Abstract
Objective
We previously examined the expression of specific C-terminal μ-opioid receptor (MOR) splice variants in human central nervous system cell types and HIV-infected brain tissue from subjects with neurocognitive impairment ± HIV encephalitis (HIVE). In the present study, we examined the N-terminal splice variant MOR-1K which mediates excitatory cellular signaling.
Methods and Results
We found segregation of expression ranging from undetectable to seemingly exclusive across nervous system cell types compared to the pool of C-terminal MOR splice variants using RT-PCR. Expression of MOR-1K mRNA was also increased in HIV-infected subjects with combined neurocognitive impairment and HIVE compared to the other groups. MOR-1K expression correlated with the level of subject neurocognitive impairment whereas the pool of C-terminal MOR splice variants did not. HIVE was also associated with increased expression of the inflammatory mediators MCP-1, MCP-2, and RANTES, but not the host HIV co-receptors CXCR4 and CCR5 or the CD4 receptor, using qRT-PCR. Network analysis of microarray data from these same subjects revealed filamin A (FLNA) as a possible interaction partner with MOR-1K, and FLNA gene expression was also found to be upregulated in HIVE using qRT-PCR. Overexpression of filamin A in HEK293 cells redistributed MOR-1K from intracellular compartments to the cell surface.
Conclusion
These results suggest that HIVE, and neurocognitive impairment depending on its severity, are associated with enhanced MOR-1K signaling through both increased expression and trafficking to the cell surface, which may alter the contribution of MOR receptor isoforms and exacerbate the effects of MOR activation in neuroAIDS.
Keywords: μ opioid receptor, splice variant, HIV, gene regulation, neural cell type, neurocognition, HIV encephalitis
Introduction
The μ-opioid receptor (MOR) is comprised of numerous alternatively spliced variants rather than a single protein entity, and most of the variation has been found to occur in the C-terminus of the receptor (for a review see [1]). One recently discovered, novel MOR splice variant termed MOR-1K is preferentially found in the brain, expressed intracellularly, and differs functionally from the C-terminal canonical MOR-1 variant [2, 3]. Unlike other MOR splice variants, which encode seven-transmembrane (7TM) spanning domains, the six-transmembrane (6TM) MOR-1K variant couples to Gαs and stimulates increases in both intracellular Ca2+ and nitric oxide (NO) release [2, 3]. Accordingly, MOR-1K may uniquely activate neuroinflammation associated with opioid-dependent hyperalgesia, tolerance, and dependence [3].
Previous studies have suggested a role for MOR in HIV replication and progression of the HIV disease leading to neurocognitive impairment, particularly in the context of opioid co-exposure [4–12]. However, most studies have focused on the canonical MOR-1 without examining the roles of other MOR splice variants (for a review see [13]). Our previous association of particular C-terminal MOR splice variants with different stages of the HIV disease process suggests a dynamic interplay between signaling pathways invoked by HIV exposure and those regulating opioid receptor levels and processing [14]. To further investigate the role of MOR variation in relation to neuroAIDS, we examined the distribution of MOR-1K among human neural cell types and asked whether MOR-1K might also play a role in HIV neuropathogenesis. By virtue of the excitatory cellular effects attributed to this variant, we questioned whether MOR-1K might underlie key aspects of opiate-dependent exacerbation of HIV neuropathogenesis [14–17]. Exaggerated MOR-1K function has been linked to aberrant opiate actions [2, 3], and may contribute to accelerated neuropathology seen with chronic opiate abuse in uninfected [18, 19] and HIV-infected individuals [17, 20]. Therefore, the purpose of this study was to identify the cells in which MOR-1K is expressed among selected neural cell types and determine whether the expression of MOR-1K is altered in HIV-infected subjects with varying levels of neurocognitive impairment which may point to a potential cell-type specific role for this unique variant in the pathogenesis of neuroAIDS.
Methods
Cells and tissue
Astrocytes, microglia, and neurons were described and characterized previously [14]. Brain vascular pericytes (catalog number 1200) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured according to the manufacturer’s instructions. Total RNA from primary human brain microvascular endothelial (catalog number 1005) and perineurial (catalog number 1715) cells was also purchased from ScienCell. Human brain tissue was obtained from the National NeuroAIDS Tissue Consortium (NNTC) Gene Array Project (http://www.nntc.org/gene-array-project) [21, 22]. The samples used in this study taken from frontal lobe white matter, frontal cortex, and basal ganglia of uninfected and HIV-infected subjects ± neurocognitive impairment with and without HIV encephalitis (HIVE) have been described in detail previously [14].
RT-PCR
RT-PCR was performed essentially as described previously [14]. For detailed methods, see Supplemental Digital Content 1.
Immunocytochemistry
Brain vascular pericytes in culture were fixed using 4 % paraformaldehyde, permeabilized with 0.5 % Triton X-100, immunolabeled, and nuclei were stained with DAPI. Primary antibody to actin alpha 2 smooth muscle (Novus Biologicals, LLC; Littleton, CO, USA; catalog number NBP1-97722) was used at a 1:25 dilution and visualized with Alexa Fluor 594 conjugated secondary antibody (Molecular Probes; Eugene, OR, USA; catalog number A-11032) used at a 1:500 dilution. Samples were imaged with a Zeiss LSM 700 laser scanning confocal microscope at 63× magnification. Images were collected using ZEN 2009 Light Edition software (Carl Zeiss, Inc.; Thornwood, NY, USA) and edited with Adobe Photoshop CS3 Extended 10.0 software (Adobe Systems, Inc.; San Jose, CA, USA). HEK293 cells (ATCC; Manassas, VA, USA) were transfected with plasmids encoding FLAG-tagged MOR-1K [3] and/or Myc-tagged filamin A (pcDNA3-Myc-FLNA WT, Addgene plasmid 8982 [23]) using Lipofectamine 2000 reagent (Invitrogen; Carlsbad, CA, USA). Forty-eight hours post-transfection, cells were fixed in −20°C methanol, permeabilized with 0.25 % Triton X-100, immunolabeled, and nuclei were stained with Hoechst 33342. Primary antibodies were anti-FLAG and anti-Myc epitope tag (Cell Signaling Technology, Inc.; Danvers, MA, USA) used at a 1:200 dilution and anti-Filamin 1 (Santa Cruz Biotechnology, Inc.; Dallas, TX, USA; catalog number sc-17749) used at a 1:100 dilution. Primary antibodies were visualized with appropriate secondary antibodies conjugated to Alexa Fluor 488 or 594 (Molecular Probes; Eugene, OR, USA). Samples were imaged using a Zeiss LSM 710 confocal microscope and images were collected and analyzed with ZEN software.
Microarray data analysis
CEL files for arrays were retrieved from the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), GEO accession number GSE35864 [21]. Probe intensity data from chips hybridized with samples from each brain region was normalized and summarized separately using robust multi-array average (RMA) analysis [24]. Probesets that were consistently absent (based on MAS 5.0 detection calls) across all arrays within a brain region were excluded from the subsequent analysis of that subset of arrays. Differential expression between subject groups was assessed using multi-class linear models for microarray data (Limma) analysis [25], and Limma output p-values were adjusted by Benjamini and Hochberg’s false discovery rate correction for multiple testing [26], setting the significance level to 0.05. Overlap in genes that were significantly different between the HIV-negative versus HIVE groups in the frontal lobe white matter, frontal cortex, and basal ganglia was assessed using Gene Weaver (http://geneweaver.org/) [27]. Additional bioinformatics analysis was performed using the ToppNet function of ToppGene Suite (http://toppgene.cchmc.org/) [28]. OPRM1 was used as the training set and overlapping genes from the above analysis were used as the test set with a K-step Markov prioritization method, step size 6 and neighborhood distance of 1.
Statistics
All statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc.; La Jolla, CA, USA). qRT-PCR data was analyzed by either one-way ANOVA and Bartlett’s test for equal variances followed by Student Neuman-Keuls post-hoc test for multiple comparisons or Student’s unpaired, two-tailed t test for paired data. A value of p < 0.05 was considered significant.
Results
MOR-1K is differentially expressed across nervous system cell types
We examined the expression profile of MOR-1K compared to the pool of C-terminal MOR splice variants (denoted MOR-1(exons 1–2)) in various nervous system cell types by RT-PCR using primer sets targeting specific exons (Fig. 1a). As the protein sequence differs from the canonical MOR-1 only in that the first 100 amino acids of the N-terminal first transmembrane domain are deleted (Fig. 1b), it is not possible to design an antibody that will specifically recognize MOR-1K. Expression profiling of MOR-1K in astrocytes, microglia, and neurons revealed detection in astrocytes, but not microglia and neurons, while MOR-1(exons 1–2) was detected in all three cell types (Fig. 1c). The expression level of MOR-1K in astrocytes was much lower compared to MOR-1(exons 1–2) (Fig. 1d).
Fig. 1. MOR-1K expression in various human nervous system cell types.
(a) Schematic representation of the 4 exons (E) comprising the MOR-1 and MOR-1K mRNA products of the OPRM1 gene. Arrows denote the locations of PCR primers used in which a common reverse primer targeted exon 2 and the forward primers targeted either exon 1 of MOR-1 or the MOR-1K specific exon 13. (b) Schematic representation of the MOR-1 and MOR-1K (100 amino acids (aa) deleted) proteins with the C-terminal sequence within exon 4 shown. (c) MOR-1 (denoted MOR-1(exons 1–2)) and MOR-1K PCR products amplified from the indicated cell types. PCR products were detected using 2 % agarose gels stained with ethidium bromide. Marker indicates 100 base pair (bp) DNA ladder marker where the 100 and 200 bp markers are shown. GAPDH served as a loading control. (d) Quantification of results from astrocytes in c. Error bars show the SEM. (e) PCR products amplified from brain microvascular endothelial cells (BMEC) and peripheral nervous system perineurial cells (PNC). (f) Quantification of results for MOR-1K from the cell types in e compared to astrocytes. (g) PCR products amplified from brain vascular pericytes (BVP). The human neuroblastoma cell line SHSY-5Y (which is heavily enriched in MORs) served as a positive control. (h) Characterization of pericytes from g by immunocytochemistry using actin alpha 2 smooth muscle as a cell type marker and DAPI to label cell nuclei. A differential interference contrast microscopy image is also shown. All experiments and error bars are representative of at least 3 individual lots of primary cells from different individuals.
We additionally examined MOR expression in brain microvascular endothelial cells and brain vascular pericytes, as well as perineurial cells from the peripheral nervous system which provide a selective barrier function likened to the blood-brain barrier within larger peripheral nerves [29– 31]. These three cell types were assessed based on reports that opiate drugs alone or in the context of HIV infection can directly affect blood-brain barrier permeability [32, 33], and to begin to explore the role of MOR-1K in known differences with the central versus peripheral actions of opiate analgesics on the nervous system [30, 31]. Interestingly, when we examined brain microvascular endothelial and perineurial cells for MOR expression, we were only able to detect MOR-1K but not MOR-1(exons 1–2) (Fig. 1e), suggesting exclusive expression of this particular MOR variant in these cell types. However, the detection level of MOR-1K was lower in these two cell types than in astrocytes suggesting a lower overall level of expression (Fig. 1f). We were unable to detect MOR-1(exons 1–2) and MOR-1K expression in cultured brain vascular pericytes (Fig. 1g,h). These results suggest that MOR-1K is differentially expressed across nervous system cell types, similar to previous findings of C-terminal MOR variant segregation across cell types from the central nervous system [14].
MOR-1K expression is increased in human subjects with HIVE
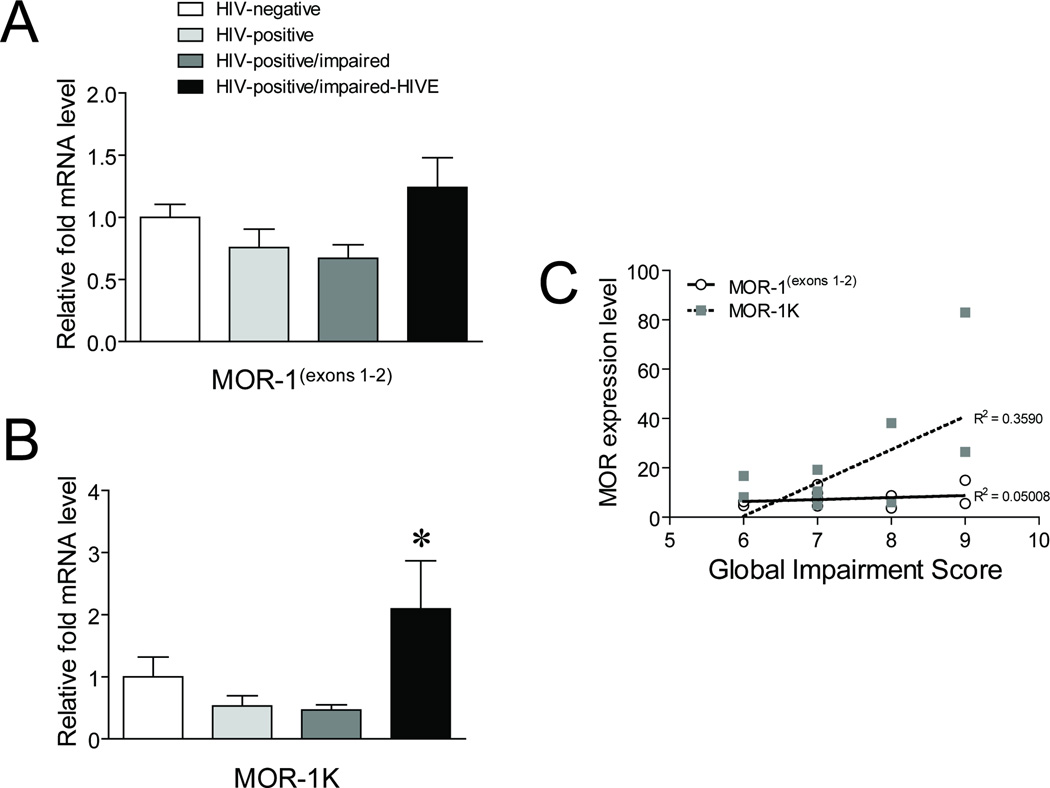
As increases in MOR expression may be a contributing factor to HIV disease pathology [14, 34–36], we next examined whether the expression level of MOR-1K differed across HIV-infected subjects with varying levels of neurocognitive impairment using qRT-PCR. While MOR-1(exons 1–2) did not show any overall significant differences, MOR1-K showed differences between HIVE subjects and the other HIV-infected groups, as well as uninfected subjects when untransformed data was analyzed (Fig. 2a,b). However, the analysis revealed that the group variances were non-homogeneous (p < 0.05; Bartlett’s test), which was anticipated considering the relative low abundance of MOR-1K transcripts, as well as challenges in obtaining consistent human samples from the same brain region, differing postmortem intervals, and RNA quality. Accordingly, the data was log transformed. We also found a non-significant trend of progressively decreasing expression with HIV-infected and HIV-infected/impaired subjects compared those that were uninfected for both MOR-1(exons 1–2) and MOR-1K. In addition, when the level of neurocognitive impairment for each HIV-infected subject with deficiencies and those with HIVE was compared to MOR expression levels, MOR-1K showed a significant correlation whereas MOR-1(exons 1–2) did not (Fig. 2c). These results suggest that the MOR-1K variant is specifically upregulated in HIVE and that the level of its expression is associated with the severity of neurocognitive deficits found in HIV patients.
Fig. 2. Expression of MOR-1K across HIV-infected subjects with varying levels of neurocognitive impairment.
Expression levels of (a) MOR-1(exons 1–2) and (b) MOR-1K were measured by qRT-PCR across the indicated groups of subjects. Error bars show the SEM. F(3,24) = 2.965, p = 0.0522 for MOR-1(exons 1–2) and F(3,23) = 4.600, p = 0.0116 (untransformed data); F(3,23) = 4.137, p = 0.0175 (log transformed data) for MOR-1K. *p < 0.05 vs. all other groups (untransformed data); *p < 0.05 vs. other HIV-infected groups (log transformed data). (c) Linear regression line modeling the correlation between expression levels of MOR-1(exons 1–2) and MOR-1K with Global Impairment Scores for individual subjects in the HIV-infected and HIVE groups with neurocognitive impairment. Pearson correlation coefficient (r) = 0.2238, p = 0.4844 for MOR-1(exons 1–2) and r = 0.5992, p = 0.0395 for MOR-1K. For the impairment score and neurocognitive diagnosis, a description of each subject included in the NNTC Gene Array Project is summarized (see Table, Supplemental Digital Content 3).
HIVE is associated with increases in mRNAs for inflammatory chemokines but not host HIV entry factors
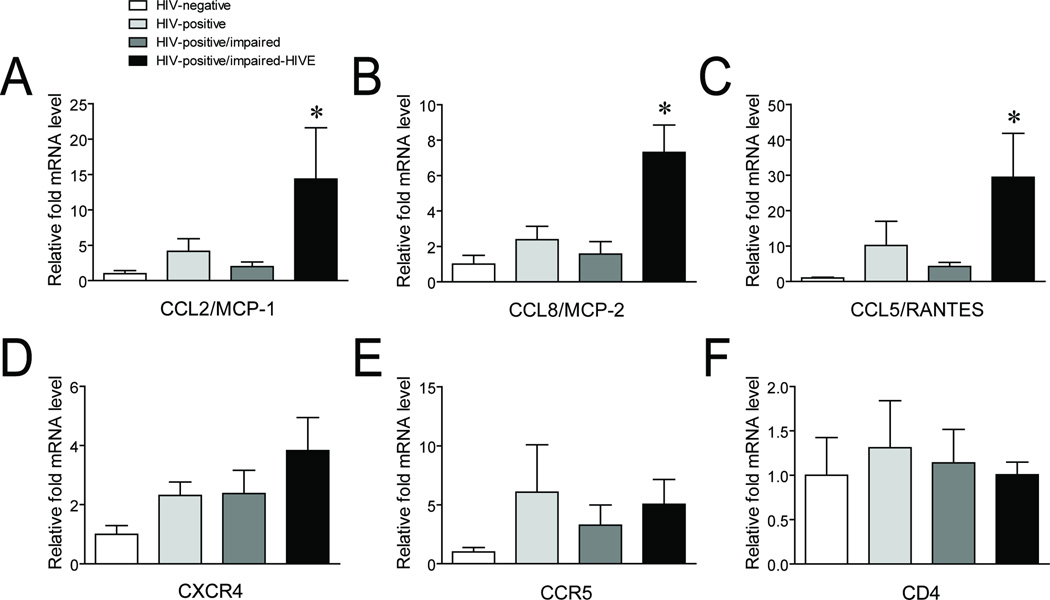
To confirm that the HIVE subjects we examined had increases in inflammation, we examined the expression levels of MCP-1, MCP-2, and RANTES using qRT-PCR. All three inflammatory mediators were significantly increased between HIVE and all the other groups of subjects examined (Fig. 3a – c). We also examined the mRNA expression levels of the HIV co-receptors CXCR4 and CCR5, as well as the CD4 receptor, since host proteins that interact with HIV have been shown to form functional complexes with opioid receptors, including MOR, to affect cellular signaling and possibly processes such as viral entry [37–43]. We did not detect any overall significant differences for these co-receptors and receptor, although there was a trend of increased CXCR4 expression across the groups of HIV-infected compared to uninfected subjects (Fig. 3d – f).
Fig. 3. Expression of mRNAs for inflammatory mediators and host HIV entry proteins in HIV-infected subjects.
Expression levels of the inflammatory mediators (a) CCL2/MCP-1, (b) CCL8/MCP-2, and (c) CCL5/RANTES and the host HIV entry proteins (d) CXCR4, (e) CCR5, and (f) CD4 were measured by qRT-PCR across the indicated groups of subjects. Error bars show the SEM. F(3,24) = 4.067, p = 0.0181 for CCL2/MCP-1; F(3,24) = 8.872, p = 0.0004 for CCL8/MCP-2; F(3,24) = 4.186, p = 0.0162 for CCL5/RANTES; F(3,24) = 1.889, p = 0.1585 for CXCR4; F(3,24) = 0.7240, p = 0.5476 for CCR5; and F(3,24) = 0.1067, p = 0.9553 for CD4. *p < 0.05 vs. all other groups.
Filamin A is an interaction target for MOR-1K in HIVE
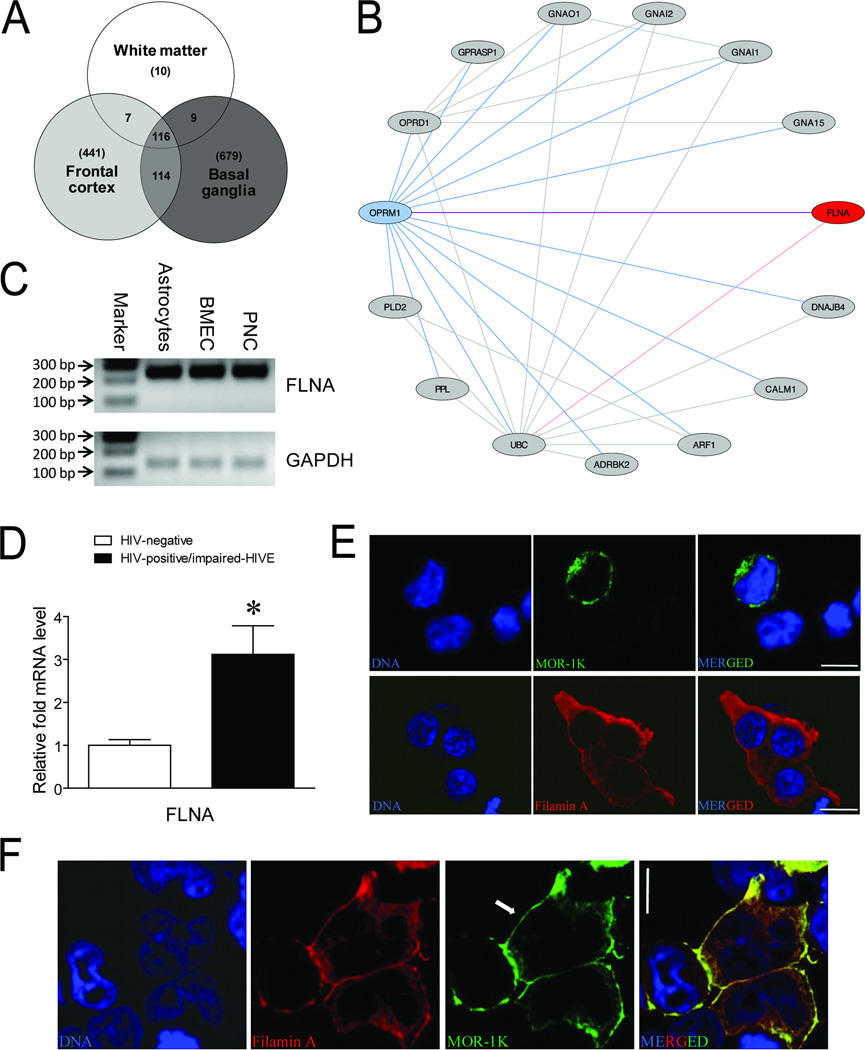
During the course of this study, microarray data was recently published on the same subjects that we examined [21]. The deposited data files were retrieved for reanalysis to determine the most likely candidate to interact with MOR in the context of HIV infection. Our analysis confirmed the previous finding that most of the differences in gene expression occurred between the uninfected and HIVE subjects [21], versus the other group pair-wise comparisons (see Figure, Supplemental Digital Content 2). For the three brain regions that were examined from uninfected and HIVE subjects, we found a total of 116 genes that overlapped between different sets of brain regions which were significantly different between these two groups (Fig. 4a). Most of the overlapping genes (114) occurred between the frontal cortex and basal ganglia which also included the genes (7) overlapped between the frontal lobe white matter and frontal cortex, with an additional two genes (GPNMB and ISG15) overlapped between the frontal lobe white matter and basal ganglia that did not occur between other regions. We then performed a proteinprotein interaction network analysis to determine which of the protein products from these 116 genes might interact with the products of the OPRM1 gene in HIVE. This analysis scored and ranked the 116 overlapping candidate genes based on topological features of global networkdistance measures on the relative location to OPRM1 (MOR) in a protein-protein interaction network generated using existing datasets from assays such as high-throughput yeast two-hybrid screens [28, 44, 45]. The resulting network revealed the product of the FLNA gene, filamin A, as a likely protein-protein interaction partner with MOR (Fig. 4b; Table 1). We then confirmed that FLNA mRNA was expressed in the cell types that we identified as also expressing MOR-1K mRNA (Fig. 4c), suggesting the possibility that filamin A could interact with MOR-1K in these cell types in vivo. Additionally, we found that FLNA expression levels were increased between uninfected and HIVE subjects using qRT-PCR (Fig. 4d).
Fig. 4. Filamin A expression in HIVE and alterations to MOR-1K subcellular trafficking.
(a) Venn diagram of significantly regulated overlapping genes between the indicated brain regions from uninfected and HIVE subjects examined in a microarray study. (b) Network analysis of protein-protein interactions between the products of the 116 overlapping genes from a with the products of the OPRM1 gene. (c) FLNA PCR products amplified from astrocytes, brain microvascular endothelial cells (BMEC), and perineurial cells (PNC). PCR products were detected using 2 % agarose gels stained with ethidium bromide. Marker indicates 100 base pair (bp) DNA ladder marker where the 100, 200, and 300 bp markers are shown. GAPDH served as a loading control. Bands are representative of 3 individual lots of primary cells from different individuals. (d) Expression levels of the filamin A gene, FLNA, were measured by qRT-PCR for the indicated groups of subjects. Error bars show the SEM. p = 0.0075. *p < 0.05 vs. uninfected group. (e) Representative images of HEK293 cells transiently transfected with plasmids encoding either epitope FLAG-tagged MOR-1K or Myc-tagged filamin A and immunolabeled with either anti-FLAG (green) or anti-Myc (red) antibodies. (f) HEK293 cells co-transfected with plasmids encoding filamin A and MOR-1K co-immunolabeled with anti-filamin A (red) and anti-FLAG (green) antibodies. Cells were counterstained for nuclear DNA (blue). Arrow denotes MOR-1K localization at the plasma membrane. Scale bars = 10 µm.
Table 1.
Protein-protein interaction ranks of the 116 genes regulated in HIVE with OPRM1.
| Rank | Gene ID | Gene Name | Interact Count | Score | Rank | Gene ID | Gene Name | Interact Count | Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2316 | FLNA | 82 | 1.25E-02 | 59 | 3913 | LAMB2 | 3 | 1.41E-05 |
| 2 | 2534 | FYN | 176 | 1.09E-03 | 60 | 51304 | ZDHHC3 | 5 | 1.37E-05 |
| 3 | 839 | CASP6 | 37 | 1.00E-03 | 61 | 64135 | IFIH1 | 5 | 1.28E-05 |
| 4 | 6352 | CCL5 | 17 | 4.80E-04 | 62 | 5696 | PSMB8 | 4 | 1.26E-05 |
| 5 | 6772 | STAT1 | 99 | 4.50E-04 | 63 | 56829 | ZC3HAV1 | 6 | 1.25E-05 |
| 6 | 7082 | TJP1 | 57 | 4.04E-04 | 64 | 54625 | PARP14 | 6 | 1.12E-05 |
| 7 | 3693 | ITGB5 | 27 | 2.44E-04 | 65 | 10365 | KLF2 | 7 | 1.11E-05 |
| 8 | 3842 | TNPO1 | 22 | 2.23E-04 | 66 | 124540 | MSI2 | 3 | 1.03E-05 |
| 9 | 6929 | TCF3 | 67 | 2.22E-04 | 67 | 23102 | TBC1D2B | 1 | 9.92E-06 |
| 10 | 596 | BCL2 | 92 | 2.16E-04 | 68 | 26524 | LATS2 | 5 | 9.73E-06 |
| 11 | 5978 | REST | 24 | 1.90E-04 | 69 | 5698 | PSMB9 | 5 | 9.66E-06 |
| 12 | 6453 | ITSN1 | 40 | 1.43E-04 | 70 | 84441 | MAML2 | 7 | 9.16E-06 |
| 13 | 19 | ABCA1 | 43 | 1.37E-04 | 71 | 8994 | LIMD1 | 4 | 8.98E-06 |
| 14 | 23513 | SCRIB | 13 | 1.11E-04 | 72 | 23541 | SEC14L2 | 2 | 8.89E-06 |
| 15 | 658 | BMPR1B | 64 | 1.09E-04 | 73 | 4038 | LRP4 | 3 | 8.58E-06 |
| 16 | 4651 | MYO10 | 13 | 1.09E-04 | 74 | 23389 | MED13L | 8 | 8.28E-06 |
| 17 | 8837 | CFLAR | 33 | 1.05E-04 | 75 | 94241 | TP53INP1 | 6 | 8.06E-06 |
| 18 | 800 | CALD1 | 9 | 8.84E-05 | 76 | 4781 | NFIB | 5 | 7.80E-06 |
| 19 | 3707 | ITPKB | 4 | 8.30E-05 | 77 | 5376 | PMP22 | 3 | 6.84E-06 |
| 20 | 6890 | TAP1 | 14 | 7.55E-05 | 78 | 81844 | TRIM56 | 3 | 6.51E-06 |
| 21 | 1523 | CUX1 | 23 | 7.24E-05 | 79 | 64761 | PARP12 | 2 | 6.19E-06 |
| 22 | 85439 | STON2 | 7 | 7.15E-05 | 80 | 8879 | SGPL1 | 2 | 5.85E-06 |
| 23 | 55707 | NECAP2 | 8 | 6.84E-05 | 81 | 26020 | LRP10 | 2 | 5.61E-06 |
| 24 | 23499 | MACF1 | 25 | 6.33E-05 | 82 | 83666 | PARP9 | 2 | 5.36E-06 |
| 25 | 10163 | WASF2 | 24 | 5.76E-05 | 83 | 54739 | XAF1 | 5 | 5.12E-06 |
| 26 | 3831 | KLC1 | 20 | 5.10E-05 | 84 | 375743 | PTAR1 | 1 | 4.64E-06 |
| 27 | 5725 | PTBP1 | 25 | 5.08E-05 | 85 | 10384 | BTN3A3 | 1 | 4.64E-06 |
| 28 | 23586 | DDX58 | 18 | 4.89E-05 | 86 | 9830 | TRIM14 | 1 | 4.64E-06 |
| 29 | 567 | B2M | 21 | 4.57E-05 | 87 | 11118 | BTN3A2 | 1 | 4.64E-06 |
| 30 | 10735 | STAG2 | 12 | 4.50E-05 | 88 | 11119 | BTN3A1 | 1 | 4.64E-06 |
| 31 | 3665 | IRF7 | 29 | 4.44E-05 | 89 | 57674 | RNF213 | 1 | 4.64E-06 |
| 32 | 3895 | KTN1 | 13 | 4.26E-05 | 90 | 54809 | SAMD9 | 1 | 4.64E-06 |
| 33 | 55701 | ARHGEF40 | 5 | 4.23E-05 | 91 | 10106 | CTDSP2 | 3 | 4.08E-06 |
| 34 | 1837 | DTNA | 15 | 4.14E-05 | 92 | 83439 | TCF7L1 | 2 | 3.97E-06 |
| 35 | 84823 | LMNB2 | 8 | 4.10E-05 | 93 | 26137 | ZBTB20 | 3 | 3.86E-06 |
| 36 | 10616 | RBCK1 | 23 | 3.95E-05 | 94 | 56987 | BBX | 2 | 3.07E-06 |
| 37 | 4299 | AFF1 | 23 | 3.86E-05 | 95 | 121512 | FGD4 | 1 | 3.05E-06 |
| 38 | 3959 | LGALS3BP | 15 | 3.73E-05 | 96 | 116071 | BATF2 | 2 | 2.81E-06 |
| 39 | 9636 | ISG15 | 17 | 3.60E-05 | 97 | 23034 | SAMD4A | 1 | 2.52E-06 |
| 40 | 2120 | ETV6 | 20 | 3.57E-05 | 98 | 6932 | TCF7 | 2 | 2.51E-06 |
| 41 | 79443 | FYCO1 | 19 | 3.43E-05 | 99 | 10457 | GPNMB | 1 | 1.73E-06 |
| 42 | 6672 | SP100 | 20 | 3.39E-05 | 100 | 4774 | NFIA | 1 | 1.21E-06 |
| 43 | 3915 | LAMC1 | 10 | 3.37E-05 | 101 | 23305 | ACSL6 | 1 | 1.15E-06 |
| 44 | 58508 | MLL3 | 18 | 3.36E-05 | 102 | 7227 | TRPS1 | 1 | 9.11E-07 |
| 45 | 4026 | LPP | 8 | 3.05E-05 | 103 | 3433 | IFIT2 | 1 | 3.19E-07 |
| 46 | 6907 | TBL1X | 24 | 2.93E-05 | 104 | 83982 | IFI27L2 | 0 | −1.00E+00 |
| 47 | 25937 | WWTR1 | 10 | 2.79E-05 | 105 | 64067 | NPAS3 | 0 | −1.00E+00 |
| 48 | 51191 | HERC5 | 10 | 2.21E-05 | 106 | 10964 | IFI44L | 0 | −1.00E+00 |
| 49 | 120 | ADD3 | 2 | 2.12E-05 | 107 | 92999 | ZBTB47 | 0 | −1.00E+00 |
| 50 | 2005 | ELK4 | 11 | 2.04E-05 | 108 | 22802 | CLCA4 | 0 | −1.00E+00 |
| 51 | 7003 | TEAD1 | 14 | 2.03E-05 | 109 | 4938 | OAS1 | 0 | −1.00E+00 |
| 52 | 151636 | DTX3L | 17 | 1.98E-05 | 110 | 26084 | ARHGEF26 | 0 | −1.00E+00 |
| 53 | 4756 | NEO1 | 6 | 1.77E-05 | 111 | 219285 | SAMD9L | 0 | −1.00E+00 |
| 54 | 64375 | IKZF4 | 14 | 1.71E-05 | 112 | 84663 | CYorf15B | 0 | −1.00E+00 |
| 55 | 3431 | SP110 | 11 | 1.57E-05 | 113 | 441108 | C5orf56 | 0 | −1.00E+00 |
| 56 | 11274 | USP18 | 8 | 1.46E-05 | 114 | 84166 | NLRC5 | 0 | −1.00E+00 |
| 57 | 84875 | PARP10 | 8 | 1.46E-05 | 115 | 221749 | PXDC1 | 0 | −1.00E+00 |
| 58 | 25836 | NIPBL | 8 | 1.43E-05 | 116 | 339005 | WHAMMP3 | 0 | −1.00E+00 |
Filamin A has previously been shown to interact with the C-terminus of the canonical MOR-1 [46, 47]. As MOR-1K has the same C-terminal sequence, it was therefore possible that this MOR variant would also form interactions with filamin A. When the overexpressed alone condition was assessed in HEK293 cells, MOR-1K localized mainly within intracellular compartments as previously reported [3], whereas filamin A showed peripheral cytoplasmic localization (Fig. 4e). However, when both proteins were overexpressed, we found that not only did filamin A co-localize with MOR-1K, but that this co-localization occurred at the cell surface (Fig. 4f). Although HEK cells endogenously express filamin A [46], basal levels were not sufficient to carry MOR-1K to the cell surface and required filamin A overexpression.
Discussion
In this study, we sought to determine the role of the N-terminal truncated MOR splice variant MOR-1K in HIV pathogenesis. We first examined the expression profile of MOR-1K in cell types from the nervous system and detected expression in astrocytes, brain microvascular endothelial and perineurial cells, but not in microglia, neurons, or brain vascular pericytes. We previously examined the expression profiles of the C-terminal MOR splice variants MOR-1A and MOR-1X, as well as MOR-1, across astrocytes, microglia, and neurons and also observed heterogeneity in expression [14]. All three variants were expressed in astrocytes, but MOR-1A was the only variant also detected in microglia and neurons. Combined with the findings of the present study, these results suggest that vastly different functions of MOR may be conferred on individual cell types by virtue of their balance of splice variants. Since astrocytes appear to be the cell type in the brain harboring most of the MOR splice variants, astroglia are thus likely to have a major contribution to the effects of morphine on interactions with HIV [16, 48–52].
In addition, our findings regarding the seemingly exclusive expression of MOR-1K in brain microvascular endothelial and perineurial cells, but lack of MOR in brain vascular pericytes, have important implications for the mechanisms of HIV-opioid interactions at the blood-brain barrier. Interestingly, it was shown that astrocytes co-cultured with brain microvascular endothelial cells had elevated calcium responses to opioid agonists compared to astrocytes cultured alone [53]. This finding suggests that the brain microvascular endothelial cells, through responses mediated by MOR-1K, could alter astroglial signaling and enhance inflammatory tone in these cells. Although placental pericytes have been shown to respond to morphine treatment [54], our inability to detect any MOR expression in the brain derivative suggests the possibly that other receptors such as TLR4 are contributing to the effects of opioids in this cell type [55, 56]. Additionally, while pericytes from human brain have recently been shown to productively infect with HIV at low levels [57], it is generally believed that although HIV can enter brain microvascular endothelial cells, productive infection does not occur in this cell type [58, 59]. Thus, if the brain microvascular endothelial cells are enhancing excitatory responses to morphine that affect astroglia, and pericytes are mediating the consequences of productive HIV infection, differential contributions from HIV and/or morphine exposure by each cell type may result in altered cellular cross-talk that could disrupt the integrity of the blood-brain barrier beyond the effects of HIV or morphine alone. Future studies will be needed to further elucidate the responses of these cell types derived from the central nervous system to HIV and morphine, and the interactive signaling pathways invoked by each that could synergistically disrupt the blood-brain barrier.
Our findings also suggest that increases in MOR-1K mRNA expression levels during HIVE occur simultaneously with increases of mRNAs from known inflammatory mediators. HIVE tissues had the highest MOR-1K levels, significantly higher than samples from cognitively impaired subjects without encephalitis. At first glance, the results suggest that MOR-1K transcript levels are differentially increased by encephalitis, irrespective of neurocognitive impairment. However, when MOR-1K mRNA expression levels were correlated with the degree of cognitive impairment, heightened MOR-1K levels were highly related to severity of the deficits (Fig. 2c). While these results appear to be disparate, they might be reconciled if we could examine a subset of HIVE patients without cognitive impairment, or if more details concerning characteristics of the HIVE and cognitively impaired groups were available. A pattern of pronounced differences in gene expression from neurocognitively-impaired HIVE(+) versus HIVE(−) individuals in these sample pools have prompted the notion that there are “two types of HIV-associated neurocognitive impairment” [21].
Interestingly, MOR-1(exons1–2) transcript levels were unaffected by HIV, HIVE, or the degree of cognitive impairment, implying fundamental differences in the response of C-terminal MOR splice variants compared to MOR-1K in neuroAIDS. Although assumed to result from encephalitis, we cannot exclude the possibility that increasing levels of MOR-1K contribute to HIVE and cognitive deficits. The onset of neurocognitive impairment may be independent of inflammation, although, if HIVE develops, it could clearly worsen a decline in cognition. It should be noted that for the 116 overlapping genes from the three brain regions examined that had significant differences between uninfected and HIVE subjects, our analysis found that CCL5/RANTES ranked fourth in protein-protein interactions with MOR (Table 1).
Opioid receptors, including MOR, have also been shown to form functional complexes with both CXCR4 and CCR5, possibly in combination with CD4, to affect cellular signaling and potentially viral entry by bidirectional heterologous interactions [37–43]. However, we did not find any overall significant differences in CXCR4, CCR5, or CD4 mRNA expression levels across the groups of HIV-infected subjects. This result suggests that MOR-1K mRNA expression levels are quite sensitive to changes in the brain that occur with HIVE, perhaps more sensitive than the major HIV co-receptors and receptor, and that MOR may be particularly important in mediating cellular signing and viral entry processes related to CXCR4 and CCR5 [60]. For example, the CCR5Δ32 mutant has been shown to be retained in the endoplasmic reticulum, similarly to MOR-1K, and to scavenge both wild-type CCR5 and CXCR4 [61]. If MOR-1K acts similarly and forms intracellular complexes with CXCR4 and CCR5, this could be another mechanism by which MOR-1K modulates HIV co-receptor signaling. Interestingly, we did not find that overexpression of either CXCR4 or CCR5 with MOR-1K resulted in the translocation of MOR-1K to the cell surface (data not shown), suggesting that such complexes would be retained intracellularly. Future studies will be needed to determine the specific MOR splice variants that form heterodimers with the HIV co-receptors and the differential effects of heterodimers containing individual MOR variants on cellular signaling and viral entry.
For MOR-1K to affect viral entry it would need to be expressed on the cell surface. Our network analysis of the genes that were significantly regulated across the three brain regions examined between uninfected and HIVE subjects showed that filamin A was the most likely candidate to interact with MOR. Filamin A has previously been shown to interact with the C-terminal tail of the canonical MOR-1 and affect receptor trafficking [46, 47]. We therefore speculated that filamin A would also interact with the same C-terminal sequence of MOR-1K and possibly affect trafficking of this MOR variant. Accordingly, we found that overexpression of filamin A trafficked MOR-1K to the surface of transfected HEK293 cells. These results are important for patients with the HIVE condition as we also found that FLNA expression levels were increased in HIVE subjects, suggesting that MOR-1K may be more highly expressed on the surface of cells during this condition. We confirmed that FLNA mRNA was expressed in all the cell types we examined that also expressed MOR-1K mRNA. Future studies will be needed to determine the effects of HIV exposure on FLNA expression levels in these individual cell types. However, as the differences we detected were in the HIVE subject group, the chronic effects of in vivo HIV infection may be difficult to model using current isolated and co-cultured cell culture systems, and particularly in murine cell systems and in vivo models [62].
In conclusion, our results highlight the potential role of a unique MOR splice variant in the central nervous system effects of HIV, particularly in the context of HIVE. From this and our previous study, we speculate that out of the MOR-1, MOR-1A, MOR-1X, and MOR-1K variants, MOR-1K is most likely to play a major role in HIV disease pathology. However, as HIV-infected individuals with the A118G (N40D) polymorphism located in exon 1 of the receptor have more severe disease progression [63], certain C-terminal MOR variants must also play a role. Characterization of the particular MOR splice variants involved and their molecular interactions could lead to unique strategies for tailored drug design directed towards targeting specific MOR variants. Moreover, while the consequences of MOR-1K expression at the plasma membrane have yet to be determined in terms of altered cellular signaling properties, many other interesting possibilities exist such as the formation of complexes with CXCR4 and CCR5 that could directly affect non-canonical processes involving MOR such as viral entry.
Supplementary Material
Acknowledgements
We thank Michael F. Miles for providing us with the SHSY-5Y cells. We are also most grateful to the subjects who provided samples that were analyzed in this study. This work was supported by the National Institutes of Health (NIH)-National Institute on Drug Abuse grants T32 DA007027, F32 DA033898 to S.M.D.; 5R41 DA032293-02 to L.D.; R01 DA024461 to P.E.K.; R01 DA018633, K02 DA027374 to K.F.H.; R01 DA034231 to P.E.K. and K.F.H.; and NextGen, University of North Carolina at Chapel Hill 5T90 DE021986-02 to A.S. The human tissue provided by the National NeuroAIDS Tissue Consortium (NNTC) for this publication was made possible from NIH funding through the National Institute of Mental Health and National Institute of Neurological Disorders and Stroke by the following grants: Manhattan HIV Brain Bank U01 MH083501, R24 MH59724; Texas NeuroAIDS Research Center U01 MH083507, R24 NS45491; National Neurological AIDS Bank 5U01 MH083500, NS38841; California NeuroAIDS Tissue Network U01 MH083506, R24 MH59745; and Statistics and Data Coordinating Center U01 MH083545, N01 MH32002. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or the NIH.
Footnotes
S.M.D. and K.F.H. designed the study, and S.M.D., B.N.C., R.X., N.E., J.B., A.S., and M.M. conducted the experiments. Microarray data were retrieved and analyzed by B.N.C. and M.A.O. S.M.D. analyzed and interpreted all other data. S.M.D. drafted the manuscript and L.D., P.E.K., and K.F.H. critically revised the work for important intellectual content. All authors approved the final version of the manuscript.
Conflicts of interest
None declared.
References
- 1.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 2.Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, et al. Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18:1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gris P, Gauthier J, Cheng P, Gibson DG, Gris D, Laur O, et al. A novel alternatively spliced isoform of the mu-opioid receptor: functional antagonism. Mol Pain. 2010;6:33. doi: 10.1186/1744-8069-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 5.Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18(Suppl 1):S69–S74. [PubMed] [Google Scholar]
- 6.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121(Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 7.Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58:154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr, Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: the cytokine connection. Adv Exp Med Biol. 1993;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- 9.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Byrd D, Murray J, Safdieh G, Morgello S. Impact of opiate addiction on neuroinflammation in HIV. J Neurovirol. 2012;18:364–373. doi: 10.1007/s13365-012-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, et al. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63:67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson-Papp J, Gelman BB, Grant I, Singer E, Gensler G, Morgello S. Substance abuse increases the risk of neuropathy in an HIV-infected cohort. Muscle Nerve. 2012;45:471–476. doi: 10.1002/mus.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regan PM, Dave RS, Datta PK, Khalili K. Epigenetics of micro-opioid receptors: intersection with HIV-1 infection of the central nervous system. J Cell Physiol. 2012;227:2832–2841. doi: 10.1002/jcp.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF. Differential expression and HIV-1 regulation of mu-opioid receptor splice variants across human central nervous system cell types. J Neurovirol. 2012;18:181–190. doi: 10.1007/s13365-012-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res. 2012;10:435–452. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31:439–448. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 19.Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 20.Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- 21.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, et al. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 25.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical Powerful Approach to multiple Testing Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 27.Baker EJ, Jay JJ, Bubier JA, Langston MA, Chesler EJ. GeneWeaver: a web-based system for integrative functional genomics. Nucleic Acids Res. 2012;40:D1067–D1076. doi: 10.1093/nar/gkr968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsson Y, Reese TS. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971;30:105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Antonijevic I, Mousa SA, Schafer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackel D, Krug SM, Sauer RS, Mousa SA, Bocker A, Pflucke D, et al. Transient opening of the perineurial barrier for analgesic drug delivery. Proc Natl Acad Sci U S A. 2012;109:E2018–E2027. doi: 10.1073/pnas.1120800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba M, Oishi R, Saeki K. Enhancement of blood-brain barrier permeability to sodium fluorescein by stimulation of mu opioid receptors in mice. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:423–428. doi: 10.1007/BF00169534. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 34.Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, et al. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadet P, Weeks BS, Bilfinger TV, Mantione KJ, Casares F, Stefano GB. HIV gp120 and morphine alter mu opiate receptor expression in human vascular endothelium. Int J Mol Med. 2001;8:165–169. doi: 10.3892/ijmm.8.2.165. [DOI] [PubMed] [Google Scholar]
- 36.Beltran JA, Pallur A, Chang SL. HIV-1 gp120 up-regulation of the mu opioid receptor in TPA-differentiated HL-60 cells. Int Immunopharmacol. 2006;6:1459–1467. doi: 10.1016/j.intimp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- 39.Heinisch S, Palma J, Kirby LG. Interactions between chemokine and mu-opioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain Behav Immun. 2011;25:360–372. doi: 10.1016/j.bbi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 42.Finley MJ, Chen X, Bardi G, Davey P, Geller EB, Zhang L, et al. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the kappa-opioid receptor. J Neuroimmunol. 2008;197:114–123. doi: 10.1016/j.jneuroim.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 44.Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 45.Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 46.Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol. 2003;64:1092–1100. doi: 10.1124/mol.64.5.1092. [DOI] [PubMed] [Google Scholar]
- 47.Simon EJ, Onoprishvili I. The interaction between the mu opioid receptor and filamin A. Neurochem Res. 2010;35:1859–1866. doi: 10.1007/s11064-010-0261-9. [DOI] [PubMed] [Google Scholar]
- 48.El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, et al. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, et al. HIV-1 Tat and opiateinduced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, et al. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 53.Hansson E, Westerlund A, Bjorklund U, Olsson T. mu-Opioid agonists inhibit the enhanced intracellular Ca(2+) responses in inflammatory activated astrocytes co-cultured with brain endothelial cells. Neuroscience. 2008;155:1237–1249. doi: 10.1016/j.neuroscience.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Luk K, Boatman S, Johnson KN, Dudek OA, Ristau N, Vang D, et al. Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy. J Oncol. 2012;2012:458385. doi: 10.1155/2012/458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med. 2012;16:2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1 Old and new insights into the mechanism. J Neurol. 2003;250:901–906. doi: 10.1007/s00415-003-1159-0. [DOI] [PubMed] [Google Scholar]
- 59.Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Hage N, Dever SM, Podhaizer EM, Arnatt CK, Zhang Y, Hauser KF. A novel bivalent HIV-1 entry inhibitor reveals fundamental differences in CCR5-μ-opioid receptor interactions between human astroglia and microglia. AIDS. 2013;27:2181–2190. doi: 10.1097/QAD.0b013e3283639804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agrawal L, Lu X, Qingwen J, VanHorn-Ali Z, Nicolescu IV, McDermott DH, et al. Role for CCR5Delta32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J Virol. 2004;78:2277–2287. doi: 10.1128/JVI.78.5.2277-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proudnikov D, Randesi M, Levran O, Crystal H, Dorn M, Ott J, et al. Association of polymorphisms of the mu opioid receptor gene with the severity of HIV infection and response to HIV treatment. J Infect Dis. 2012;205:1745–1756. doi: 10.1093/infdis/jis264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.