Abstract
Background
Human herpesvirus-8 (HHV-8) infection in early childhood is common throughout sub-Saharan Africa with prevalence increasing throughout childhood. Specific routes of transmission have not been clearly delineated, though HHV-8 is present in high concentrations in saliva.
Methods
To understand the horizontal transmission of HHV-8 within households to children we enrolled for cross-sectional analysis, 251 households including 254 children, age two and under, in Lusaka, Zambia. For all children, plasma was screened for HHV-8 and HIV-1 and health and behavioral questionnaires were completed. Multi-level logistic regression analysis was conducted to assess independent factors for HHV-8 infection in children.
Results
Risk factors for HHV-8 infection included increasing number of HHV-8 positive household members [OR 2.5 (95% CI: 1.9, 3.3) P < 0.01] and having a primary caregiver who tested the temperature of food with their tongue prior to feeding the child [OR 2.4 (95% CI: 1.93, 3.30) P =0.01]. Breastfeeding was protective against infection with HHV-8 for children [OR 0.3 (95% CI: 0.16, 0.72) P <0.01].
Conclusions
These results indicate that exposure to HHV-8 in the household increases risk for early childhood infection with specific feeding behaviors likely playing a role in transmission.
Impact
Interventions to protect children from infection should emphasize the possibility of infection through sharing of foods.
Keywords: HHV-8, Horizontal transmission, KSHV, Risk factors, Zambia
INTRODUCTION
Human herpesvirus 8 (HHV-8) is the most recently discovered member of the gamma- herpesvirus family (1). Also known as Kaposi’s sarcoma-associated herpesvirus (KSHV), it is known to be the causative agent of Kaposi’s sarcoma (KS), as well as other malignancies such as primary effusion lymphoma (2) and multicentric Castleman’s disease (3). Seroprevalence of HHV-8 depicts uneven distribution worldwide but is generally high in areas where non-HIV associated forms of KS (classic or endemic forms) are common (4). Results of epidemiological studies on HHV-8 seroprevalence in African adults varies from 29% to 48% based on region and population group (5–7). In children, seroprevalence differs significantly with age and between different geographical regions, and the prevalence of infection increases consistently throughout childhood and adolescence in young children in some endemic areas of Africa such as Zambia (8).
Prior to the onset of the HIV/AIDS epidemic in the mid 1980’s, KS was rare in children even in KS endemic areas such as sub-Saharan Africa (9–13). During the HIV/AIDS epidemic, KS incidence rates in Africa increased dramatically in both adults and children (14). In Zambia, pediatric KS occurrence increased from 3.2% of total childhood cancers to 19.0% as a direct result of the HIV/AIDS epidemic (10), and by 1990 constituted 20–25% of all Zambian pediatric malignancies (12). Given the high prevalence of HIV infection in the population estimated to be 14.1% in 2004 (15), this may have been associated with either an increased risk of acquisition of HHV-8 infection or an increased risk of HHV-8 transmission to young children. Hence, there is a need for a better understanding of the frequency of HHV-8 transmission during early childhood.
In both Western and developing countries, the risk for HHV-8 infection is likely associated with horizontal transmission through saliva however; in children there is a paucity of data regarding when and how transmission occurs and, in particular, which behavioral factors are associated with an increased risk of transmission. Several studies have shown that horizontal transmission occurs within families, most likely through saliva exchange (16–18).. Our laboratory has previously reported the isolation of HHV-8 viral DNA in saliva samples from seropositive mothers in Zambia. Results from these studies strongly suggest that child rearing behaviors associated with saliva exposure could be a risk factor for increased HHV-8 transmission (19). Results from a recent study conducted in rural Uganda lend credence to this theory when it reported a possible, albeit weak association between variables associated with saliva exposure such as sharing of food and/or sauce plates and increased risk for infection with HHV-8 (20).
Our earlier longitudinal cohort study demonstrated that children in Zambia acquire HHV-8 infection early in life, with up to 40% of children being infected by 48 months of age (21). These results provide compelling evidence that horizontal transmission of HHV-8 infection during early childhood is associated with the high incidence of infection in children. Therefore, we hypothesized that behaviors associated with saliva exchange, such as sharing food, would increase the risk for HHV-8 seropositivity. To date, no large epidemiological study has been conducted in an endemic area such as Zambia to investigate horizontal transmission of HHV-8 infection from within a household to children. In the present analysis we evaluated behavioral risk factors associated with household saliva exposure to delineate risk factors for HHV-8 infection in young children in Zambia.
MATERIALS AND METHODS
Study design and population
The present study is a part of a larger longitudinal cohort study recruited for the purpose of determining of risk factors for early childhood infection with HHV8 in Zambia. This is a collaborative study among investigators at the University of Nebraska-Lincoln and the University Teaching Hospital (UTH) of University of Zambia School of Medicine in Lusaka, Zambia to study HHV-8 and HIV infections in Zambia. Details of the study design, rationale and recruitment process for this study have been previously described in detail (22). In brief, participant recruitment was conducted from August of 2004 to April of 2007 at the KS/HHV8 Study Clinic situated within UTH. Community workers were hired from eligible participants in our previous mother to child HHV-8 cohort transmission study specifically to inform community members about the study and to help with the screening and enrollment process (22).
Inclusion criteria for enrollment were as follows; families with a child less than 2 years of age (the index child), a resident of Lusaka, all family members should be willing to participate in annual follow-up visits and the primary caregiver and index child should commit to 4-month follow-up visits. The primary caregiver was the family member self-described as the individual with the most individual contact with and providing care for the index child. Of the enrolled children, 75 HHV-8 positive children chosen at random during the screening process were also enrolled for the present analysis to understand the risk factors associated with HHV-8 prevalence in this cohort. These children were randomly selected if the caregiver expressed the willingness to participate.
Written consent was obtained from the study participants after describing the purpose of the study. Informed consent was obtained from all adult participants, and primary caregivers provided consent for children in the household. Study approval was granted by the Institutional Review Boards of the University of Zambia and the University of Nebraska and all suggestions and modifications were incorporated in the study protocols.
Data collection and measures
The primary caregiver in each household was interviewed by trained study nurses using structured questionnaires that included questions on socio-demographic variables (gender, age, education of the primary caregiver, household size, number of playmates), household living conditions (electricity, water source, toilet type, number of rooms/sleeping areas, household density), behaviors involving food and drink (premastication of food, sharing sweets and/or drinks), and healthcare and personal care practices (bathing habits, use of traditional medicine, and use of saliva to clean children’s faces, soothe injuries or insect bites). The questionnaires were written, designed and initially tested on focus groups in 2004 before study enrollment was initiated (23).
Laboratory testing
Sample collection
Blood samples were collected by venipuncture from all members of the household within eight months (mean 2.3 months) of each other. Family size is often large in Zambian households and everyone in the family is often unable to attend the clinic at the same time. HIV-1 testing was performed at the UTH clinic in Lusaka, Zambia and PBMC’s were isolated for PCR analysis for HIV-1 positive individuals. Plasma samples were shipped to University of Nebraska-Lincoln, Nebraska for HHV-8 serological analysis.
HIV-1 Serology
Plasma was screened for HIV-1 antibodies using both Capillus (Cambridge Biotech), and Determine (Abbott Laboratories) according to the manufacturer’s instructions. A result was considered positive if one or both of these rapid screening assays revealed a positive result. For children under 18 months of age, early infant diagnosis using dried blood spot (DBS) was conducted. If children under 18 months of age were found to be seropositive but no confirmatory DBS data was available, serostatus was based on serology results that were obtained at subsequent follow-up visits (>18 months of age). If follow-up visits were not completed, the HIV-1 result was considered indeterminant.
HHV-8 Serology
HHV-8 serological testing was conducted using a monoclonal antibody-enhanced immunofluorescence assay (mIFA). Plasma samples were diluted 1:40 in phosphate buffered saline, and screening was performed on BC-3 cells (an Epstein-Barr virus negative and HHV-8 positive cell line) (American Type Culture Collection, Manassas, Virginia). BC-3 cells were grown in RPMI media supplemented with 20% heat-inactivated fetal calf serum, and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). Cells were stimulated to promote HHV-8 lytic cycle with 20 ng/ml of tetradecanoyl phorbal ester acetate. At 48 hours post stimulation, cells were fixed using 4% paraformaldehyde, permeablized using 0.1% Triton X-100, and then spotted on 12-well teflon coated slides. The mIFA was performed with mouse monoclonal anti-human IgG antibody (CRL 1786) (American Type Culture Collection, Manassas, Virginia) as secondary antibody, and DyLight 488-conjugated donkey anti-mouse IgG (Thermo Scientific) as tertiary antibody. A plasma sample was considered to be positive if two readers independently determined the sample to be positive on two independent mIFA tests. Positive samples were tested on BJAB cells (an Epstein-Barr virus negative and HHV-8 negative B cell line) which performed the role of negative controls to rule out any non-specific binding of antibodies to cells. For all children under 18 months of age found to be HHV-8 positive, serology testing was repeated at subsequent visits (>18 months of age) to eliminate early positivity due to maternal antibodies.
Data analysis
Dataset for the present analysis was built and statistical analysis was conducted using SAS (v9.2) (Cary, NC, USA). Logistic regression model with manual forward stepwise selection was conducted to explore the strength and significant association between HHV-8 seroprevalence (outcome) and a range of household behavioral habits (covariates). Associations between covariates and outcome were also evaluated to identify potential confounders. Odds ratios (ORs), 95% confidence intervals (CI) and P values were calculated to identify risk factors for HHV-8 infection in children under 2 years of age. Variables with a P value <0.05 in univariable analysis were included in a multivariable logistic regression model utilizing a manual forward selection process, to control for possible confounders and identify independent associations. Anthropometric Z scores were calculated using the NUTSTAT anthropometric software package (Epi Info, version 3.2.2; Centers for Disease Control and Prevention). Three households had multiple index children in one household. To account for a clustering affect, the data was also analyzed utilizing GEE logit link type 3 analyses though we did not observe any influence of clustering on the final results.
RESULTS
We screened index children under the age of 24 months from 1,600 households within the city of Lusaka, Zambia. Eligibility criteria were met by participants of 1,023 households. Of these, 622 primary caregivers along with their index children returned for enrollment and 368 of these returned with the complete household. There were 117 households that had incomplete data and record completion at enrollment, thus information from 251 households was available for analysis. Total enrollment in the study included 251 households with 254 index children, and 877 family members as outlined in Figure 1. Of the 254 index children enrolled, 75 (29.5%) were HHV-8 positive. Table 1 summarizes the characteristics of the HHV8 positive and negative children, their household sizes, primary caregivers, and the HHV-8 status of the household members. With univariable analysis, we found the HHV-8 serostatus of the primary caregiver, mother, father, youth and children in the household to be positively associated with HHV-8 infection in the index child (Table 2). Specifically, an increased risk for HHV-8 infection was associated with a child having a primary caregiver who was HHV-8 positive [OR = 2.5 (95% CI: 1.44, 4.32) P <0.01]. There was a slightly stronger association if the mother was HHV-8 positive as opposed to other caregivers [OR = 2.7 (95% CI: 1.48, 4.77) P <0.01]. However, an increased risk for HHV-8 prevalence was associated with having any HHV-8 positive adult household member [OR = 2.1 (95% CI: 1.11, 3.92) P = 0.03], and there was an increased odds of 1.8 [(95% CI: 1.22, 2.51) P <0.01], for every additional adult household member who was HHV-8 positive. The increasing total number of HHV-8 positive household members, including youth, was associated with an even higher odds of 2.5 (95% CI: 1.91, 3.21) P <0.01.
Figure 1.
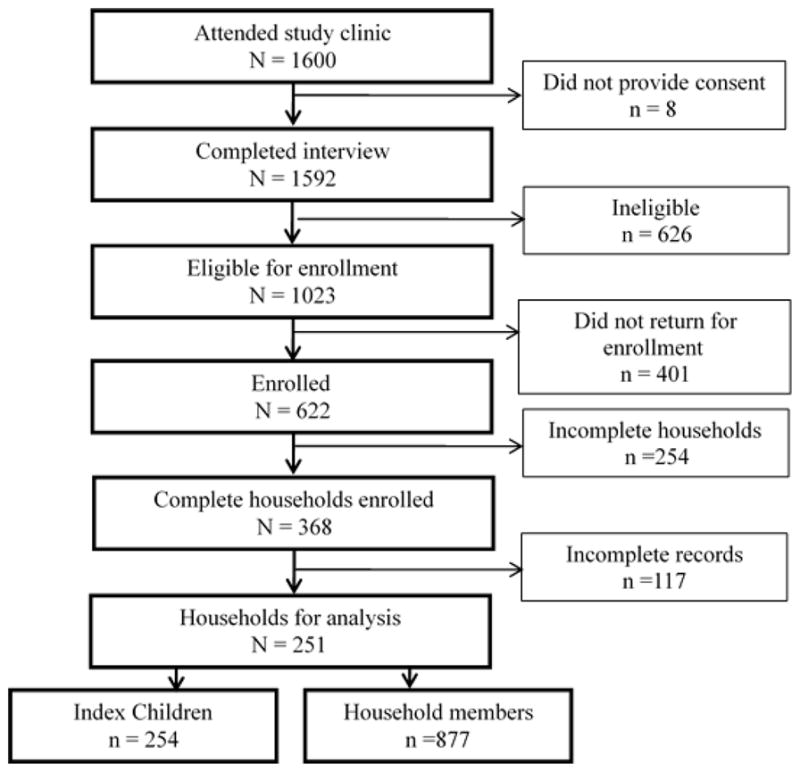
Flow Chart outlining the screening and recruitment of study cohort in Lusaka, Zambia, 2004–2007.
Table 1.
Demographics and HHV-8 Characteristics of HHV-8 Positive and HHV-8 Negative Children in Lusaka, Zambia, 2004–2007.
| Characteristic | HHV8 + (n=75) | HHV8 − (n=179) |
|---|---|---|
| Demographics | ||
| Age of index child (months): | ||
| Range | 4–25 | 2–28 |
| Mean | 13.4 | 13.5 |
| Sex of index child: | ||
| Males | 47 | 93 |
| Females | 28 | 86 |
| Number of household members: | ||
| Range | 2–8 | 2–8 |
| Mean | 4.7 | 4.5 |
| Number of adults: | ||
| Range | 1–4 | 1–4 |
| Mean | 1.8 | 1.7 |
| Age of primary caregiver (years): | ||
| Range | 17–49 | 14–58 |
| Mean | 28.9 | 27.3 |
| Education of primary caregiver: | ||
| None | 7 | 18 |
| Primary school | 47 | 105 |
| Secondary school | 21 | 56 |
| Household HHV-8 | ||
| Primary caregiver HHV-8+ | 52/75 (69.3%) | 82/179 (45.8%) |
| Mother HHV-8+ | 51/72 (70.8%) | 79/175 (45.1%) |
| Father HHV-8+ | 23/36 (63.9%) | 44/74 (59.5%) |
| ≥1 other household member HHV-8+ | 68/75 (90.7%) | 143/179 (79.9%) |
Table 2.
Univariable Analysis to Investigate the Association of HHV-8 Serological Status of Household Members with Child HHV-8 Seropositivity in Lusaka, Zambia, 2004–2007.
| Characteristic | Odds Ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Adult and Household HHV-8 Risk Factors | |||
| ≥ 1 household member HHV-8+ | 2.7 | 1.23, 5.78 | 0.01a |
| Increasing number of HHV-8+ household members | 2.5 | 1.91, 3.21 | <0.01a |
| Primary caregiver HHV-8+ | 2.5 | 1.44, 4.32 | <0.01a |
| Mother HHV-8+ | 2.7 | 1.48, 4.77 | <0.01a |
| Father HHV-8+ | 1.2 | 0.49, 2.67 | 0.66 |
| ≥ 1 adult household member HHV-8+ | 2.1 | 1.11, 3.92 | 0.03a |
| Increasing number HHV-8+ adult household members | 1.8 | 1.22, 2.51 | <0.01a |
| Youth HHV-8 Risk Factors | |||
| ≥ 1 household youth HHV-8+ | 1.4 | 0.70, 2.84 | 0.30 |
| Increasing number of household youth HHV-8+ | 1.3 | 0.90, 1.99 | 0.16 |
| Any other household child HHV8 + | 1.3 | 0.66, 2.74 | 0.52 |
Significant at α level of 0.05
Total numbers vary reflecting family members enrolled in study
We found no associations between child anthropometrics (weight and height percentiles), and risk for HHV-8 prevalence (Table 3). Additionally, there was no association between CD4 and CD8 cell count, hematocrit and hemoglobin levels and risk for HHV-8 prevalence in children (Table 3). The association between HHV-8 prevalence and socio-demographic variables was also examined, including age, gender, number of household members categorized by age, relationship of primary caregiver to index child, parental age, and primary caregiver education. There was an increased risk associated with having a greater number of adults in the household (Number of household members > 15 years of age) [OR = 1.5 (95% CI: 0.99, 2.18) P = 0.05] (Table 3), however we found no association between the other variables including child age, gender, adult age, education level and increased risk of infection.
Table 3.
Univariable Analysis to Investigate the Association of Socio-Demographic Factors and Nutritional and Immunological Factors with Child HHV-8 Seropositivity in Lusaka, Zambia, 2004–2007.
| Characteristic | Odds Ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Child specific characteristics | |||
| Age of index child (months) | 1.0 | 0.96, 1.04 | 0.99 |
| Gender of index child (Reference: male) | 0.6 | 0.37, 1.19 | 0.12 |
| Household specific characteristics | |||
| Number of household members | 1.1 | 0.94, 1.36 | 0.18 |
| Number of household adults > 15 yrs | 1.5 | 0.99, 2.18 | 0.05a |
| Number of household youth (age > 5, ≤ 15yrs) | 1.1 | 0.85, 1.37 | 0.51 |
| Number of household children (age ≤ 5 yrs) | 1.0 | 0.68, 1.52 | 0.93 |
| Two parents vs. single parent household | 1.6 | 0.86, 2.93 | 0.45 |
| Extended family parenting vs. single parent household | 1.6 | 0.75, 3.58 | 0.45 |
| Caregiver and adult specific characteristics | |||
| Relationship of primary caregiver to index child: | |||
| - Aunt vs. Mother | 5.0 | 0.44, 56.0 | 0.99 |
| - Grandmother vs. Mother | 0.7 | 0.27, 10.2 | 0.98 |
| Maternal age (years) | 1.0 | 0.99, 1.08 | 0.13 |
| Paternal age (years) | 1.0 | 0.98, 1.06 | 0.42 |
| Primary caregiver age (years) | 1.0 | 0.99, 1.08 | 0.10 |
| Primary caregiver education: | |||
| - No school vs. junior primary | 1.5 | 0.48, 4.53 | 0.30 |
| - No school vs. upper primary | 1.1 | 0.41, 2.79 | 0.93 |
| - No school vs. junior secondary | 1.1 | 0.38, 3.19 | 0.87 |
| - No school vs. upper secondary | 0.7 | 0.21, 2.59 | 0.37 |
| Nutritional status indicators | |||
| Length-for-age or stature-for-age percentile | 1.0 | 0.99, 1.01 | 0.70 |
| Weight-for-length or weight-for-stature percentile | 1.0 | 0.99, 1.00 | 0.42 |
| Weight-for-age percentile | 1.0 | 0.99, 1.00 | 0.13 |
| Laboratory values | |||
| CD4 count | 1.0 | 0.99, 1.00 | 0.27 |
| CD8 count | 1.0 | 0.99, 1.00 | 0.18 |
| Hematocrit | 1.1 | 0.86, 1.48 | 0.39 |
| Hematocrit (normal) | 1.2 | 0.44, 3.11 | 0.76 |
| Hemoglobin | 1.3 | 0.76, 2.30 | 0.33 |
Significant at α level of 0.05
We also examined for an association between HHV-8 seroprevalence in children and household and community specific living condition covariates including crowding, water source, toilet availability, number of rooms and sleeping areas in the home, crowding of persons in sleeping areas, number of playmates and number of playmates under the age of 5, and number of times weekly the index child spends overnight with friends. No associations were found with any of these covariates and child HHV-8 serostatus (Supplementary Table S1). Similarly, we found no association between HHV-8 seroprevalence in children and health and hygiene exposures including number of full-body baths, daily face cleanings, use of traditional medicine, and use of saliva to clean child’s face, soothe childhood injuries, or insect bites (Supplementary Table S1) although having a toilet within the household was marginally significant for protecting against HHV-8 infection [OR = 0.1 (95% CI: 0.01, 1.34) P=0.09).
We also investigated covariates related to feeding practices to investigate the association with HHV-8 seroprevalence and observed that there was decreased odds of HHV-8 seropositivity when the children was currently being breastfed [OR = 0.5 (95% CI: 0.27, 0.88) P = 0.01] or if the child had ever been breastfed [OR = 0.4 (95% CI: 0.21, 0.74) P <0.01] (Table 4). A number of feeding practices that involved exchange of saliva were evaluated to assess for association with HHV-8 prevalence including premastication and sharing of foods. Having a HHV-8 positive caregiver premasticate the food prior to feeding the child did not demonstrate significantly increased odds for HHV-8 prevalence, although a small number of caregivers indicated that this practice was performed limiting our overall sample size (data not shown).
Table 4.
Univariable Analysis to Investigate the Association of Child Feeding Behavioral Habits of Household with Child HHV-8 Seropositivity in Lusaka, Zambia, 2004–2007.
| Characteristic | Odds Ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Breastfeeding practices | |||
| Child has ever been breastfed | 0.4 | 0.21, 0.74 | <0.01a |
| Child is currently being breastfed | 0.5 | 0.27, 0.88 | 0.01a |
| Primary caregiver moistens nipples with saliva prior to breastfeeding | 1.0 | 0.38, 2.21 | 0.96 |
| Premastication | |||
| Adult premasticates food prior to sharing with children | 1.2 | 0.60, 2.72 | 0.61 |
| Primary caregiver performs premastication | 1.1 | 0.54, 2.47 | 0.86 |
| Other household members perform the premastication | 1.8 | 0.51, 5.68 | 0.35 |
| Adult feeding variables | |||
| Primary caregiver testing temp of food with tongue prior to sharing w/ child | 1.6 | 0.91, 2.89 | 0.09 |
| Blowing on food prior to sharing with child | 1.1 | 0.67, 1.90 | 0.69 |
| Sucking on sweets prior to sharing with child | 1.1 | 0.61, 1.89 | 0.80 |
| Child and household food sharing variables | |||
| Child shares sweets with other neighborhood children | 0.9 | 0.44, 2.02 | 0.71 |
| Child shares sweets with other household members | 1.1 | 0.64, 1.89 | 0.87 |
| Child shares drinks with other children | 0.5 | 0.24, 1.37 | 0.19 |
| Household members share common utensils for meals | 0.8 | 0.34, 1.88 | 0.58 |
| Household members share drinks with child | 1.0 | 0.50, 1.84 | 0.89 |
Significant at α level of 0.05
Upon multivariable logistic regression analysis, an independent association for HHV-8 prevalence included total number of HHV-8 positive household members [OR = 2.5 (95% CI: 1.93, 3.30) P< 0.01] or having the primary caregiver taste the temperature of the food prior to feeding [OR = 2.4 (95% CI: 1.19, 4.73) P = 0.01] (Table 5). Current or past breastfeeding [OR = 0.3 (95% CI: 0.16, 0.72) P <0.01] was protective against infection with HHV-8.
Table 5.
Multivariable Analysis to Investigate Independently Associated Risk Factors Associated with HHV-8 Seropositivity in Children in Lusaka, Zambia, 2004–2007.
| Characteristic | Odds Ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Age of index child (in months) | 1.0 | 0.94, 1.10 | 0.75 |
| Total number of HHV-8+ household members | 2.5 | 1.93, 3.30 | <0.01a |
| Primary caregiver testing temperature of food prior to feeding | 2.4 | 1.19, 4.73 | 0.01a |
| Child breastfed currently or in the past | 0.3 | 0.16, 0.72 | <0.01a |
Significant at α level of 0.05
DISCUSSION
In sub-Saharan Africa children become infected with HHV-8 at an early age. Our cohort studies have reported that by 13.4% of Zambian children were infected 12 months of age (24). An additional Ugandan study found comparable levels of infection by 2 years of age (25). Zambia is a part of the “KS belt”, an area with endemic KS that has been severely impacted subsequently by the HIV epidemic, underscoring the need for epidemiologic studies to better understand patterns of HHV-8 transmission in young children. Although sexual transmission in adults and mucosal shedding of HHV-8 along with salivary exposure has been implicated in the transmission of HHV-8 in adults, there is little information about routes of transmission in children, and behavioral habits that could be potential risk factors and sources of transmission within the family.
Although other studies have examined childhood HHV-8 infection as a result of transmission between mother and child or between siblings (18, 26, 27), few have examined specific household behaviors that could contribute to virus transmission to a susceptible child within a household. The strengths of our study include utilization of a detailed questionnaire designed to assess behavioral habits not only engaged by the mother, but by household members and even the child’s interaction with neighborhood children. Ours is one of the first studies demonstrating an association between behavioral factors associated with saliva exchange. We observed that in the multivariable analysis a specific behavior i.e. the primary caregiver testing temperature of food prior to feeding was associated with HHV-8 prevalence, which potentially supports Butler et al’s findings in a previous study with an association with food sharing behaviors in children up to 14 years of age (20).
In the present study, we observed that presence and number of HHV-8 positive household members was consistently associated with childhood HHV-8 infection. This suggests person-to-person contact with HHV-8 positive members is likely to play a key role in transmission. These results have been further confirmed by a study completed in our lab utilizing molecular analysis of the K1 gene sequence data of KSHV-positive individuals from nine households in our cohort (28). Olp et al found that in six of the nine households, the child had 100% sequence identify to all household members, supporting that intra-household transmission occurs (28).
We observed that both the primary caregiver’s and the mother’s HHV-8 status were the most significant factors, most likely due to more frequent and close person-to-person contact. This contact likely occurs during food exchange behaviors, when possible saliva exchange occurs. But in this study, some inconsistancies exist in that none of the child rearing behaviors, including premastication, individually were associated with HHV-8 prevalence with the exception of the primary caregiver testing the temperature of the food before feeding the child. It is likely that for a HHV-8 transmission event to occur the amount of saliva and the amount of viral shedding at the time of the behavior may be key factors. Seroreversion, the fluctuation in antibody titers to undetectable levels, in adults is well documented, and could correlate to viral shedding and likelihood of transmission. Also, the frequency of occurrence of each behavior over time could be critical but this was not fully explored in this study. It is also likely that the behavioral habits analyzed in this study such as sharing drinks and foods, involve exposure to only minimal amount of saliva. Similarly, Butler et al’s (2011) paper, reporting only a marginal association of sharing food and/or sauce plates with HHV-8 infection in children could support the fact that the amount or frequency of saliva sharing could be a factor (20). Of interest, 9 HHV-8 positive index children did not have any family members that were HHV-8 positive. Because of seroreversion in adults, the influence of family members on childhood HHV-8 acquisition may be underestimated. Alternatively, HHV-8 exposures may come from contacts outside the household.
Breastfeeding as a protection against HHV-8 infection is a novel finding among herpesviruses. Breast milk has been implicated in mother to child transmission of several viruses, including cytomegalovirus and HIV-1 (29, 30). However, to our knowledge, this is the first report of breastfeeding as protection against childhood infection of HHV-8 or any herpesvirus. It is likely that there may be non-specific immune factors responsible for breast milk protection, including lactoferrin, complement components, or even commensal organisms. No correlation was found with maternal seropositivity and breastfeeding with childhood HHV-8. We feel that this may be due to seroreversion in adults, and a longitudinal study might better identify antibody presence in breast milk and serologically over time. Overall, our finding of the decreased risk of HHV-8 infection associated with breastfeeding is intriguing and warrants further studies to identify the protective factors that may be responsible.
HIV-1 infection has been documented to be a risk factor associated with HHV-8 infection. However, we did not analyze the association of HIV-1 with HHV-8 infection in the current study, because our subjects were selected to be HHV-8 negative as part of a larger longitudinal study with an aim to study HHV-8 incidence in early childhood. Our earlier cohort studies have clearly demonstrated that HIV infected children are at a significantly higher risk for acquiring HHV-8 infection (24). Therefore, during the enrollment of this cohort, in which HHV-8 negative children were being recruited, it is likely that HIV-1 positive children were under-represented because they were already HHV-8 infected.
A limitation of our study is that the behavioral questions were self-reported which could potentially result in recall bias. HIV-1 status of the household members was reported to the study subjects at study enrollment and counseling was done regarding transmission of HIV-1 and preventative health measures. However, since HHV-8 status was not known by the caregivers at the time of the study, we do not believe that this potential for bias was a significant factor.
In summary, we have demonstrated that the presence of HHV-8 in the household plays a key role in transmission of the virus to a susceptible child. This likely occurs through saliva sharing during behaviors such as testing the temperature before sharing to the child. This finding has implications for public health risk, and the need for education of families of potential transmission of the virus when sharing food with young children, and needed behavior changes. Although the current analysis supports the view that the primary caregiver poses the most significant risk of transmitting HHV-8 infection to a child, other results from the same cohort also demonstrate that HHV-8 transmission to a child can also occur from other household members and even from others outside of the household (28). Whether similar factors are also associated with HHV-8 incident infection in “at-risk” children is the current focus of this study. Longitudinally followed children in this cohort up to 48 months after enrollment will improve our understanding of the nature of interpersonal contact with a child and its role in HHV-8 transmission.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (PHS grant number RO1 CA75903); Fogarty International Training Grant (grant number D43 TW01492); T32 AI060547; and the National Institute for General Medical Science (NIGMS) Centers of Biomedical Research Excellence grant (grant number P30 GM103509) to CW. K.L.C. was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Allergy and Infectious Diseases and by NIGMS 8P20GM103427.
We would like to thank the field staff including nurses and community workers who helped with patient recruitment and follow-up. We thank Chafye Siuluta for his help in coordinating the study activities. We also thank all the study participants for their consent and willingness to participate in this study.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- 1.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, et al. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae [published erratum appears in J Virol 1996 Dec;70(12):9083] J Virol. 1996;70:549–58. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 4.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–8. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Bhat G, Kankasa C, Chintu C, Mitchell C, Duan W, et al. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi’s sarcoma (KS) and mother-child pairs with KS. J Infect Dis. 1998;178:1787–90. doi: 10.1086/314512. [DOI] [PubMed] [Google Scholar]
- 6.Newton R, Ziegler J, Bourboulia D, Casabonne D, Beral V, Mbidde E, et al. The sero-epidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in adults with cancer in Uganda. Int J Cancer. 2003:103. doi: 10.1002/ijc.10817. [DOI] [PubMed] [Google Scholar]
- 7.Campbell TB, Borok M, Ndemera B, Fiorillo S, White IE, Zhang XQ, et al. Lack of evidence for frequent heterosexual transmission of human herpesvirus 8 in Zimbabwe. Clin Infect Dis. 2009;48:1601–8. doi: 10.1086/598978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarmati L. HHV-8 infection in African children. Herpes. 2004;11:50–3. [PubMed] [Google Scholar]
- 9.Ziegler JL, Katongole-Mbidde E. Kaposi’s sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996:65. doi: 10.1002/(SICI)1097-0215(19960117)65:2<200::AID-IJC12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. BMJ. 1995;73:100–04. doi: 10.1136/adc.73.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutz W, Stout AP. Kaposi’s sarcoma in infants and children. Cancer. 1960:13. doi: 10.1002/1097-0142(196007/08)13:4<684::aid-cncr2820130408>3.0.co;2-g. [DOI] [PubMed]
- 12.Athale UH, Patil PS, Chintu C, Elem B. Influence of HIV epidemic on the incidence of Kaposi’s sarcoma in Zambian children. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:96–100. [PubMed] [Google Scholar]
- 13.Chitsike I, Siziya S. Seroprevalence of human immunodeficiency virus type 1 infection in childhood malignancy in Zimbabwe. Cent Afr J Med. 1998;44:242–5. [PubMed] [Google Scholar]
- 14.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. Lancet Oncol. 2008;9:683–92. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS. Global Report 2010: AIDSinfo. 2012 [Google Scholar]
- 16.Borges JD, Souza VA, Giambartolomei C, Dudbridge F, Freire WS, Gregorio SA, et al. Transmission of human herpesvirus type 8 infection within families in american indigenous populations from the brazilian Amazon. J Infect Dis. 2012;205:1869–76. doi: 10.1093/infdis/jis278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancuso R, Brambilla L, Agostini S, Biffi R, Hernis A, Guerini FR, et al. Intrafamiliar transmission of Kaposi’s sarcoma-associated herpesvirus and seronegative infection in family members of classic Kaposi’s sarcoma patients. J Gen Virol. 2011;92:744–51. doi: 10.1099/vir.0.027847-0. [DOI] [PubMed] [Google Scholar]
- 18.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–5. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 19.Brayfield BP, Kankasa C, West JT, Muyanga J, Bhat G, Klaskala W, et al. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J Infect Dis. 2004;189:2260–70. doi: 10.1086/421119. [DOI] [PubMed] [Google Scholar]
- 20.Butler LM, Were WA, Balinandi S, Downing R, Dollard S, Neilands TB, et al. Human herpesvirus 8 infection in children and adults in a population-based study in rural Uganda. J Infect Dis. 2011;203:625–34. doi: 10.1093/infdis/jiq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minhas V, Crabtree KL, Chao A, M’Soka TJ, Kankasa C, Bulterys M, et al. Early Childhood Infection by Human Herpesvirus 8 in Zambia and the Role of Human Immunodeficiency Virus Type 1 Coinfection in a Highly Endemic Area. Am J Epidemiol. 2008;168:311–20. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minhas V, Crabtree KL, Chao A, Wojcicki JM, Sifuniso AM, Nkonde C, et al. The Zambia Children’s KS-HHV8 Study: rationale, study design, and study methods. Am J Epidemiol. 2011;173:1085–92. doi: 10.1093/aje/kwq465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojcicki JM, Kankasa C, Mitchell C, Wood C. Traditional practices and exposure to bodily fluids in Lusaka, Zambia. Trop Med Int Health. 2007;12:150. doi: 10.1111/j.1365-3156.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- 24.Minhas V, Crabtree KL, Chao A, M’Soka TJ, Kankasa C, Bulterys M, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol. 2008;168:311–20. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler LM, Dorsey G, Hladik W, Rosenthal PJ, Brander C, Neilands TB, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis. 2009;200:430–8. doi: 10.1086/600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malope BI, Pfeiffer RM, Mbisa G, Stein L, Ratshikhopha EM, O’Connell DL, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–5. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 27.Dedicoat M, Newton R, Alkharsah KR, Sheldon J, Szabados I, Ndlovu B, et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–75. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 28.Olp LN, Shea DM, White MK, Gondwe C, Kankasa C, Wood C. Early childhood infection of Kaposi’s sarcoma-associated herpesvirus in Zambian households: A molecular analysis. Int J Cancer. 2012 doi: 10.1002/ijc.27729. [DOI] [PMC free article] [PubMed]
- 29.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–8. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 30.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–8. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


