Abstract
Background
Although COPD is a major cause of disability worldwide, its determinants remain poorly defined.
Objective
We hypothesized that both pulmonary and extra-pulmonary factors would predict prospective disablement across a hierarchy of activities in persons with COPD.
Methods
609 participants were studied at baseline (T0) and 2.5 years later (T1). The Valued Life Activities (VLA) scale quantified disability (10-point scale; 0=no difficulty, 10=unable to perform), defining disability as any activity newly rated “unable to perform” at T1. Predictors included pulmonary (lung function, six-minute walk distance, and COPD severity score) and extra-pulmonary (quadriceps strength, lower extremity function) factors. Prospective disability risk was tested by separate logistic regression models for each predictor (baseline value and its change, T0 to T2; odds ratios were scaled at 1 standard deviation per factor. Incident disability across a hierarchy of obligatory, committed, and discretionary VLA subscales was compared.
Results
Subjects manifested a 40% or greater increased odds of developing disability for each predictor (baseline and change over time). Disability in discretionary activities developed at a rate 2.2-times higher than observed in committed activities, which was in turn, 2.5-times higher than the rate observed in obligatory activities (p<0.05 for each level).
Conclusions
Disability is common in COPD. Both pulmonary and extra-pulmonary factors are important in predicting its development.
Keywords: Chronic obstructive pulmonary disease, disability, exercise capacity, functional limitation, peripheral muscle weakness
INTRODUCTION
Disability among working-aged adults is a critical, yet under-studied health outcome that has been identified as a priority for further research.1 Chronic obstructive pulmonary disease (COPD) currently ranks within the top five causes of disability among working-aged adults in the United States and, by 2020, is projected to rank fifth wordwide.2, 3 Indeed, persons with COPD have a 10-fold greater risk of disability than the general population.4 Despite its importance, however, the pathways leading to COPD-related disability remain poorly characterized.
COPD is particularly relevant to the disablement process because it manifests as a systemic disease with both pulmonary and extra-pulmonary features.5 These manifestations include elevated biomarkers of systemic inflammation,6 poorer muscle function,7 and frailty8. Moreover, persons with COPD experience a myriad of co-morbidities, including atherosclerosis,9 depression,10 and osteoporosis11. To date, disability in COPD has been predominantly studied from the narrow perspective of activities necessary for survival or basic functioning such as (instrumental) activities of daily living ([I]ADLs).12–16 The inability to perform such activities, however, typically develops late, in relatively advanced disease. Not only does this narrow construct of disability underestimate the burden of COPD-related morbidity, but it also provides little insight into earlier stages of disablement that might be more amenable to intervention.
Nagi advanced disability research by proposing a conceptual model of disablement that was modified by Verbrugge and that has since been adopted widely.17, 18 This model proposes that disability begins with alterations in function of a body organ affected by disease resulting in impairment. Impairment brings about reductions in physical or mental actions conceptualized as functional limitations. Functional limitations, in turn, lead to disability across a hierarchy of activity levels. Findings from our previous work analyzing disablement in COPD have been consistent with this model.19
We conducted a prospective longitudinal study of working-aged adults with COPD to characterize the development of disability. We aimed to determine whether changes over time in pulmonary and extra-pulmonary impairment and functional limitations predicted the prospective development of disability. We further aimed to discriminate the development of such disability across a hierarchy of activity domains: obligatory activities that are required for survival and independence (e.g., [I]ADLs); committed activities that define one’s principal social roles (e.g., working for pay or caring for family); and discretionary activities (e.g., involvement in hobbies, socializing or travel).
METHODS
Subjects and design
We used data from the Function, Living, Outcomes, and Work (FLOW) study, an ongoing prospective longitudinal cohort study of working-aged adults (40–65 years at baseline) recruited from an integrated health care delivery system. The FLOW cohort consists of 1,202 Kaiser Permanente Medical Care Program (KPMCP) members with COPD recruited using a validated algorithm based both on recent health-care utilization linked to a COPD diagnostic code and pharmacy dispensing for COPD-related medications; recruitment methods have been described previously.20 At baseline study Wave 1 (T0), we conducted structured telephone interviews that ascertained sociodemographic characteristics, COPD clinical history, and health status. We also conducted a study clinic visit to perform spirometry and other physical assessments. Approximately 2.5 years later (T1), we successfully conducted Wave 2 follow-up interviews on 1,051 (90%) of those studied at baseline (Figure 1). After exclusions for ineligibility for or inability to follow-up with clinic visits, we performed repeat clinic visits on 677 (69%) of 987 participants. For this study, we excluded 68 subjects (10%) because of unacceptable spirometric data at either T0 or T1.
Figure 1.
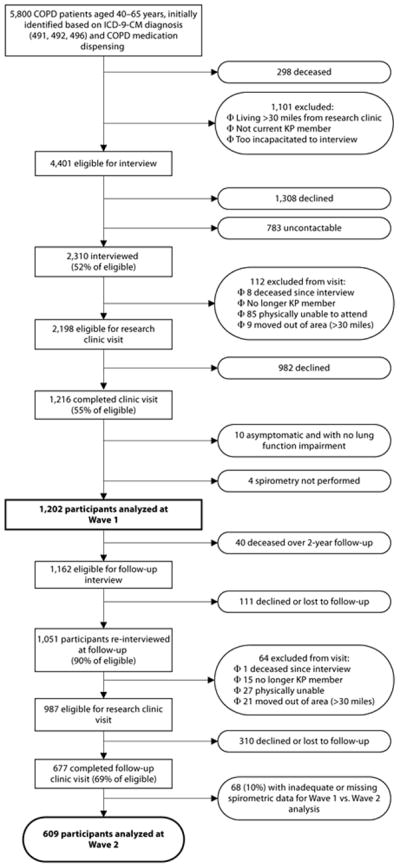
FLOW Study Recruitment and Retention
At the time of baseline assessments, we recruited 302 age- and gender-matched referents who were KPMCP members without a COPD diagnosis or obstruction on spirometry. We used these referent data to derive normative values for quadriceps strength,8 but did not include them otherwise in the analyses presented herein.
Protocols were approved by the UC San Francisco Committee on Human Research and the Kaiser Foundation Research Institute institutional review board.
Independent Predictor Variables
Respiratory impairment
Pulmonary function
We assessed respiratory function by spirometry according to American Thoracic Society (ATS) Guidelines.21 Spirometry was performed with the EasyOne™ Frontline spirometer (ndd Medical Technologies, Chelmsford, MA). We applied FEV1 % predicted values from the regression equations developed from the National Health and Nutrition Examination Survey III.22
COPD Severity Score
The COPD Severity Score is a novel, validated survey-based disease severity instrument that does not require physiologic measures of respiratory function or exercise capacity.23 This feature makes it useful for epidemiologic studies and telephone administration. The COPD Severity Score is based on items spanning five domains: (1) severity of respiratory symptoms, (2) prior use of systemic corticosteroids (3) use of other COPD medications, (4) previous hospitalization or intubation for respiratory causes, and (5) use of long term oxygen therapy. The COPD Severity Score ranges from 0–35; higher scores reflect greater disease severity and correlate with FEV1, BODE Index, exercise capacity, and health-related quality of life.24, 25
Non-respiratory impairment
Exercise capacity
Exercise capacity was measured using the Six-Minute Walk Test (6MWT).26 We used a standardized flat, straight course of 30 meters in accordance with American Thoracic Society guidelines. Every two minutes, a technician used standardized phrases to encourage effort.
Quadriceps strength
Decreased quadriceps strength is associated with poorer exercise capacity and lower extremity functioning across a spectrum of COPD severity.8, 27 Isometric quadriceps strength was assessed by standard manual muscle testing procedures using a hand-held dynamometer (MicroFet2 dynamometer, Saemmons Preston, Bolingbrook, IL).28 Examiners trained in manual muscle testing by the same experienced physical therapist practiced testing until there was agreement between the raters 90% of the time within 2.3 kilograms of force. We focused on quadriceps strength because these muscles are considered essential for walking and previous work has suggested the importance of quadriceps weakness as a predictor of reduced exercise capacity in COPD.29
Lower extremity functioning
Lower extremity function was quantified using the validated Short Physical Performance Battery (SPPB).30 Poorer SPPB performance is predictive of incident disability, institutionalization, and mortality in older persons, independent of co-morbidity or socioeconomic factors.30, 31 This battery includes 3 performance measures of balance, chair stands and a 4-meter walk, each scored from 0 to 4 points. A summary score ranges from 0 to 12.
Outcome Variables
Disability
Disability was measured by the Valued Life Activities disability scale (VLA).32 The VLA scale makes operational the broad conceptual hierarchy of disability proposed by Verbrugge.18 Originally developed in rheumatoid arthritis, the VLA scale measures complex functioning in daily life. Subsequently, it has been validated in asthma and COPD.33 Comprised originally of 32-items, refinements over the past decade have resulted in shorter scales. For this study, a 22-item scale was employed; respondents rate on a 10-point scale how difficult activity performance is across 22 obligatory, committed, and discretionary domains because of their breathing problems (0=no difficulty, 10=unable to perform the activity). The VLA scale was administered at T0 and T1 and change scores were derived. Incident disability was defined in two ways: (1) a new rating of “unable” in any activity domain from T0 to T1 or, (2) a ½ standard deviation increase in the mean difficulty rating across all rated items, which we defined as a “meaningful change” in mean disability consistent with prior definitions.33 We evaluated the overall scale in this manner as well as within the hierarchy of obligatory, committed, and discretionary subscales.
Other Covariates
We included variables that might confound the relationships between the predictor and outcomes measures of interest. These included sociodemographic characteristics (age, sex, and race), as well as cigarette smoking history using questions refined from the National Health Interview Survey and second-hand smoke exposure using items we originally developed.34, 35
Statistical analysis
Categorical variables were analyzed with the 2 test. Continuous variables were analyzed with the students t-test (by follow-up status) or the paired t-test (for change T0 to T1). We examined the impact of baseline (T0) and change (T0 toT1) in 5 respiratory and non-respiratory predictors on the prospective risk of VLA disability. Predictors, including FEV1, 6MWT, COPD Severity Score, quadriceps strength and SPPB were tested in separate multivariable logistic regression models that included the baseline value of the predictor as well as its change over time. We tested the impact of each predictor on the two definitions of VLA disability. Odds ratios were expressed per Z unit (1 standard deviation) change in each predictor. Each model was tested for two prospective VLA outcomes: incident disability and a meaningful (0.5 SD) increase in mean difficulty rating. All models included gender, age (continuous variable), race (categorized as White/non-Hispanic [referent], Black, or all others), BMI (continuous variable), change in BMI from T0 to T1, smoking (packs per day), and second-hand smoke exposure (hours per week). Since they were the most consistent predictors of VLA disability, we used multivariable logistic regression to test the impact of 6MWT and COPD Severity Score on the risk of disability in the obligatory (e.g., ADLs), committed (e.g., working for pay) and discretionary (e.g., socializing or travel) VLA subscales controlling for gender, age, BMI, race, smoking status, and second-hand smoke exposure. In sensitivity analyses, we defined BMI dichotomously as obese (BMI>30) versus not and change in BMI categorically as a ≥10% gain, ≥10% loss, or other (referent]). We also repeated analyses replacing the baseline value of each predictor with its average between T0 and T1.
Lastly, we hypothesized that discretionary activities would be more vulnerable to the development of incident disability than committed activities and, similarly, committed activities would be more vulnerable to disablement than obligatory activities. We compared the rates of disability in each activity domain as a ratio of a Poisson variable to its expected value based on the denominator rate.36
Analysis was conducted using STATA/ICv11.2 (StataCorp, College Station, TX).
RESULTS
Among 609 study participants analyzed (Table 1), mean age was 59.3±6.1 years, 367 (60%) were female, and mean baseline FEV1 was 1.79±0.74 liters (64%±23 predicted). Most subjects (85%) were either current or former smokers. Mean time between study visits was 2.4±0.5 years. Compared to subjects included in the analysis, re-interviewed subjects without follow-up research clinic data (n=310) were more likely (p<0.05) to be current smokers and have lower baseline 6MWT distances, but did not otherwise differ by any of the other variables shown in Table 1 (data not shown).
Table 1.
Baseline subject characteristics of the FLOW cohort study (N=609)
| Subject Characteristics | n (%) or Mean ± SD |
|---|---|
| Age in years | 59.3 ± 6.1 |
| Female sex | 367 (60) |
| Body mass index | 31.8 ± 8.3 |
| Race / ethnicity | |
| White, non-Hispanic | 421 (69) |
| Black | 105 (17) |
| Other | 83 (14) |
| Cigarette Smoking (packs per day) | 0.85 ± 0.35 |
| Second hand smoke exposure (hours per week) | 1.10 ± 5.02 |
| Pulmonary Function | |
| FEV1 in liters | 1.79 ± 0.74 |
| FEV1 % predicted | 64 ± 23 |
| FEV1/FVC | 0.61 ± 0.15 |
| Six minute walk test, in meters | 412 ± 117 |
| Skeletal muscle strength | |
| Quadriceps (kilograms of force) | 27.1 ± 9.3 |
| Quadriceps % predicted† | 84.4 ± 25.6 |
| Short Physical Performance Battery | 10.6 ± 1.8 |
| COPD Severity Score | 10.1 ± 6.0 |
FEV1 % predicted values derived directly from the linear regression equations developed from the National Health and Nutrition Examination Survey (NHANES III).22
Muscle strength % predicted values generated from 302 age and sex matched control subjects without COPD employing linear regression controlling for age, gender, body mass index (BMI), and height.8
Changes in the independent predictors from T0 to T1 are presented in Table 2. FEV1 and FEV1% predicted declined by 0.10±0.25L and 1.9±8.7%, respectively (both p<0.0001). These declines, however, were not consistently observed. Over the follow-up period, 40% of subjects manifested essentially stable lung function.
Table 2.
Change in Pulmonary Physiology, Exercise Capacity, Muscle Strength, lower extremity functioning, COPD Severity Score, and BMI from baseline to follow-up (N=609)
| Subject Characteristics | mean ± SD | p-value |
|---|---|---|
| FEV1 in liters | −0.10 ± 0.25 | <0.0001 |
| FEV1 % predicted | −1.9 ± 8.7 | <0.0001 |
| Six minute walk distance, in meters | −36.1 ± 84.1 | <0.0001 |
| Quadriceps (kilograms of force) | 0.8 ± 7.5 | 0.01 |
| Quadriceps % predicted† | 1.8 ± 23.0 | 0.01 |
| Short Physical Performance Battery | 0.1 ± 1.5 | 0.02 |
| COPD Severity Score | 0.1 ± 4.7 | 0.70 |
| BMI | −0.2 ± 3.2 | 0.15 |
FEV1 % predicted values derived directly from the linear regression equations developed from the National Health and Nutrition Examination Survey (NHANES III).22
Muscle strength % predicted values generated from 302 age and sex matched control subjects without COPD employing linear regression controlling for age, gender, body mass index (BMI), and height.8
Strong, consistent associations were identified between each physical performance measure (FEV1, 6MWT, quadriceps strength, and SPPB) and the development of incident disability, defined as any VLA activity newly reported as “unable to perform” (Table 3). These predictive associations were observed for both baseline measures as well as their change over time. Odds ratios (ORs) for incident disability per standard deviation (SD) decrement in each performance measure were ≥1.43 (95%CI ranges:1.00–3.75; p-values<0.04). Similarly, for each SD decrement in baseline COPD Severity Score as well as change in the COPD Severity Score over time, subjects had a 2.19 (95%CI: 1.65–2.89) and 1.94 (95%CI: 1.45–2.58) increased odds of developing incident disability, respectively (p-values<0.01).
Table 3.
Impact of change in characteristics on the development of incident disability in Valued Life Activities (VLA).
| Incident Disability in Valued Life Activities (VLA)
| ||||
|---|---|---|---|---|
| Characteristic | VLA, Newly Unable to Perform* n = 55/609 (9%) |
Mean VLA Rating, Meaningful Increase* n = 91/619 (15%) |
||
|
| ||||
| OR (95% CI)** | p-value | OR (95% CI)** | p-value | |
| Baseline FEV1 | 1.76 (1.21 – 2.56) | <0.01 | 1.11 (0.84 – 1.45) | 0.48 |
|
| ||||
| Decrement in FEV1† | 1.57 (1.15 – 2.15) | <0.01 | 1.58 (1.24 – 2.00) | <0.01 |
|
| ||||
| Baseline 6MWT | 2.65 (1.87 – 3.75) | <0.01 | 1.46 (1.10 – 1.92) | <0.01 |
|
| ||||
| Decrement in 6MWT‡ | 1.43 (1.10 – 1.85) | <0.01 | 1.37 (1.11 – 1.69) | <0.01 |
|
| ||||
| Baseline quadriceps strength | 1.75 (1.18 – 2.58) | <0.01 | 1.08 (0.80 – 1.46) | 0.63 |
|
| ||||
| Decrement in quadriceps strength | 1.39 (1.00 – 1.95) | 0.05 | 1.09 (0.85 – 1.42) | 0.49 |
|
| ||||
| Baseline SPPB | 1.85 (1.42 – 2.41) | <0.01 | 1.32 (1.05 – 1.66) | 0.02 |
|
| ||||
| Decrement in SPPB†† | 1.43 (1.11 – 1.84) | <0.01 | 1.14 (0.91 – 1.41) | 0.26 |
|
| ||||
| Baseline COPD Severity Score | 2.19 (1.65 – 2.90) | <0.01 | 1.37 (1.09 – 1.73) | <0.01 |
| Increase in COPD Severity Score | 1.94 (1.45 – 2.59) | <0.01 | 1.37 (1.09 – 1.72) | <0.01 |
FEV1 = forced expiratory volume in one second;
6MWT = Six minute walk distance;
SPPB = Short Physical Performance Battery
Newly unable to perform defined as a new rating of “unable” in any activity domain from T0 to T1; a Meaningful increase defined as a ½ standard deviation increase in the mean difficulty in rated items from T0 to T1
Odds ratios expressed per standardized per Z unit (one standard deviation) decrement in FEV1, 6MWT, Quadriceps Strength or SPPB or increase in COPD Severity Score. FEV1, 6MWT, COPD Severity Score, quadriceps strength and SPPB were tested in separate multivariable logistic regression models that included the baseline value of the predictor as well as its change over time. All models also include gender, age, body mass index, change in body mass index, race, smoking status, and second-hand smoke exposure.
For VLA disability defined alternatively as a meaningful increase (½SD) in the mean difficulty rating across activities, predictive associations of the 6MWT and COPD Severity Score with VLA disability remained strong, but were less consistent for FEV1 and SPPB (Table 3). Moreover, quadriceps strength did not predict new disability by this definition. Overall, point estimates for the ORs for incident disability were lower when disability was defined as a meaningful increase in mean difficulty compared to previous analyses based on the new rating of “unable” in any activity domain.
We next examined the impact of the COPD Severity Score and 6MWT, the two most consistent predictors of overall VLA disability, on the development of disability in the obligatory, committed, and discretionary VLA subscales (Table 4). Both baseline COPD Severity Score and change in the COPD Severity Score over time were consistently predictive of incident disability across all subscales: the ORs for incident disability across scales per SD decrement in the COPD Severity Score were all ≥1.90 (p-values ≤0.01). Additionally, baseline 6MWT predicted incident disability across all VLA subscales with estimated ORs of ≥2.6 per SD decrement in 6MWT (p-values <0.01). Change in 6MWT, however, only predicted incident disability in the discretionary subscale.
Table 4.
Impact of change in 6MWT and COPD Severity Score on the development of incident disability in subcategories of Valued Life Activities (VLA).
| Incident Disability in newly unable to perform Valued Life Activities (VLA) by VLA Subcategory* | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Obligatory n = 11/609 (2%) |
Committed n = 27/609 (4%) |
Discretionary n = 60/609 (10%) |
|||
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI)* | p-value | |
| Change in 6MWT‡ | 1.12 (0.66 – 1.94) | 0.66 | 1.38 (0.96 – 1.96) | 0.08 | 1.35 (1.04 – 1.75) | 0.02 |
| Baseline 6MWT | 2.68 (1.37 – 5.23) | <0.01 | 2.86 (1.80 – 4.54) | <0.01 | 2.92 (2.08 – 4.11) | <0.01 |
| Change in COPD Severity Score | 2.06 (1.16 – 3.67) | 0.01 | 2.25 (1.51 – 3.36) | <0.01 | 1.92 (1.45 – 2.55) | <0.01 |
| Baseline COPD Severity Score | 2.02 (1.17 – 3.50) | 0.01 | 1.98 (1.36 – 2.88) | <0.01 | 2.44 (1.84 – 3.23) | <0.01 |
Newly unable to perform defined as a new rating of “unable” in any activity domain from T0 to T1
Odds ratios expressed per standardized per Z unit (one standard deviation range) decrement in 6MWT or increase in COPD Severity Score. 6MWT and COPD Severity Score were tested in separate multivariable logistic regression models that included the baseline value of the predictor as well as its change over time. All models also include gender, age, body mass index, change in body mass index, race, smoking status, and second-hand smoke exposure.
6MWT = Six minute walk distance.
All models also include gender, age, body mass index, change in body mass index, race, smoking status, and second-hand smoke exposure.
Eleven subjects (2%) developed incident disability in the obligatory subscale, 27 (4%) developed disability in the committed subscale, and 60 (10%) developed disability in the discretionary subscale. Disability in committed activities was 2.5 times more likely than obligatory activities disability, taking that as the expected rate (95% CI: 1.27–4.54). Further, disability in discretionary activities was 2.2 times more likely to develop than in committed activities (95% CI: 1.5–3.2).
The results of the sensitivity analyses including alternative definitions of BMI and in other analyses replacing T0 predictor variables with the mean of T0 to T2 were not substantively different from the results presented (data not shown).
DISCUSSION
We found that, in working-aged adults with COPD, greater impairments and poorer pulmonary and extra-pulmonary functioning predicted the development of incident disability. Although spirometic lung function was predictive of disability, so too was 6MWT and an integrative COPD Severity Score that does not require either lung function or exercise testing. Moreover, measures of extra-pulmonary impairment (quadriceps strength) and function (SPPB) also predicted incident disability. Finally, within a hierarchy of activities, those considered discretionary were the most vulnerable to the development of disability and manifested the most consistent relationship with both baseline and change in the independent predictors studied. Notably, discretionary activities are those least commonly assessed in traditional measures of ADL functioning.
These findings offer important insights into the COPD disablement process. Not only is COPD a respiratory disease, it is also a systemic process with effects on body systems distant from the lungs. Our study provides prospective epidemiological evidence that these effects on extra-pulmonary body systems predict the development of disability in patients with COPD. It is likely, therefore, that interventions aimed exclusively at improving pulmonary function are unlikely to fully mitigate COPD-related disablement.
We also identified a gradient in the development of disability that is similarly relevant to preventive strategies. Discretionary activities appear to represent a particularly vulnerable and “sensitive” measure of the impact of COPD on disability. Over a follow-up period of only 2.5 years, 10% of subjects developed disability in discretionary activities. Moreover, this risk of disablement was five-fold higher than the risk observed in the obligatory category, a category that subsumes [I]ADLs. Additionally, in COPD, the disability in discretionary activities is strongly associated with the development of depression.33 Thus, narrowly defining disability as (I)ADLs substantially underestimates the burden of COPD on daily life.19 Thus, interventions aimed at COPD disability prevention should measure disability broadly across a spectrum of activities considered important to patients.
Our study builds upon previous work to advance the understanding of the disablement process in COPD. Indeed, the growing appreciation of COPD as a systemic disease process is reflected in our study; we systematically quantified the impact on COPD-related disability of both pulmonary and extra-pulmonary body systems at baseline and over time. Further, most longitudinal studies of disability in COPD have focused on advanced disease37–39 for which interventions to prevent disablement may be less effective, the elderly40 or hospitalized subjects37, 41, or on [I]ADLs39, 40. By studying longitudinally a working-aged population with a wide range of disease severity, our findings are particularly relevant to ambulatory COPD populations at early risk for disability. Further, most previous studies of COPD have defined disability based on [I]ADLs. Although widely used to study disability in debilitated populations, [I]ADLs have limited utility in ambulatory populations because of a “floor” effect in which most subjects score rather well and do not appear to change over time. By defining disability across a broad range of activities, we identified a heretofore unobserved gradient in the prospective development of disability. Finally, we demonstrated that the COPD Severity Score, a method of disease severity assessment that does not require measuring pulmonary function, is as strong a predictor of disability as lab-based measures of pulmonary and extra-pulmonary functioning. This may be useful for epidemiological studies aiming to risk-adjust for disease severity or identify subjects at higher risk of developing disability.
Our study also faces limitations. Of the 1051 subjects re-interviewed, 69% completed follow-up clinic visits. Of these, 10% were excluded from this analysis due to inadequate/missing spirometry data. It is possible death, refusal to continue study participation, or loss to follow-up may have introduced selection bias. The 310 subjects who did decline a follow-up visit were more likely to be current smokers and had worse exercise capacity. Thus, it is likely that any selection bias introduced would have resulted in an underestimation of disability risk. Further, our method of ascertaining a COPD diagnosis may have resulted in misclassification, although our algorithm required utilization of COPD services, concomitant treatment with COPD medications, and a physician diagnosis of COPD and was validated against a sample chart review.20 Additionally, the primary aim of this longitudinal study is to identify predictors of COPD-related disability. Driving this aim, subject recruitment was limited to working-aged adults. Thus, while our findings are particularly applicable this population, our results may not be generalizable to older patients. Finally, there was, on average, little change in lung function over the observation period even though within the group there were some who declined rapidly; this appears to be consistent, however, with the heterogeneous natural history of COPD.42 Despite these potential limitations, we identified factors that predict the development of disability over a relatively short period of time in an ambulatory COPD population and across a broad range of activities.
In summary, decrements in lung function as well as body-systems distant from the lungs are important predictors of the development and progression of COPD-related disablement. Further, we delineated a hierarchy of disablement in which discretionary activities are most vulnerable. Our findings suggest that interventions designed to prevent disability in COPD should comprehensively target both pulmonary and extra-pulmonary factors and should be initiated at the time disability appears in discretionary activities.
Acknowledgments
Funded by: National Heart, Lung, and Blood Institute / National Institutes of Health R01HL077618, K24 HL 097245, F32 HL107003-01; Flight Attendant Medical Research Institute (FAMRI Bland Lane Center of Excellence on Secondhand Smoke); and the University of California Tobacco-Related Diseases Research Program (17RT-0101). A portion of this study was also supported by the National Center for Research Resources at the National Institutes of Health (UCSF-CTSI UL1 RR024131)
Footnotes
Work was conducted at Kaiser Permanente Division of Research and UC San Francisco
References
- 1.Iglehart JK. Prioritizing comparative-effectiveness research--IOM recommendations. N Engl J Med. 2009;361:325–8. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 2.Verbrugge LM, Patrick DL. Seven chronic conditions: their impact on US adults’ activity levels and use of medical services. Am J Public Health. 1995;85:173–82. doi: 10.2105/ajph.85.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Eisner MD, Yelin EH, Trupin L, Blanc PD. The influence of chronic respiratory conditions on health status and work disability. Am J Public Health. 2002;92:1506–13. doi: 10.2105/ajph.92.9.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121:789–96. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159:S1–40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 8.Singer J, Yelin EH, Katz PP, et al. Respiratory and skeletal muscle strength in chronic obstructive pulmonary disease: impact on exercise capacity and lower extremity function. J Cardiopulm Rehabil Prev. 2011;31:111–9. doi: 10.1097/HCR.0b013e3182033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speizer FE, Fay ME, Dockery DW, Ferris BG., Jr Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140:S49–55. doi: 10.1164/ajrccm/140.3_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 10.Antoniu SA. Predictors of depression in chronic obstructive pulmonary disease patients. Expert Rev Respir Med. 2011;5:333–5. doi: 10.1586/ers.11.30. [DOI] [PubMed] [Google Scholar]
- 11.Ionescu AA, Schoon E. Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:64s–75s. doi: 10.1183/09031936.03.00004609. [DOI] [PubMed] [Google Scholar]
- 12.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez Gonzalez-Moro JM, de Lucas Ramos P, Izquierdo Alonso JL, et al. Impact of COPD severity on physical disability and daily living activities: EDIP-EPOC I and EDIP-EPOC II studies. Int J Clin Pract. 2009;63:742–50. doi: 10.1111/j.1742-1241.2009.02040.x. [DOI] [PubMed] [Google Scholar]
- 14.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–7. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 15.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–72. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 16.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177:743–51. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 17.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–67. [PubMed] [Google Scholar]
- 18.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 19.Katz PP, Gregorich S, Eisner M, et al. Disability in valued life activities among individuals with COPD and other respiratory conditions. J Cardiopulm Rehabil Prev. 2011;30:126–36. doi: 10.1097/HCR.0b013e3181be7e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 21.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Eisner MD, Trupin L, Katz PP, et al. Development and validation of a survey-based COPD severity score. Chest. 2005;127:1890–7. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- 24.Eisner MD, Omachi TA, Katz PP, Yelin EH, Iribarren C, Blanc PD. Measurement of COPD severity using a survey-based score: validation in a clinically and physiologically characterized cohort. Chest. 2010;137:846–51. doi: 10.1378/chest.09-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omachi TA, Yelin EH, Katz PP, Blanc PD, Eisner MD. The COPD severity score: a dynamic prediction tool for health-care utilization. COPD. 2008;5:339–46. doi: 10.1080/15412550802522700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Coronell C, Orozco-Levi M, Mendez R, Ramirez-Sarmiento A, Galdiz JB, Gea J. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J. 2004;24:129–36. doi: 10.1183/09031936.04.00079603. [DOI] [PubMed] [Google Scholar]
- 28.Kendall F, McReary E, Provance P. Muscles: Tsting and Function. 4. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- 29.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–80. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 32.Katz PP, Yelin EH. Life activities of persons with rheumatoid arthritis with and without depressive symptoms. Arthritis Care Res. 1994;7:69–77. doi: 10.1002/art.1790070205. [DOI] [PubMed] [Google Scholar]
- 33.Katz PP, Gregorich S, Eisner M, et al. Disability in valued life activities among individuals with COPD and other respiratory conditions. J Cardiopulm Rehabil Prev. 2010;30:126–36. doi: 10.1097/HCR.0b013e3181be7e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cigarette smoking among adults--United States, 1997. MMWR Morb Mortal Wkly Rep. 1999;48:993–6. [PubMed] [Google Scholar]
- 35.Eisner MD, Katz PP, Yelin EH, Hammond SK, Blanc PD. Measurement of environmental tobacco smoke exposure among adults with asthma. Environ Health Perspect. 2001;109:809–14. doi: 10.1289/ehp.01109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailar JC, Ederer F. 202. Note: Significance Factors for the Ratio of a Poisson Variable to Its Expectation. Biometrics. 1964;20:639–43. [Google Scholar]
- 37.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–67. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 38.Graydon JE, Ross E, Webster PM, Goldstein RS, Avendano M. Predictors of functioning of patients with chronic obstructive pulmonary disease. Heart Lung. 1995;24:369–75. doi: 10.1016/s0147-9563(05)80057-3. [DOI] [PubMed] [Google Scholar]
- 39.Okubadejo AA, O’Shea L, Jones PW, Wedzicha JA. Home assessment of activities of daily living in patients with severe chronic obstructive pulmonary disease on long-term oxygen therapy. Eur Respir J. 1997;10:1572–5. doi: 10.1183/09031936.97.10071572. [DOI] [PubMed] [Google Scholar]
- 40.Isoaho R, Puolijoki H, Huhti E, Laippala P, Kivela SL. Chronic obstructive pulmonary disease and self-maintaining functions in the elderly--a population-based study. Scand J Prim Health Care. 1995;13:122–7. doi: 10.3109/02813439508996748. [DOI] [PubMed] [Google Scholar]
- 41.Peach H, Pathy MS. Follow-up study of disability among elderly patients discharged from hospital with exacerbations of chronic bronchitis. Thorax. 1981;36:585–9. doi: 10.1136/thx.36.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casanova C, de Torres JP, Aguirre-Jaime A, et al. The Progression of Chronic Obstructive Pulmonary Disease is Heterogeneous: The Experience of the BODE Cohort. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]


