Abstract
Recently we demonstrated that extracts of bitter melon (BME) can be used as a preventive/therapeutic agent in colon cancers. Here, we determined BME effects on anticancer activity and bioavailability of doxorubicin (DOX) in colon cancer cells. BME enhanced the effect of DOX on cell proliferation and sensitized the cells towards DOX upon pretreatment. Furthermore, there was both increased drug uptake and reduced drug efflux. We also observed a reduction in the expression of Multidrug resistance conferring proteins (MDRCP) P-glycoprotein, MRP-2 and BCRP. Further BME suppressed DOX efflux in MDCK cells overexpressing the three efflux proteins individually, suggesting that BME is a potent inhibitor of MDR function. Next, we determined the effect of BME on PXR, a xenobiotic sensing nuclear receptor and a transcription factor that controls the expression of the three MDR genes. BME suppressed PXR promoter activity thereby suppressing its expression. Finally, we determined the effect of AMPK pathway on drug efflux because we have previously demonstrated that BME affects the pathway. However, inhibiting AMPK did not affect drug resistance, suggesting that BME may use different pathways for the anticancer and MDR modulating activities. Together, these results suggest that BME can enhance the bioavailability and efficacy of conventional chemotherapy.
Keywords: PXR, Efflux Transporters, Drug Interaction, Drug-Food Interaction, Drug resistance, colon cancer
INTRODUCTION
Colorectal cancer is the second leading cause of cancer related deaths and the third most commonly occurring non-cutaneous carcinoma in the United States of America1. Studies have suggested that use of certain foods such as bitter melon is associated with lower prevalence of the disease, especially in certain parts India and central Asia4, 5. Bitter melon (Momordica charantia) is a tropical and subtropical vine, widely grown in Asia, Africa, and the Caribbean for its edible fruit. The fruit is recommended in ancient Indian and Chinese medicine for prevention and treatment of diabetes6, 7, though all parts of the plant (fruit, seed, and leaves) have been shown to possess hypoglycemic properties 8. Studies have also shown that bitter melon extracts are well tolerated in both acute and chronic doses in animals9–11. We have recently demonstrated that an extract of bitter melon has potent anti-colon cancer activity12. Similarly other labs 13–15 have demonstrated that bitter melon extracts can inhibit the growth of breast and prostate cancer cells. Although bitter melon extracts was shown to induce apoptosis of prostate and breast cancer cells, our studies, for the first time demonstrated that the extracts induce autophagic cell death in colon cancer cells via the activation AMPK signaling pathway.
Multiple chemotherapeutic agents are used for the treatment of cancer but they often fail due to insufficient concentration being achieved in the cancer tissues. Sub-therapeutic concentrations in the tumors can be a result of multi-drug resistance (MDR) associated efflux pumps. Many chemotherapeutic agents including doxorubicin (DOX) have been shown to be substrates of multiple efflux transporters16–18. These ATP binding cassette (ABC) transporters derive their energy from the hydrolysis of ATP and utilize this energy to transport the xenobiotic molecules out of the cells, thus imparting drug resistance. These proteins are also referred to multidrug resistance conferring isoforms/proteins (MDRCP). Though these MDRCP are considered as drug-resistant pumps in cancer tissues, they are expressed in many normal tissues suggesting their role in cellular transport of many endogenous substrates and therapeutic agents19. Several members of MDRCP including P-glycoprotein (P-gp/MDR1/ABCB1), multidrug resistance associated protein 2 (MRP2/ABCC2/cMOAT) and breast cancer resistance protein (BCRP/ABCG2/MXR) have been identified to be expressed on multiple healthy tissues such as the intestine, lungs, liver, cornea20, 21. They are also overexpressed in cancer tissues especially in cancer stem cells resulting in enhanced chemoresistance22.
Due to the therapeutic properties present in multiple food products, patients often complement their chemotherapy with medicinal dietary agents23. However, what one should be cognizant about is that this often results in pharmacokinetic drug: food interactions which can either be synergistic (resulting in enhanced drug activity) or antagonistic (by reducing the active drug concentration at the target site. MDRCP based drug-food interactions are very common and lead to multiple therapy failures24, 25. Not much is known about the potential effects of bitter melon extracts in pharmacokinetic drug interactions. There have been previous reports suggesting potential interaction of bitter melon with P-gp26, however, none of these studies have been comprehensive, and no major studies have been performed studying both long and short-term effects in colon cancer cells.
In this article, we present the results of our in vitro experiments showing that methanolic extracts of bitter melon (BME) act synergistically with DOX in inhibiting colon cancer (HT-29) cell growth. We show that this synergy is both in case of co-treatment as well as pretreatment of the cells with BME. Mechanistically, we have determined that the synergy upon co-administration is due to inhibition of multiple efflux transporters by BME. In addition, the extracts modulate the activity of nuclear hormone receptor PXR (pregnane X receptor) which in turn regulates the expression of MDR proteins and thus maybe the mechanism of action for BME regulation of drug resistance.
METHODS
Cell culture and preparation of bitter melon extracts (BME)
HT-29 cells (American Type Culture Collection, Manassas, VA) and MDCK cells overexpressing P-gp (MDCK-MDR1), MRP-2 (MDCK-MRP2), BCRP (MDCK-BCRP) (A gracious gift from Dr. Peter Borst, Netherland's Cancer Institute) were grown in Dulbecco’s modified eagle medium containing 10% heat inactivated fetal bovine serum (Sigma Chemical Co, St. Louis, MO) and 2% antibiotic-antimycotic solution (Mediatech Inc, Herndon, VA) at 37°C in a humidified atmosphere with 5% CO2. Methanol extracts of bitter melon whole fruit (BME) were prepared from the raw and green variety of young bitter melons (Momordica charantia Linn, subcontinent variety). For the preparation of the bitter melon whole fruit extracts (BME), Pre-weighed amount of fruits were finely chopped and placed in 1:1 w/v methanol for 72 h at 4°C. These were then homogenized, centrifuged and the supernatant freeze dried at −45°C for 72 h and stored at −80°C. These dried extracts were dissolved in dimethyl sulfoxide (DMSO) to prepare 100 mg/mL stocks, which were utilized for further experiments. To limit batch-to-batch variation, the weight of the final extract was measured and the batches with more than 10% variation in extraction efficiency vs the initial weight of the fruit were discarded. Previously we have demonstrated that among the selected batches of the methanolic extract no significant batch-to-batch variations were observed based on proliferation assays12.
Proliferation assay
To assess the effect of pre and co-treatment of BME on proliferation, 5,000 cells per well were seeded on to 96 well plates and grown overnight. For co-treatment with DOX cells were incubated with either increasing concentrations of doxorubicin alone or in the presence of increasing concentrations of BME (0–150 µg/mL) in DMEM media containing 10% FBS. The treatment period for the co-treatment studies was 1 h. For the pre-treatment studies with BME cells pretreated with 25µg/mL bitter melon extract for 24 h. Proliferation studies carried out 4 h post exposure to increasing concentrations of DOX. . For the pre-treatment studies with DOX, cells were pretreated with DOX 1µM for 12 h. Proliferation studies carried out 12 h post exposure to increasing concentrations of BME. Analysis of cell proliferation after the treatment period was estimated by the hexosaminidase assay as previously described 27. Briefly, the medium was removed and hexosaminidase substrate solution in citrate buffer pH 5 (7.5 mM), p-nitrophenol-N-acetyl-beta-D-glucosaminidase (Sigma Aldrich) was added at 75 µl per well. The plate was incubated at 37 °C in 100 % humidity for 30 min. The reaction was stopped with 112.5 µl of 50 mM glycine containing 5 mM of EDTA (pH 10.4). The absorbance was measured at 405 nm. Experiments were conducted at n=6, and independently repeated at least thee times. The data were analyzed as percent of control, where the control wells were treated with equivalent amounts of DMSO alone, and the analyzed data was presented as average ± standard error of mean. The differences among mean values were deemed significant at p<0.05. For IC50 calculations, a plot between the drug concentration and hexosaminidase activity was generated and the data was fitted either linearly or exponentially. The best fit was used for further processing of data. IC50 was obtained by determining the concentration of compounds resulting in 50% of cell death after 48 h of treatment by using GraphPad PRISM™ software (GraphPad Software, Inc.).
Uptake studies & Efflux studies
Uptake studies were performed in 12-well tissue culture-treated plates with confluent cells, usually 5 d post seeding. The medium was aspirated from wells and cells were washed (3 ×10 min) with 2ml of Dulbecco’s phosphate-buffered saline (DPBS) containing 130 mM NaCl, 2.5 mM KCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, 5 mM glucose, and 20 mM HEPES at pH 7.4. Then 1ml of drug solutions (each containing 05 µM of DOX) were added to the cells and incubated at 37°C for 30 min. For uptake studies at 4°C the cells were incubated in a 4°C refrigerator. Then, solutions were removed and uptake was terminated with ice-cold stop solution (200 mM KCl and 2 mM HEPES). Later, cells were lysed overnight at room temperature with 1 mL of 0.1% (v/v) Triton-X in 0.3 N sodium hydroxide solution. Aliquots (100µL) of the cell lysate were then added to 96 well clear bottom black plates and fluorescence was measured at excitation of 480nm and emission wavelength of 570nm. Uptake in each well was normalized to protein content, estimated by the Bio-Rad protein estimation kit using bovine serum albumin as the standard. For uptake studies is presence of specific inhibitors of efflux transporters PSC833 (SB-PSC833-1, Xenotech, Lenexa, KS), MK571 and KO143 (Sigma, St. Louis, MO) were used at concentrations 3–5 times their reported IC50 value.
For efflux studies the cells were pre-incubated with 1ml of the drug solutions containing DOX for 30 min. The cells were rapidly washed (3 × 30 sec) with 2ml of stop solution. Then the cells were incubated with 500µL of DPBS for 30 min. The DPBS was then collected and stored in −80°C till further analysis. 100 µL aliquots were analyzed for fluorescence using plate reader using same protocol as for uptake studies.
Quantitative RT-PCR Analysis
Total RNA was isolated from cells using Trizol-LS® reagent according to manufacturer’s instructions. RNA was measured by UV absorbance at 260 nm. The quality of RNA was verified by using 260/280 nm absorbance ratio. Briefly, 1 µg of total RNA was mixed with 2 µl of oligo dT15 primer and denatured at 70 °C for 10 min. After denaturation, it was reverse transcribed with 1 µl (10 units) per reaction mixture of MMLV Reverse Transcriptase (Promega, Madison, WI). PCR was carried out in 20-µL volumes containing 20 ng of each tissue cDNA as a template and 0.5 µM of each primer with Taq polymerase (Promega). The primer sequences used are summarized in Table 1. cDNAs were then used for Real Time PCR using Jumpstart Taq DNA polymerase (Sigma-Aldrich) and SYBR Green nucleic acid stain (Molecular Probes, Eugene, OR). Crossing threshold values for individual genes were normalized to β-Actin. Changes in mRNA expression were expressed as fold change relative to control.
Table 1.
Primer sequences for quantitative RT-PCR
| Gene | Primer Sequence | Product length |
|---|---|---|
| BCRP | Forward: TCAGCAGCTCTTCGGCTTGC Reverse: TTCCAACCTTGGAGTCTGCCACT |
112 |
| P-gp | Forward: TGCCTTCATCGAGTCACTGC Reverse: TGACGTGGCTTCATCCAAAA |
148 |
| MRP-2 | Forward: TGTGACCACAGATACCAGGA Reverse: AAATATTTTGCCTGGGAACC |
147 |
| GAPDH | Forward: AACAGCGACACCCACTCCTC Reverse: ACCACCCTGTTGCTGTAGCC |
116 |
Western Blot Analysis
Cell lysates were subjected to polyacrylamide gel electrophoresis and blotted onto Immobilin polyvinylidene difluoride membranes (Millipore, Bedford, MA). Antibodies were purchased from Cell Signaling Technology (Beverly, MA), Abcam Inc (Cambridge, MA) and Santa Cruz Biotechnology Inc (Santa Cruz, CA) and specific proteins were detected by the enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ). The densitometry study to quantitatively compare band intensity between various treatments was performed using ImageJ software from NIH.
Reporter gene assays
To test activation of human PXR, HT-29 cells were transiently transfected with PXR-response element cloned in a luciferase vector (a gracious gift from Dr. Grace Guo, Rutgers University, New Jersey) using lipofectamine 2000 (Invitrogen) as per manufacturers protocol. 12 h post transfection, cells were treated with different concentrations of BME, 10 µM MK571, 5 µM KO143, or 1 µM PSC833 in DMEM for 12 h. BME. MK571, KO143, and PSC833 are specific inhibitors of MRP2, BCRP and P-glycoprotein, respectively. Activation of PXR was determined by measuring the firefly luciferase activity 12 h later using Luciferase reporter assay kit (Invitrogen), followed by normalization of luciferase activity by protein concentrations. Further PXR activity assays were also carried out in the presence of 10 and 20 µM Metformin (Spectrum Chemical Mfg. Corp., Gardena, CA) and 5 µM AICAR (Sigma, St. Louis, MO).
Statistical analysis
All experiments were conducted at least in triplicate and results were expressed as mean ± SD. Statistical comparisons of mean values were evaluated by Student’s t-test using GraphPad InStat version 3.1 (GraphPad, La Jolla, CA). Values of P < 0.05 were considered significant.
RESULTS
DOX interacts synergistically with BME to inhibit colon cancer cell growth
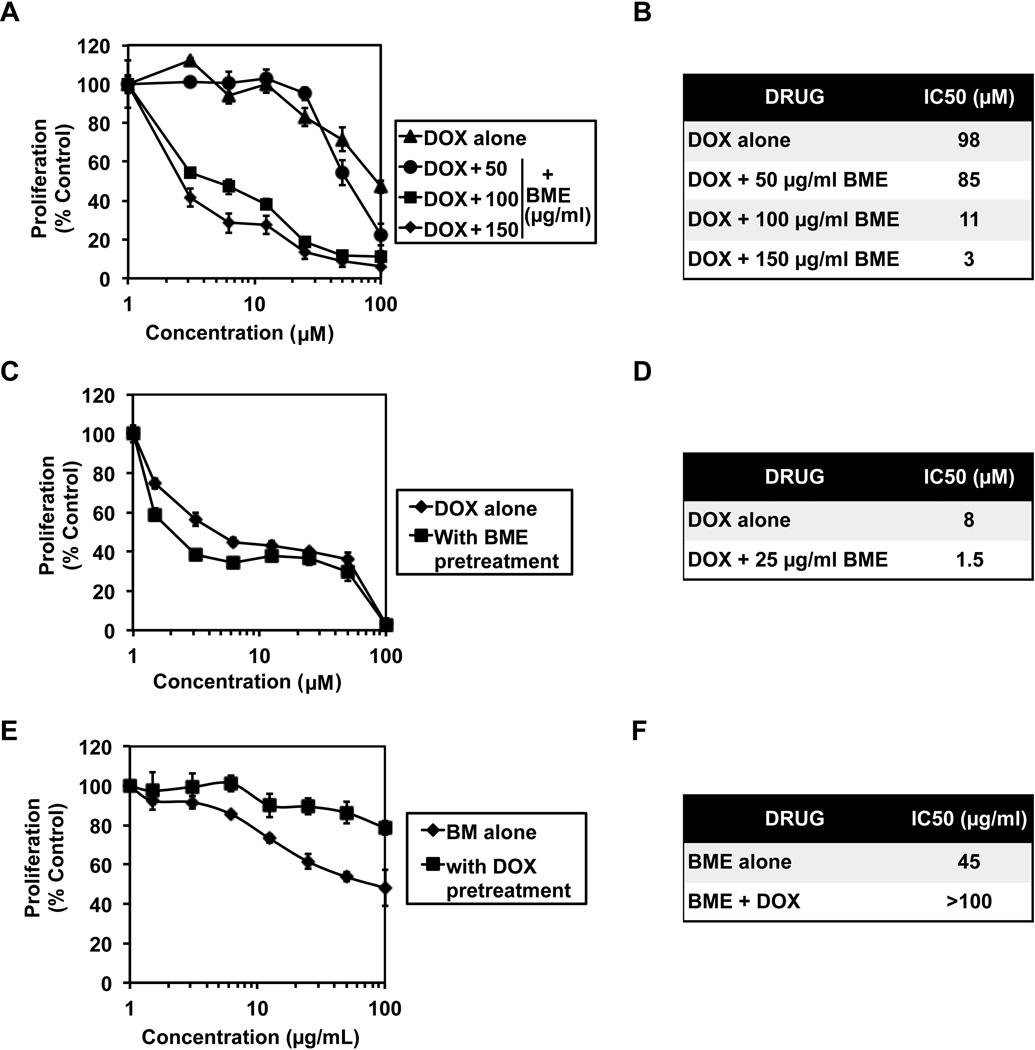
We first determined the sensitivity of HT-29 colon cancer cells to DOX alone and in combination with BME to determine whether the extracts enhance the cytotoxic effects of DOX. For this, the cells were incubated with either doxorubicin (0 to 100 µM) alone or in the presence of increasing concentration (0--50 µg/mL) BME for 1 h and the cell proliferation was measured by hexosaminidase assay. BME inhibited HT29 cell proliferation in a dose-dependent manner in combination with DOX (Figure 1 A). The IC50 dose of 1 h incubation of DOX for proliferation of HT-29 cells was observed to decrease from 98 µM, to almost 10 fold less in presence of BME. (Figure 1 B). To study whether BME pretreatment sensitizes colon cancer cells to DOX, the HT-29 cells were pretreated with 25 µg/mL bitter melon extract for 24 h. The cells were then incubated with increasing concentrations of DOX (0–100 µM) and then levels of proliferation were assessed. BME pre treatment was found to increase the sensitivity of HT-29 cells to DOX (Figure 1 C). The IC50 dose of DOX for proliferation of HT-29 cells was observed to decrease from 8 µM to 1.5 µM upon BME pretreatment (Figure 1 D). We then determined whether the order addition affects the sensitivity, and also whether pretreatment with DOX affects BME mediated suppression of cell proliferation. For this, HT-29 cells were pretreated with 1 µM DOX for 12 h. Subsequently the cells were incubated with increasing concentrations of BME (0–100 mg/mL) for 12 h and proliferation assessed. BME was found to be less effective in cells presensitized to DOX (Figure 1 E) with the IC50 increase from 45 µg/mL to more the 100 µg/mL. These data suggest that preincubation with DOX reduces the susceptibility of the cancer cells to BME.
Figure 1. Doxorubicin interacts synergistically with BME to inhibit colon cancer cell growth.
(A & B) Doxorubicin co-incubation with BME (BME) increases its cytotoxicity. Proliferation studies were carried out 1 h after exposure to the drug combination. Cells were incubated with either doxorubicin alone or in the presence of BME (0–150 µg/mL). The IC50 values decreased significantly when cells were incubated with increasing concentrations of BME; (C & D) BME pretreatment sensitizes colon cancer cells to DOX. Cells were pretreated with 25 µg/mL BME for 24 h followed by 4 h incubation with DOX. Pretreatment with BME significantly reduced the IC50values for DOX. (E & F) DOX desensitizes colon cancer cells to BM. Cells were pretreated with 1 µM DOX for 12 h and then treated with BME. Pretreatment with DOX affected BME activity.
BME inhibits the interaction of DOX with multidrug resistance proteins altering its bioavailability within the cancer cells
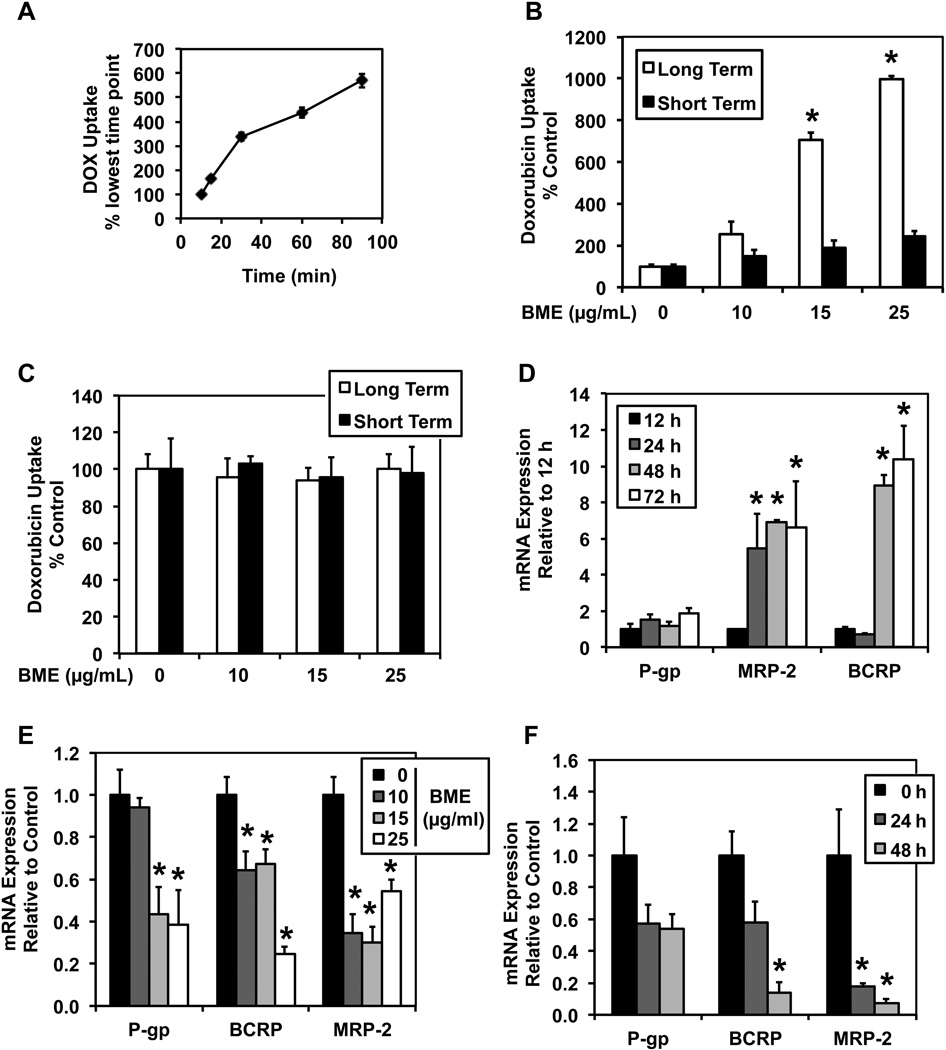
The cytotoxicity of a chemotherapeutic drug depends on the amount of drug reaching within the cells to cause the cytotoxic effects. To study whether the synergistic effects of BME on DOX was due to increased bioavailability within the cancer cells, drug uptake studies were performed. To optimize the drug uptake study protocol, time dependent uptake of DOX was carried out. The uptake was found to be linear for first 30 min of exposure in HT-29 cells and hence all further studies were carried out for 30 min (Figure 2 A). To study the effects of BME on the uptake of DOX, cells were either pretreated or co-treated with various concentrations of BME. DOX uptake was found to increase in both long term pre-incubation as well as co-incubation (short term) with increasing concentrations of BME (Figure 2 B). To identify if the effects of BME on DOX uptake were due to alteration of active transport of DOX or due to changes in passive transport, similar uptake studies were carried out at 4°C where all active transports shut down. There was no significant change in uptake observed in either short term or long term uptake studies carried out at low temperature (Figure 2 C). These data suggest that DOX uptake was an active process. Since DOX is a substrate of efflux transporters, which may also be responsible for the changes in its active transport, the effects of BME on multidrug resistance genes (MDR) was studied. To optimize the time point to study the MDR expression levels, we first performed a time dependent PCR study. All three MDR-related genes normalized at 48 h post seeding (Figure 2 D). Further to study the effects of BME on the MDR gene expression, two studies (concentration dependent and a time dependent) were performed. MDR gene expression was found to decrease with increase in concentration of BME pretreatment (Figure 2 E). Furthermore, MDR gene expression was found to decrease in a time dependent manner upon pretreatment with 25 µg/mL BME (Figure 2 F).
Figure 2. BME inhibits the interaction of DOX with multidrug resistance proteins altering its bioavailability within the cancer cells.
(A) Time dependent uptake of doxorubicin. The uptake is linear for first 30 min of exposure in HT-29 cells. (B) Both pre-incubation and co-incubation of BME increases the uptake of DOX at 37°C. The white bars represent the doxorubicin uptake at 50 µM for 30 min in cells pretreated with different concentrations of BME for 48 h. The black bars represent doxorubicin uptake at 50 µM for 30 min when incubated in combination with different concentrations of BME. All these studies were carried out at 37°C. *p<0.05. (C) BME treatment of DOX affects active transport of DOX as there is no difference in the uptake of DOX at 4°C. The white bars represent the doxorubicin uptake at 50 µM for 30 min in cells pretreated with different concentrations of BME for 48 h. The black bars represent the doxorubicin uptake at 50µM for 30 min in combination with different concentrations of BME. All these studies were carried out at 4°C. (D) Highest MDR gene expression is achieved 48 h post seeding which then normalizes by 72 h. *p<0.05. (E & F) BME pretreatment reduces the expression of MDR proteins both in a concentration dependent (panel E) and time dependent manner (panel F). In panel E, cells were treated with different concentrations of BME for 48 h, while in panel F, they were treated with 25 µg/mL BME for different time points. *p<0.05.
BME may interact with multiple efflux transporters in HT-29 cells
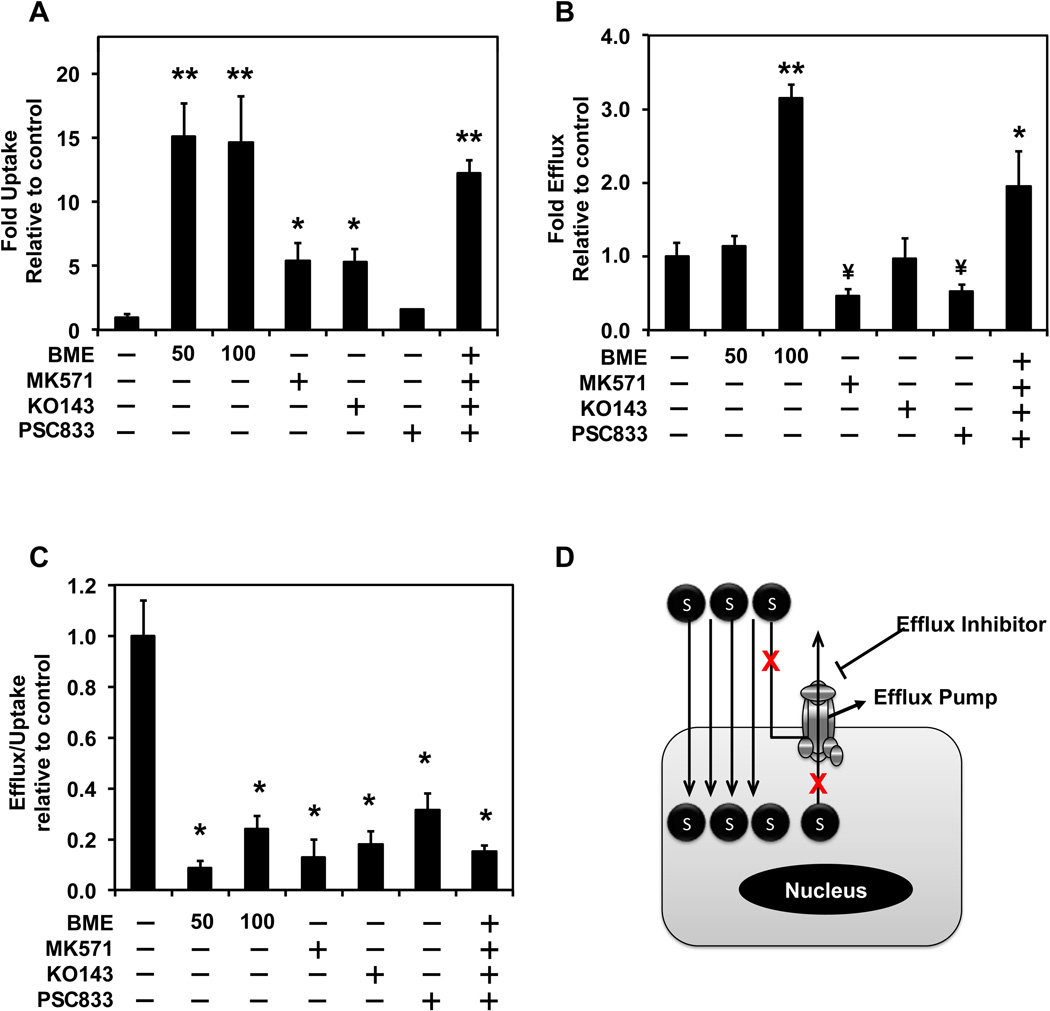
Since short term exposure to BME resulted in increased uptake of DOX, and given that DOX is a substrate of multiple efflux transporters, we needed to determine whether BME could interact with any efflux transporters. Uptake studies of DOX were carried out in HT-29 cells in presence of BME (50 or 100 mg/ML) and compared to the uptake of DOX in presence of specific inhibitors of MDR proteins. PSC833 (specific inhibitor of P-gp), MK-571 (specific inhibitor of MRP-2) and KO143 (specific inhibitor of BCRP) were all observed to increase the uptake of DOX in HT-29 cells. None of the inhibitors increased the uptake of DOX similar to the levels achieved with bitter melon when treated individually (Figure 3 A). However, when a combination of all three specific inhibitors was used, there was a change in DOX uptake levels as that observed with BME. These data suggest that that BME may inhibit all three MDR proteins. Further, a drug efflux study was carried out in presence of BME and specific MDR inhibitors to additionally confirm the effects on efflux transporters. The efflux data did not show a similar trend as the uptake data because the inhibitor treated cells showed more efflux than control (Figure 3 B). However, when the efflux levels were normalized to the amount of drug originally taken up by the cells, the expected trend of reduced efflux was observed (Figure 3 C). These data suggest that BME is a potent inhibitor of efflux pumps (Figure 3 D).
Figure 3. BME interacts with multiple efflux transporters in HT-29 cells.
(A) Uptake of DOX in presence of BME. MK571, KO143, PSC833 were used as specific inhibitors of MRP2, BCRP and P-glycoprotein, respectively. Uptake was carried out for 30 min with 50 µM DOX alone or in combination with either BME (50 or 100 µg/mL) or the indicated specific inhibitors (10 µM MK571, 5 µM KO143, 1 µM PSC833). *p<0.05 and **p<0.01 for upregulation and ¥p<0.01 for downregulation when compared to untreated controls. (B) Efflux of doxorubicin in the presence of BME or specific inhibitors of P-gp, MRP2 and BCRP. Efflux into PBS was carried out after 30 min preincubation with 50 µM DOX alone or in combination with either BME (50 or 100 µg/mL) or the specific inhibitors (10 µM MK571, 5 µM KO143, 1 µM PSC833). (C) Ratio of Efflux of doxorubicin to the uptake of doxorubicin. The ratio normalizes the efflux to the amount of drug within the cells at the time when efflux was initiated. (D) A summary schematic of drug movement in and out of cells and effects of efflux transporters on drug movement.
BME interaction with individual efflux transporters in MDCK cells overexpression specific efflux transporters
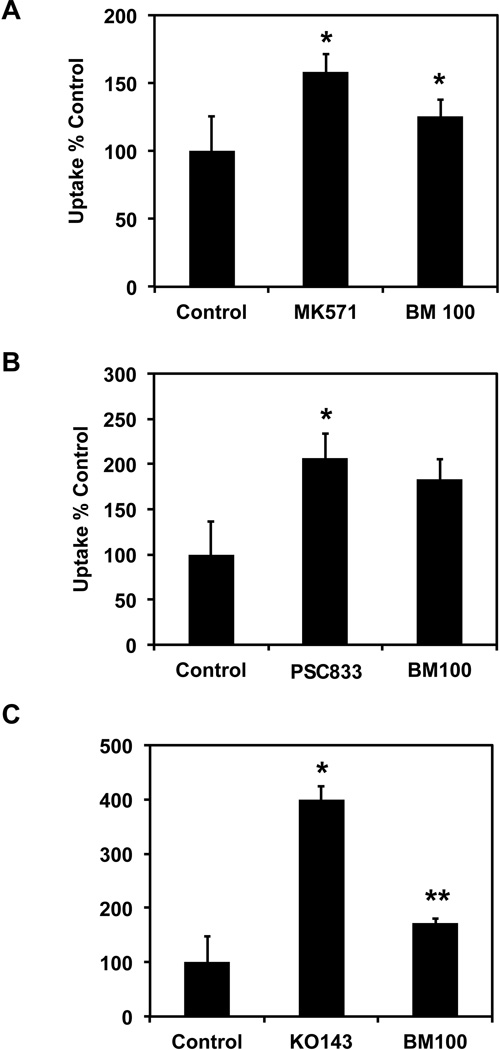
To further confirm the interaction of BME with individual efflux transporters, uptake studies of DOX were carried out in MDCK stably cells transfected with and overexpressing P-gp, MRP-2 or BCRP. MDCK cells were chosen for these studies because they have been previously shown to not express either one of these transporters in sufficient amounts to interfere significantly with the overexpressed protein activity28. BME was found to increase DOX uptake in all three cell lines i.e. MDCK-MRP2 (Figure 4 A), MDCK-MDR1 (Figure 4 B) and MDCK-BCRP (Figure 4 C). To confirm that the effect was indeed due to BME mediated suppression of the individual transporters, we also performed studies where the cells were incubated with its specific inhibitor. Again, MK571, PSC833 and KO143 were used as positive controls for the inhibition of MRP-2, P-gp and BCRP, respectively. In all three cases, the inhibitors increased DOX uptake.
Figure 4. BME interaction with individual efflux transporters in MDCK cells overexpressing specific efflux transporters.
(A) Uptake of DOX alone or in the presence of either BME 100 µg/mL or 10 µM MK571 used as a specific inhibitor for MRP2. *p<0.05 (B) Uptake of DOX alone or in presence of either BME 100 µg/mL or 1 µM PSC833 used as a specific inhibitor for P-gp. *p<0.05 (C) Uptake of DOX alone or in presence of either BME 100 µg/mL or 5 µM KO143 used as a specific inhibitor for BCRP. *p<0.01, **p<0.05. In all three cases, uptake was carried out for 30 min with 50 µM DOX alone or in combination with either BME or the specific inhibitors.
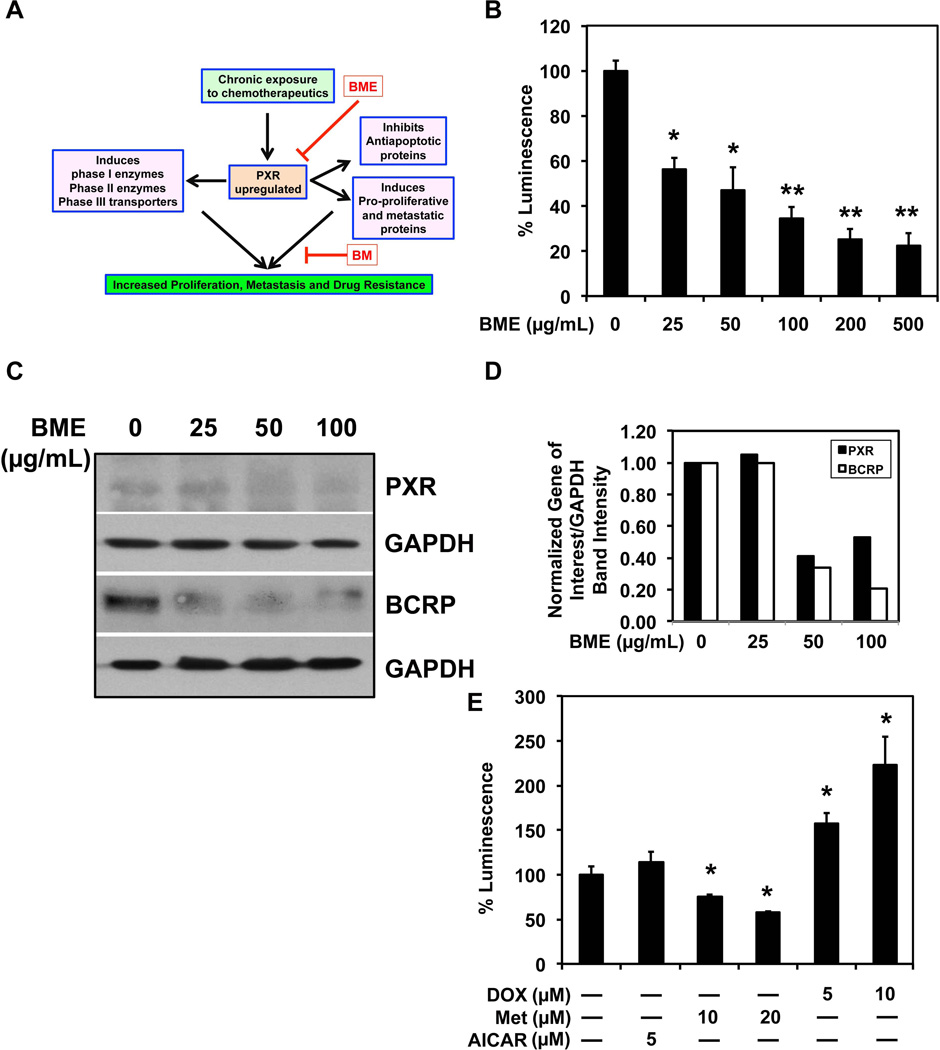
BME inhibits PXR expression and activity leading to reduced multidrug resistance
The direct interaction with the efflux transporters can explain the effectiveness of BME in short-term interaction studies, but it does not explain the reason for long-term interactions. As shown previously, pretreatment with BME results in decrease in MDR gene expression (Figure 2 E,F). In addition to inducing cell proliferation and inhibiting apoptosis, PXR is known to induce the expression of drug metabolizing enzymes and efflux transporters in the presence of xenobiotics (Figure 5 A). To study the potential role of BME on PXR activity, a luciferase based PXR promoter activity assay was performed in presence of increasing concentrations of BME (0–500 mg/mL). BME was found to decrease PXR promoter activity in a concentration dependent manner (Figure 5 B). Pretreatment with BME was also found to decrease PXR expression and one of its downstream targets BCRP (Figure 5 C). This change was quantified using densitometry (Figure 5D). Since there is no known mechanism by which BME may inhibit PXR activity, we tested the effect of different drugs with similar activity to BME for their interaction with PXR. In our studies we observed that metformin (an anti-diabetic drug with mechanism of action similar to BME 29, 30) was also found to decrease PXR activity. But AICAR another AMPK activator similar to BME31 was found to increase the PXR activity in a statistically insignificant manner (Figure 5 E). As expected, DOX was also observed to induce PXR activity.
Figure 5. Chronic exposure to BME inhibits PXR expression and activity leading to reduced multidrug resistance.
(A) Schematic representation of the role of PXR in cancer cell growth and resistance to therapeutic agents, and the proposed mechanism of BME action. (B) BME inhibits PXR-driven promoter activity. Cells were transfected with luciferase reporter plasmid under the control of the PXR promoter and then treated with BME for 12 h. There is a dose dependent inhibition of PXR promoter activity by BME. *p<0.05 and **p<0.01 (C) Western blot analyses for PXR and its target BRCP protein. Both PXR and BRCP are reduced at higher doses of BME. (D) Quantitative estimation of band intensity of western blots by densitometry. Band intensity of both PXR and BRCP were reduced at higher doses of BME treatment when compared to control and normalized to loading control. (E) PXR promoter activity in response to various drugs. Cells were transfected with the plasmid containing PXR promoter driving the luciferase gene and then incubated with doxycycline (0–10 µM), metformin (10–20 µM) or AICAR (5 µM) for 12 h. Doxycycline induced, while metformin inhibited PXR promoter activity. *p<0.05 and **p<0.01.
DISCUSSION
The data presented in this manuscript demonstrates that methanolic extract of bitter melon works in synergism with chemotherapeutic drug doxorubicin in inhibiting the growth of colon cancer cells. Since, cancer is a disease involving uncontrolled proliferation of cells, if a drug combination ultimately deters this cell division, as compared to a single drug, it can potentially show better anticancer activity. Given the potent inhibition of proliferation of HT-29 colon cancer cells by the combination of bitter melon extracts and doxorubicin, we proceeded with determining the mechanism of action.
One of the biggest problems with combination studies is how the two agents will interact. Hence it is really important to determine whether the compounds should be added together or sequentially. Also, if sequential addition is required, then the order of treatment needs to be determined. Hence, as a first step, we tested whether the bitter melon extracts interact with the chemotherapeutic doxorubicin upon co-administration or upon pre-exposure. We chose doxorubicin as a model chemotherapeutic for two important reasons. First is that the compound is easy to detect and measure32. More importantly, doxorubicin is a substrate of multiple active transport proteins, especially efflux transporters16–18. We observed that the anti-proliferative activity of doxorubicin increased considerably in the presence of increasing concentrations of bitter melon extracts resulting in ten-fold decrease in the IC50 values when BME is present. There are multiple possibilities of this synergy one of which can be enhanced bioavailability of the drug within the cells resulting in increased activity. More importantly, the results demonstrate that co-administration of doxorubicin and bitter melon extracts has a synergistic effect on inhibiting cell viability. We also determined whether sequential administration was effective. In this case, there was a really interesting finding. While pretreatment with bitter melon extracts significantly enhanced doxorubicin activity, the reverse did not. In fact, when cells were treated with doxorubicin first and then with bitter melon extracts, the cells were not affected, with a significant increase in IC50 values. These data suggest that the order of addition of the two agents is really important.
What is very interesting is that treatment with bitter melon extracts enhances doxorubicin activity in killing cancer cells. This suggests a long-term interaction between bitter melon extracts and doxorubicin, wherein the extracts can inhibit the expression of active transporters, especially those responsible for the efflux of doxorubicin. If this were the case, then the synergy should result in increased doxorubicin accumulation in the cells. Indeed, our studies, both with short term and long-term pre-treatment with bitter melon extracts resulted in increased intracellular levels of doxorubicin. This increase in uptake can be either an end result of effects of the extracts on active transport mechanisms or by influencing other routes of permeation. Again, our studies point to active uptake and not passive diffusion because when studies were performed at 4°C, wherein all the active transport processes slows down significantly and passive diffusion becomes the predominant pathway for drug uptake, there was no significant change in doxorubicin uptake.
There has been a lot of attention on drug efflux proteins because they shunt out the drugs form inside the cells reducing the effectiveness of the compounds. Three MDRCP that are important and use doxorubicin as substrate are P-glycoprotein, MRP-2 and BCRP33, 34. Expression studies demonstrated that treatment with bitter melon extracts reduced the expression of these three proteins in a dose and time dependent manner. This is the fist demonstration that extracts of bitter melon have significant effects on expression of multiple drug efflux proteins. A related question we had is whether the extracts only affected the expression of the genes or was there also an effect on efflux activity. For this, we performed studies with specific inhibitors of the three transporters. Interestingly, neither one of the inhibitors alone showed the decrease in doxorubicin efflux activity to the levels seen with bitter melon extracts. It was only when all three inhibitors were added together that the activity was similar to that observed with bitter melon extracts. There data suggest that all three transporters need to be suppressed. To further confirm this, we planned on performing studies with overexpression of the transporters. Given that expression of all three transporters was reduced, we had to employ a strategy where the proteins were overexpressed individually. More importantly, we had to perform the studies in a cell line where there will be no interference with endogenous proteins. Hence, we settled on the MDCK cell line because as the endogenous expression of any of the three efflux transporters is insignificant and the model has been established for these kinds of studies28. Using cells where each transporter was individually overexpressed using stable transfection, and with specific inhibitors, we again determined that bitter melon extracts were potent inhibitors of all the three efflux proteins.
The co-incubation uptake studies support our hypothesis that bitter melon extracts interacts with MDR proteins allowing for increased uptake of doxorubicin in cancer cells. A closer look at these studies suggests that there may be a possibility that BME may be affecting other transporters in addition to the efflux transporters tested. This may the reason for the significantly higher uptake and toxicity in presence of high concentrations of extracts with doxorubicin than specific inhibitors or their combination. More detailed studies are further required to completely delineate the effects of BME on other transporters present on these cells. Another possible mechanism by which bitter melon extracts may be affecting efflux protein activity is through its effects on ATP. Our previously published studies have shown that the extracts reduce the levels of available ATP in the cells through the activation of the AMPK pathway12. Other studies from members of this group have also shown that decrease in levels of ATP can significantly alter the activity of efflux transporters as they belong to the ATP binding cassette family of transporters and require ATP to perform their activity35, 36.
PXR is a xenobiotic receptor that regulates the expression of various multiple drug resistance associated genes. Upon the activation of PXR it is known to induce the expression of Phase I and II metabolizing enzymes as well as efflux transporters37. PXR is known to target the promoter region of the three MDR genes P-glycoprotein, MRP-2 and BCRP38–40. However, more recent studies have pointed to the ability of PXR to also activate genes that promote cell proliferation while inhibiting apoptosis41. Taken together, PXR appears to be a central protein that promotes cancer cell growth and drug resistance. Hence, targeting PXR would be an excellent strategy for cancer prevention and therapy. Indeed, our studies demonstrate that bitter melon extracts suppress PXR expression upon prolonged exposure to cancer cells. In our previously published studies, we showed that bitter melon extracts produces its anticancer effects through energy modulation similar to the anti-diabetic drug metformin by the activation of AMPK12. We also tested the effect of AICAR, an AMPK activator. Although metformin showed a decrease in PXR activity, AICAR resulted in a slight but not significant increase in PXR activity. These results indicate towards a possibility that bitter melon extracts may use different pathways to regulate PXR activity and an alternate mechanism to demonstrate its anticancer activity. Since bitter melon extract is a mixture of several compounds, different components may perform different functions within the cancer cells. This also raises the possibility that the active ingredient within the extract may be a substrate/competitive inhibitor for efflux transporters. Hence, additional purification studies are warranted to identify the active compound(s) in the extracts. Once such compounds are identified we may be able to answer these potential questions.
One other thing that is important is that we performed the current studies with doxorubicin. We chose doxorubicin as the compound for our proof of principle studies because it is an excellent substrate for the three efflux transporters. However, additional studies are required to determine whether the bitter melon extracts enhance the activities of current approved colon cancer therapeutics such as 5-fluorouracil, irinotecan and oxaloplatin. Once the individual components of bitter melon extracts are identified, our future studies will focus on determining their effects on the activities of these therapeutic agents.
ACKNOWLEDGEMENTS
This work was supported by the NIH grants CA109269 and CA135559 to S. Anant and grant support from the Thomas O'Sullivan Foundation and NCI-designated University of Kansas Cancer Center (P30CA168524-01). S. Anant is an Eminent Scientist of the Kansas Biosciences Authority. D. Kwatra was supported by a postdoctoral fellowship from the University of Kansas Medical Center Biomedical Research Training Program. We thank Dr. Grace Guo of Rutgers University and Dr. Peter Borst of the Netherland's Cancer Institute for the gracious gifts of PXR plasmids and transfected MDCK cell lines, respectively. We also thank all members of the Anant Laboratory for their discussion during the course of this study.
Footnotes
CONFLICT OF INTEREST
The authors of the manuscript declare that there is no conflict of interest with any identity used in the manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Erlanson-Albertsson C. High-fat diet not harmless--serious health risks have been surveyed. Atherosclerosis, breast and colonic cancer, depression, reduced memory, dependence... Lakartidningen. 2011;108(51–52):2713–2717. [PubMed] [Google Scholar]
- 3.Mandong BM, Ngbea JA. Cancer prevention strategies. Niger J Med. 2011;20(4):399–405. [PubMed] [Google Scholar]
- 4.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutrition and cancer. 2010;62(7):919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 5.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocrine, metabolic & immune disorders drug targets. 2008;8(2):99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120(3):451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 8.Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. The British journal of nutrition. 2009;102(12):1703–1708. doi: 10.1017/S0007114509992054. [DOI] [PubMed] [Google Scholar]
- 9.Nerurkar PV, Lee YK, Motosue M, Adeli K, Nerurkar VR. Momordica charantia (bitter melon) reduces plasma apolipoprotein B-100 and increases hepatic insulin receptor substrate and phosphoinositide-3 kinase interactions. The British journal of nutrition. 2008;100(4):751–759. doi: 10.1017/S0007114508937430. [DOI] [PubMed] [Google Scholar]
- 10.Nerurkar P, Ray RB. Bitter melon: antagonist to cancer. Pharm Res. 2010;27(6):1049–1053. doi: 10.1007/s11095-010-0057-2. [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2003;60(4):356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 12.Kwatra D, Subramaniam D, Ramamoorthy P, Standing D, Moran E, Velayutham R, Mitra A, Umar S, Anant S. Methanolic extracts of bitter melon inhibit colon cancer stem cells by affecting energy homeostasis and autophagy. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:702869. doi: 10.1155/2013/702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray RB, Raychoudhuri A, Steele R, Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 2010;70(5):1925–1931. doi: 10.1158/0008-5472.CAN-09-3438. [DOI] [PubMed] [Google Scholar]
- 14.Nagasawa H, Watanabe K, Inatomi H. Effects of bitter melon (Momordica charantia l.) or ginger rhizome (Zingiber offifinale rosc) on spontaneous mammary tumorigenesis in SHN mice. Am J Chin Med. 2002;30(2–3):195–205. doi: 10.1142/S0192415X02000302. [DOI] [PubMed] [Google Scholar]
- 15.Ru P, Steele R, Nerurkar PV, Phillips N, Ray RB. Bitter melon extract impairs prostate cancer cell-cycle progression and delays prostatic intraepithelial neoplasia in TRAMP model. Cancer Prev Res (Phila) 2011;4(12):2122–2130. doi: 10.1158/1940-6207.CAPR-11-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, van Tellingen O, Vainchtein LD, Rosing H, Beijnen JH, Schellens JH, Schinkel AH. Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. The Journal of pharmacology and experimental therapeutics. 2006;318(1):319–327. doi: 10.1124/jpet.106.101774. [DOI] [PubMed] [Google Scholar]
- 17.Szaflarski W, Sujka-Kordowska P, Januchowski R, Wojtowicz K, Andrzejewska M, Nowicki M, Zabel M. Nuclear localization of P-glycoprotein is responsible for protection of the nucleus from doxorubicin in the resistant LoVo cell line. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2013;67(6):497–502. doi: 10.1016/j.biopha.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Kuang YH, Patel JP, Sodani K, Wu CP, Liao LQ, Patel A, Tiwari AK, Dai CL, Chen X, Fu LW, Ambudkar SV, Korlipara VL, Chen ZS. OSI-930 analogues as novel reversal agents for ABCG2-mediated multidrug resistance. Biochemical pharmacology. 2012;84(6):766–774. doi: 10.1016/j.bcp.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 20.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatmaitan ZC, Arias IM. Structure and function of P-glycoprotein in normal liver and small intestine. Adv Pharmacol. 1993;24:77–97. doi: 10.1016/s1054-3589(08)60934-5. [DOI] [PubMed] [Google Scholar]
- 22.Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open biology. 2012;2(5):120066. doi: 10.1098/rsob.120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treasure J. Herbal medicine and cancer: an introductory overview. Seminars in oncology nursing. 2005;21(3):177–183. doi: 10.1016/j.soncn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Jatoi A. Cancer chemotherapy: with or without food? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18(Suppl 2):S13–S16. doi: 10.1007/s00520-009-0666-7. [DOI] [PubMed] [Google Scholar]
- 25.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clinical pharmacokinetics. 2004;43(15):1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 26.Konishi T, Satsu H, Hatsugai Y, Aizawa K, Inakuma T, Nagata S, Sakuda SH, Nagasawa H, Shimizu M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. British journal of pharmacology. 2004;143(3):379–387. doi: 10.1038/sj.bjp.0705804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK, Houchen CW, Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68(6):1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 28.Kwatra D, Boddu SH, Mitra AK. MDCK Cells and Other Cell Culture Models of Oral Drug Absorption. Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications. 2011:443–459. [Google Scholar]
- 29.Hardie DG. AMPK: A Target for Drugs and Natural Products With Effects on Both Diabetes and Cancer. Diabetes. 2013;62(7):2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Molecules and cells. 2013 doi: 10.1007/s10059-013-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosilio C, Lounnas N, Nebout M, Imbert V, Hagenbeek T, Spits H, Asnafi V, Pontier-Bres R, Reverso J, Michiels JF, Sahra IB, Bost F, Peyron JF. The metabolic perturbators metformin, phenformin and AICAR interfere with the growth and survival of murine PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells. Cancer letters. 2013;336(1):114–126. doi: 10.1016/j.canlet.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Wiench B, Eichhorn T, Korn B, Paulsen M, Efferth T. Utilizing inherent fluorescence of therapeutics to analyze real-time uptake and multi-parametric effector kinetics. Methods. 2012;57(3):376–382. doi: 10.1016/j.ymeth.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Campa D, Muller P, Edler L, Knoefel L, Barale R, Heussel CP, Thomas M, Canzian F, Risch A. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int J Cancer. 2012;131(12):2920–2928. doi: 10.1002/ijc.27567. [DOI] [PubMed] [Google Scholar]
- 34.Tian C, Ambrosone CB, Darcy KM, Krivak TC, Armstrong DK, Bookman MA, Davis W, Zhao H, Moysich K, Gallion H, DeLoia JA. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecologic oncology. 2012;124(3):575–581. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo S, Pal D, Shah SJ, Kwatra D, Paturi KD, Mitra AK. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Molecular pharmaceutics. 2010;7(2):412–420. doi: 10.1021/mp900193e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwatra D, Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. International journal of pharmaceutics. 2010;395(1–2):114–121. doi: 10.1016/j.ijpharm.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochemical pharmacology. 2012;83(8):1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer chemotherapy and pharmacology. 2010;66(4):765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. The Journal of biological chemistry. 2002;277(4):2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 40.Anapolsky A, Teng S, Dixit S, Piquette-Miller M. The role of pregnane X receptor in 2-acetylaminofluorene-mediated induction of drug transport and -metabolizing enzymes in mice. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(3):405–409. doi: 10.1124/dmd.105.006197. [DOI] [PubMed] [Google Scholar]
- 41.Pondugula SR, Mani S. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer letters. 2013;328(1):1–9. doi: 10.1016/j.canlet.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]