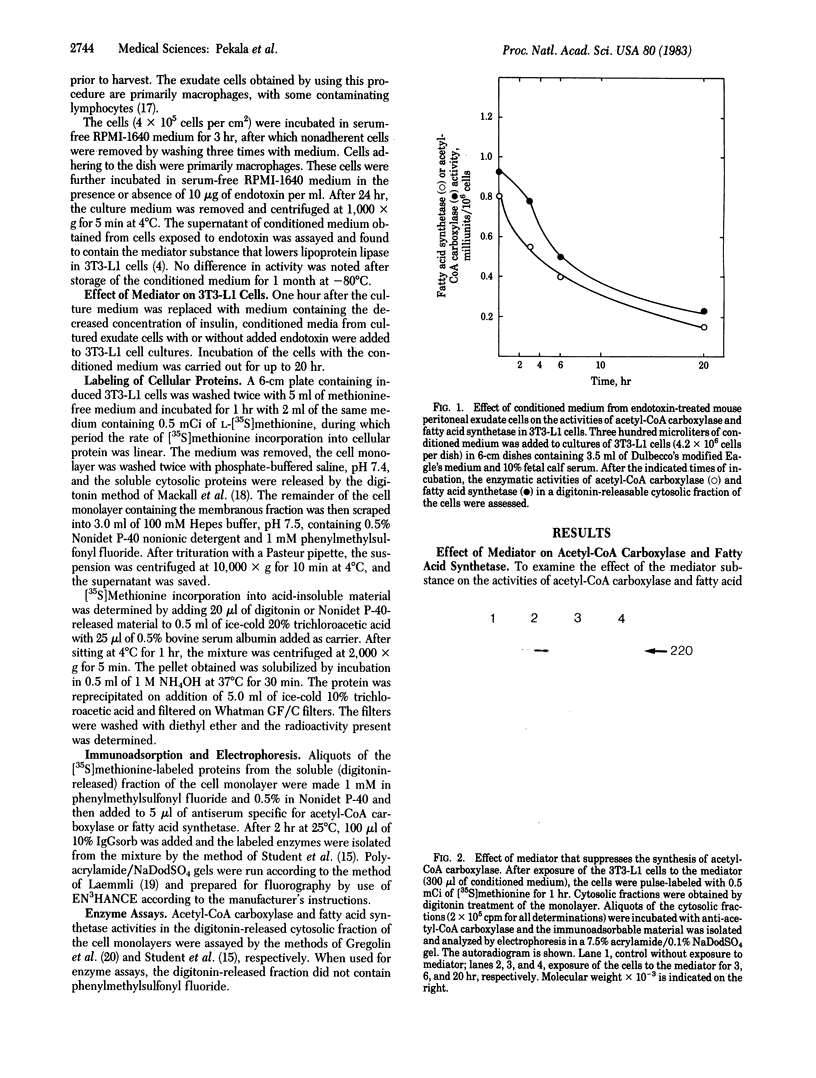
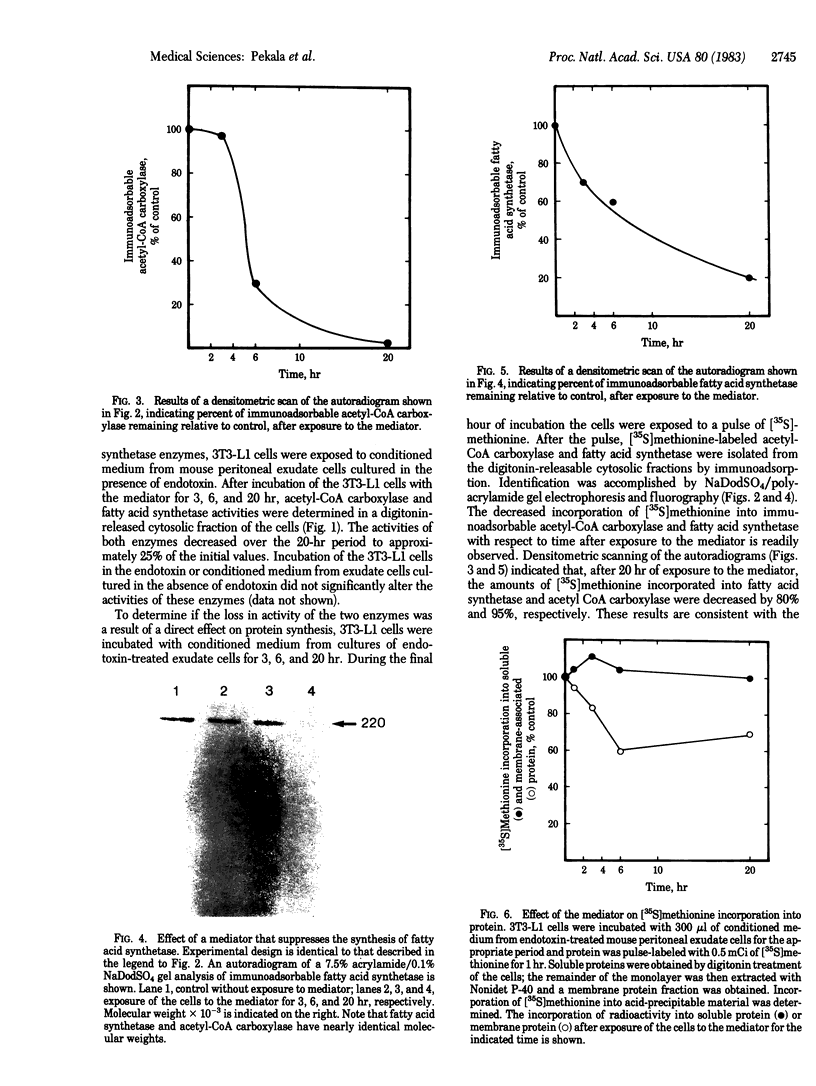
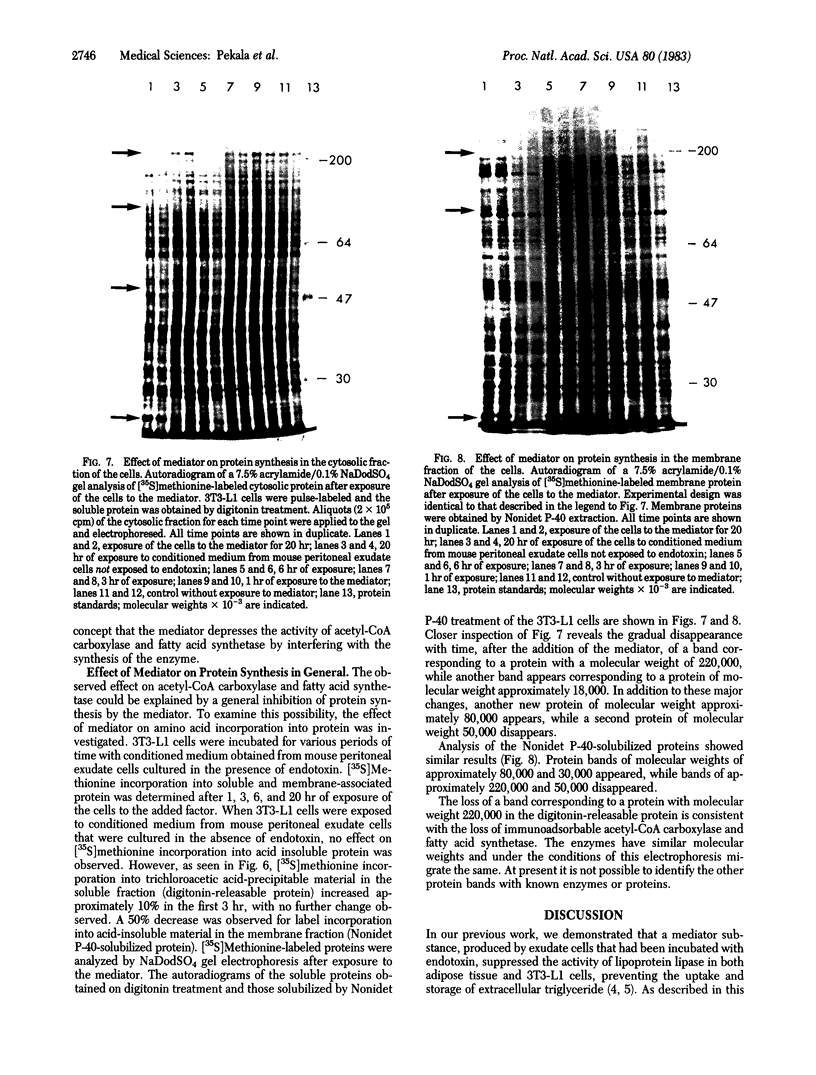
An endotoxin-induced mediator from exudate cells markedly suppresses the activities of the key enzymes for de novo fatty acid biosynthesis--acetyl-CoA carboxylase [acetyl-CoA:carbon dioxide ligase (ADP-forming), EC 6.4.1.2] and fatty acid synthetase--in differentiating 3T3-L1 murine preadipocytes. The loss in activity, at least in part, appears to be due to a specific effect on the synthesis of the enzymes, as determined by a decreased incorporation of [35S]methionine into immunoadsorbable acetyl-CoA carboxylase and fatty acid synthetase when the cells were exposed to the mediator. During this exposure, the radiolabeling of proteins with [35S]methionine in a particulate fraction was decreased by nearly 50% with little change in the soluble protein fraction. Sodium dodecyl sulfate/polyacrylamide gel analysis of the labeled protein indicated no major disturbances of protein synthesis in general; however, the syntheses of specific proteins in both the soluble and particulate fractions were enhanced or depressed. The present study demonstrates that endotoxin promotes the release of a mediator from exudate cells that regulates key anabolic activities in adipose cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977 Sep 9;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Insulin regulation of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1978 Oct 30;84(4):1069–1075. doi: 10.1016/0006-291x(78)91692-3. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Warner R. C., Kleinschmidt A. K., Chang H. C., Lane M. D. Liver acetyl coenzyme A carboxylase. II. Further molecular characterization. J Biol Chem. 1968 Aug 25;243(16):4236–4245. [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Mackall J., Meredith M., Lane M. D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979 May;95(1):270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Cerami A. Hypertriglyceridemia associated with Trypanosoma brucei brucei infection in rabbits: role of defective triglyceride removal. Mol Biochem Parasitol. 1980 Oct;2(1):31–38. doi: 10.1016/0166-6851(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Development of lipoprotein lipase activity and accumulation of triacylglycerol in differentiating 3T3-L1 adipocytes. Effects of prostaglandin F2alpha, 1-methyl-3-isobutylxanthine, prolactin, and insulin. J Biol Chem. 1979 Feb 25;254(4):1305–1311. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. Separation and dependence of hormonal effects on hexose metabolism and synthesis of RNA and protein. J Biol Chem. 1979 Oct 25;254(20):10021–10029. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]