Abstract
Innate immune system has been known to play an important role in inhibiting the malignant transformation, tumor progression and invasion. However, the mechanistic basis remains ambiguous. Despite polyclonality of human γδ T cells, Vγ2Vδ2 T cell subset was shown to recognize and limit the growth of various tumors at various degrees. The differential recognition of the tumor cells by Vγ2Vδ2 T cells are yet to be defined. Our study reveals that γδ T cells limit in vitro growth of most breast tumor cells, such as SkBr7 (HER2+), MCF7 (ER+) and MDA-MB-231 (ER−) by inhibiting their survival and inducing apoptosis, except BrCa-MZ01 (PR+) cells. To investigate detail mechanisms of antineoplastic effects, we found that cell death was associated with the surface expression levels of MICA/B and ICAM1. Molecular signaling analysis demonstrated that inhibition of cell growth by γδ T cells was associated with the lower expression levels of cell survival-related molecules such as AKT, ERK and concomitant upregulation of apoptosis-related molecules, such as PARP, cleaved caspase 3 and tumor suppressor genes PTEN and P53. However, opposite molecular signaling was observed in the resistant cell line after coculture with γδ T cells. In vivo, antineoplastic effects of γδ T cells were also documented, where tumor growth was inhibited due to the downregulation of survival signals, strong induction of apoptotic molecules, disruption of microvasculature and increased infiltration of tumor associated macrophages. These findings reveal that a complex molecular signaling is involved in γδ T cell-mediated antineoplastic effects.
Keywords: Vγ2Vδ2 T cell, breast cancer, cell survival, apoptosis, angiogenesis, xenotransplant, NOD/SCID mice
Breast cancer is most frequently diagnosed cancer among women and leading cause of death in women worldwide.1 It is heterogeneous in nature categorized by origin (luminal or basal), and by hormonal receptor status of mammary tissue (negative or positive for the expression of receptors, usually estrogen receptor (ER) or progesterone receptor (PR) or human epidermal growth factor (HER2/ErbB/neu)). Breast tumors expressing ER were shown to have good clinical prognosis compared to ER−/PR+/Her2+ or triple negative tumors, which show poor prognosis.2,3
Observations from breast tumor biopsies have shown that innate immune system plays a critical role in breast tumor biology. Most of the human peripheral blood T cells express T cell receptor (TCR) αβ but only 1–5% express TCR γδ receptors,4 and infiltration of γδ T cells were found in breast tumor sites.5,6 Majority of the peripheral blood γδ T cells express Vγ2Vδ2 TCRs, mediate antitumor activity without MHC-or CD1-dependent antigen presentations.7,8 Vγ2Vδ2 T cells also express NKG2D receptor that exert cytolytic effect via interaction with stress-induced MHC class-I A/B (MICA/B) ligands expressed on tumor cells. Higher MICA expression was linked to the poor prognosis in breast cancer patients while other tissue carcinomas seemed to have no correlation.9,10 It was reported that γδ T cells within the epithelia contributed to downregulation of epithelial malignancies. The existence αβ T cells promoted the development of cutaneous tumors, and after elimination of αβ T cells, γδ T cells-mediated cytotoxicity was enhanced. Juxtaposition of γδ T cells with the tumor cells was critical in enhancing their cytototoxic effects.11 Furthermore, presence of γδ T cells was correlated with the disease-free survival.12
Clinical trial showed that patients treated with adoptive transfer of γδ T cells in combination with IL-2 had delayed cancer growth; however, molecular mechanisms are yet to be defined.13 Therefore, it is important to understand the mechanistic basis of γδ T cells interaction with breast tumor cells to develop newer therapy with increased efficacy. We reported earlier that γδ T cells interact with ovarian cells and limit their growth in vitro via inhibition of MICA-dependent survival pathways. We showed that activation of MICA renders the resistant cells susceptible to cytotoxic lysis with downregulation of ERK signaling pathways and upregulation of apoptotic gene expression.8 However, if similar molecular pathways are involved in γδ T cells-mediated apoptosis of breast cancer cells is not yet known.
Immune system plays a significant role in repression of neoplasms, which involves interplay of various chemokines within the tumor.14,15 Macrophages play a pleiotropic role by producing pro- and anti-inflammatory cytokines. It is well known that tumor associated macrophages (TAM) exert immunosuppressive effects by synthesizing excessive L-arginine catabolizing arginase (Arg1).16 TAMs are of two types; classically (M1) activated, characterized by expression of inducible nitric oxide synthase (iNOS), and alternatively (M2) activated macrophages, characterized by Arg1 and mannose receptor (CD206) expressions.17 TAM accumulation at tumor site is correlated with the expression of chemokine monocyte chemoattractant protein-1 (MCP1), which negatively influences tumor growth.18,19 Furthermore, breast tumor cells secrete pro-angiogenic factors, which increases microvessel density in the tumors. The increased level of vascular endothelial growth factor (VEGF), a pro-angiogenic factor was correlated with poor clinical prognosis.20 It was shown that tumor growth could be regressed by inhibition of angiogenic pathways and simultaneous activation of apoptotic pathways, which further leads to apoptosis of blood vessel.21,22
Herein, we investigated the antineoplastic effects of γδ T cells on the breast cancer cells in vitro and in vivo and identified the pathways in induction of cytotoxicity. Specifically, we studied modulation of pro- and antisurvival pathways on γδ T cell induction in breast cancer cells. Furthermore, we investigated the role of macrophages and angiogenesis-related factors promoting tumor cell death on γδ T cell induction. These novel findings will help us develop targeted combination therapy includes small molecule and immune cells.
Material and Methods
Isolation and in vitro culture of γδ T cells
Human peripheral blood (30 ml) was collected from adult healthy donors after obtaining the IRB approval from Wexner Medical Center at The Ohio State University and obtaining consents from donors. Peripheral blood mononuclear cells (PBMC) were isolated from fresh blood as described earlier.23–25 γδ T cells from four different healthy donors were used in most of the experiments.
Morphology and cell viability of breast tumor cells after coculture with γδ T cells
Ten thousand breast tumor cells (SkBr7, BrCa-MZ01, MCF7 and MDA-MB-231) were cultured in a 96-well cell culture plate in a media supplemented with 10% fetal bovine serum (FBS). The γδ T cells (T) were added to the well and cocultured with breast tumor (C) cells with a ratio of C:T = 1:1, 1:7.5, 1:15, 1:30 for 24 and 48 hr at 37°C humid incubator with 5% CO2. Phase contrast micrographs were taken at 24 hr. The images were captured under epifluorescence microscope (Axioplan2; Carl Zeiss) using Zeiss Axiovision imaging software. Similar set-up of the experiments was used to perform tumor cell viability using MTT (3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide) cell proliferation assay kit as described earlier.8
Cell cytotoxicity of breast tumor cells after coculture with γδ T cells
Breast tumor cell cytotoxicity assay was performed by using “Live/Dead Cell” cytotoxicity kit and following their protocol (L7010, Molecular Probes, Life technologies, NY). Briefly, half a million breast tumor cells (SkBr7 and BrCa-MZ01) were plated for 6–8 hr, and then breast tumor cultures were labeled with 3 mM of 3,3′-dioctadecyloxacarbocyanine [DiOC18 (3)] overnight. Next day, the dye was washed and then incubated with five million γδ T cells to make a ratio C:T = 1:10. After 24 hr, both γδ T cells and breast cancer cells were collected by centrifugation (250g) for 10 min. Collected cell pellet was gently dissociated and stained with propidium iodide for 20 min in 37°C water bath. The cells were immediately analyzed by flowcytometry (FACS). For analyses, γδ T cells were gated out, and percentage of live and dead breast tumor cells was calculated from three different independent experiments.
Xenografts of the tumor cells
Immunocompromised (NOD/SCID) male mice (8 weeks old) were subdivided in four groups. Each of the NOD/SCID mouse was subcutaneously injected with either tumor cells, SkBr7 (one million) or γδ T cells (30 million) separately, or coinjected with SkBr7 or BrCa-MZ01 cells plus γδ T cells in ratio 1:15 or 1:30 in 200 μl (1× PBS). The γδ T cells (Day 17 of expansion) 15 million or 30 million in 200 μl (1× PBS) were injected. After 4 weeks of cell injection, tumors were harvested and weighed, divided into two equal halves for further experiments. One half was formalin fixed for histology and immunohistochemistry or used for isolation of total protein and RNA.
Protein analyses
Total protein analyses were performed using standard western blot technology for SkBr7 and BrCa-MZ01 cells cocultured with γδ T cells as described earlier.8 Western blot analysis was performed using total pAKT, pan AKT, pERK, ERK, CDK2, PARP, GAPDH, pPTEN, cleaved caspase 3, GAPDH, eNOS antibodies (Cell Signaling Technology) and p53, CDK4, VEGF (Santa Cruz Biotech) to detect their expression levels. For isolation of proteins from tumor tissues, frozen tumors were thawed on ice, minced and homogenized using protein lysis buffer.26
Hematoxylin and eosin staining and immunohistochemistry of tumors
The harvested tumor tissues were formalin fixed, paraffin-blocked, sectioned and stained with standard hematoxylin and eosin (H&E) or immunostained by pathology core facility personnel’s, College of Veterinary Medicine, The Ohio State University. The tumors were immunostained with cleaved Caspase 3, VEGF, F4/80 or anti-CD3 primary antibodies and stained using horseradish peroxidase conjugated DAB staining. Slides were observed under a microscope (Axioplan2; Carl Zeiss) and images were captured with Zeiss, Axiovision imaging software (Carl Zeiss).
Real time polymerase chain reaction analysis
Tumors were harvested from SkBr7 and BrCa-MZ01 cells, and total RNA was isolated by using TRIzol reagent (Invitrogen, Grand Island, NY). One microgram of RNA was used for the synthesis of cDNA using oligo dT (Invitrogen). Real time polymerase chain reaction (RT-PCR) was performed using 1 μl of cDNA for the gene specific primers. Expression of genes analyzed by quantitative PCR (qPCR) was normalized to 18SRNA using the 2 (−ΔCt) method.27 Primers used for RT-PCR are listed in the Supporting Information.
Data analyses
Values were expressed as mean ± SEM, and statistical analysis was performed by using Students t test, and the results were considered significant when values of p < 0.05.
Results
Effect of γδ T cells on breast tumor cell survivability in vitro
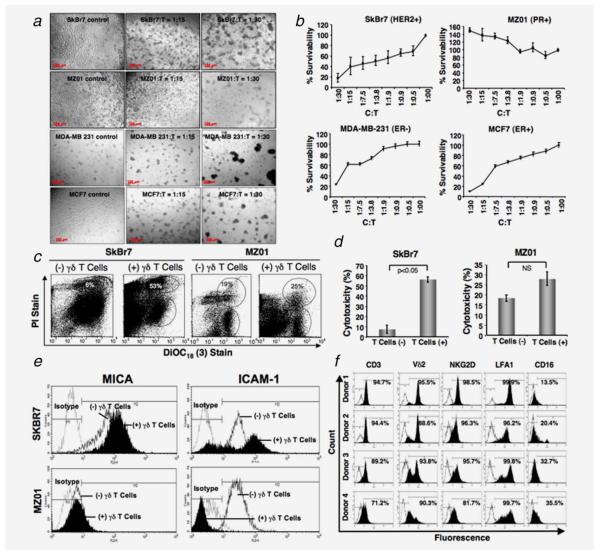
We have confirmed our ability to expand human γδ T cells from healthy donors (~88% CD3+ and ~92% Vδ2+, Fig. 1f). These expanded γδ T cells were used for in vitro assays, and for in vivo orthotropic models in NOD/SCID mice to determine their ability to limit cancer cell growth. To investigate the innate immune response of the γδ T cells (T) against breast tumor cells (C) of various phenotypes, such as SkBr7 (HER2+), BrCa-MZ01 (PR+), MCF7 (ER+) and MDA-MB-231 (ER−), cells were cocultured with γδ T cells (T) at various concentrations. At concentrations of C:T (1:15 or 1:30), visible floating clumps of cells were observed in all breast tumor cells except BrCa-MZ01cells (showed considerable resistance to lysis), after 24 hr of coculture with γδ T cells (Fig. 1a).
Figure 1.
Morphological changes of breast cancer cells after incubation with γδ T cells. (a) Various human breast cancer cell lines (SkBr7, BrCa-MZ01, MDA-MB-231 and MCF7) were cocultured with ex vivo expanded γδ T cells for 24 hr with a ratio of cancer: γδ T cells, C:T = 1:0; 1:15; 1:30 and the morphology of cells was imaged under a phase contrast microscope. (b) Breast cancer cell lines (SkBr7, BrCa-MZ01, MDA-MB-231 and MCF7) were cocultured with various ratios of γδ T cells for 24 hr. MTT assay was performed to evaluate tumor cell survival and proliferation after gentle removal of γδ T cells. (c) Cytotoxicity of breast cancer cells in presence of γδ T cells. Breast cancer cell lines (SkBr7, BrCa-MZ01) were cocultured with various ratios of γδ T cells for 24 hr. Flowcytometric analysis was performed to evaluate breast cancer cell cytotoxicity induced by γδ T cells after 24 hr of coculture using a LIVE/DEAD cell-mediated viability/cytotoxicity kit. In brief, breast cancer cells were labeled with DiOC18 (3), washed and then exposed to γδ T cells for 24 hr. After 24 hr, cells were labeled with propidium iodide (PI) and then subjected to flowcytometric evaluation. (d) Graphical presentation of cumulative cytotoxic lysis (%) of SkBr7 and BrCa-MZ01 cells of three independent experiments using γδ T cells. (e) The surface expression of molecules on breast cancer cells in presence or absence of γδ T cells was assessed by flowcytometry. SkBr7 and BrCa-MZ01 cells were cocultured with γδ T cells for 24 hr at 1:10 ratios and assessed for MICA and ICAM1 levels, isotype AB was used as a control. (f) Flow cytometric analysis of ex vivo expanded γδ T cells from four different healthy donors at Day 17 of ex vivo expansion. Each experiment was performed at least three times using γδ T cells from 3–4 donors. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We next wanted to see whether tumor cell survivability and proliferation was affected in presence of γδ T cells. Breast tumor cells (C) of various phenotypes were cocultured with γδ T cells (T) at various concentrations for 24 hr, and MTT assay was performed. A dose-dependent inhibition of cell survivability and proliferation was observed in most of the breast tumor cells except BrCa-MZ01 (Fig. 1b). Interestingly, an increase in the proliferation of BrCa-MZ01 cells was observed when cocultured with γδ T cells, probably due to influence of unidentified cytokines and growth factors. Therefore, we considered that SkBr7, MCF7 and MDA-MB-231 cells could possibly be designated as sensitive cells, and BrCa-MZ01 cells could be designated as resistant cells toward γδ T cell-mediated cytotoxicity. To further understand the molecular mechanism of γδ T cell-mediated cell death, we choose SkBr7 as a representative sensitive cell line and BrCa-MZ01as a resistant cell line.
Cell cytotoxicity of breast tumor by γδ T cells
To analyze cytotoxic lysis of breast tumor cells by γδ T cells, we used a dual color fluorescence flowcytometry technique. The breast cancer cells were labeled with the green fluorescent dye that binds to the plasma membrane of live cells and then were exposed to γδ T cells for 24 hr. After coculture, the mixture of both cells was collected and exposed to propidium iodide. During analyses, the γδ T cells were gated according to their size. We found that 56% of the SkBr7 cells were killed as opposed to only 10% of the BrCa-MZ01 cells when exposed to γδ T cells (Figs. 1c and 1d). Thus, this technique also confirms that SkBr7 cells are more susceptible to lysis by γδ T cells compared to BrCa-MZ01 cell line.
Surface expression of molecules on tumor cells on exposure to γδ T cells
To examine the molecules expressed on the tumor cells, which might have role for recognition by the γδ T cells to exert their downstream cytotoxic effects, we analyzed some relevant surface markers on tumor cells, cultured with or without coculture of γδ T cells. We and others have reported that MICA/B expressed on most tumor cells engages with NKG2D receptor present on γδ T cells.8,23,28 This recognition resulted in substantial enhancement of TCR-dependent T cell response in various pathogenic infections and ovarian tumors and gliomas.8,25,29 We further explored whether the expression levels of MICA/B was different in sensitive versus resistant tumor cells, which might result in differential cytotoxic response. As expected, flowcytometric data revealed that only SkBr7 cell line expressed MICA/B on its surface and BrCa-MZ01 was negative for the expression of MICA/B (Fig. 1e). On exposure to γδ T cells, MICA/B expression was further upregulated in SkBr7 cell line, however, MICA/B expression did not change in BrCa-MZ01 cell line after 24 hr. Next, we examined the expression level of intercellular adhesion molecule 1 (ICAM1/CD54), which is known to play role in interaction of tumor cells with the immune cells or cytotoxic cells via lymphocyte function associated antigen-1, LFA1 (CD11a).30 ICAM1 was expressed on both cell lines, however, after cocultures with γδ T cells for 24 hr, ICAM expression was upregulated in SkBr7 cells and was downregulated in BrCa-MZ01 cells (Fig. 1e). The induction of ICAM1 expression was reported to be cytokine-dependent and upregulation might be tumoricidial.31 Collectively, the increased expression of MICA/B and ICAM1 in SkBr7 cells on γδ T cell induction suggests that these molecules might be responsible for cytotoxicity of SkBr7 cells, and the absence of expression or unaltered status of MICA/B and downregulation of ICAM1 in BrCa-MZ01 cells could explain why these cells were able to proliferate and evade γδ T cells recognition.
Molecular signaling in tumor cells in response to γδ T cell induction
As SkBr7 and BrCa-MZ01 cell lines showed differential sensitivity to γδ T cells, we next sought to investigate whether they modulate different pro-and antisurvival signaling pathways differentially on γδ T cell induction. To assess the changes at the molecular levels in tumor cells after coculture with γδ T cells, the levels of various signaling molecules and their activation by phosphorylation (p) were analyzed by western blot after proteins were isolated from the SkBr7 and BrCa-MZ01 cell lines at 4 and 24 hr (Fig. 2 and Supporting Information Fig. S1). As γδ T cells were removed by gentle washing, the chance of contamination of γδ T cells was minimized. First, we analyzed the activation of survival pathways such as AKT and ERK pathways by using phospho-specific antibodies. We found that AKT phosphorylation was markedly reduced at 4 hr and ERK phosphorylation was significantly reduced at both time points in SkBr7 cells after coculture with γδ T cells. We noted that these findings correlated with the visible clumping and reduced survivability and proliferation in MTT assays. However, in BrCa-MZ01 cells both pAKT and pERK were significantly upregulated as early as 4 hr, and this upregulation was maintained till 24 hr, which correlated with the reduced visible clump formation in these cells and enhanced survivability observed in MTT assay. Furthermore, as downregulation of pPTEN tumor suppresssor gene has been shown to deactivate the expression of pro-apoptotic genes in an AKT-dependent or -independent manner, next we analyzed pPTEN level in both of these cell lines. However, we did not observe any significant change in pPTEN at 4 and 24 hr time point in SkBr7 and BrCa-MZ01 cells. Accordingly, activation of pro-apoptotic signals such as PARP and cleaved Caspase 3 was significantly enhanced in SkBr7 cells in both 4 and 24 hr time points. However, in BrCa-MZ01 cells, PARP was unchanged at both the time points; however, cleaved Caspase 3 was significantly upregulated in both time points. Taken together, these data support that γδ T cells induces an apoptotic stress in SkBr7 cells after coculture, whereas BrCa-MZ01 cells shows a very subtle response to this treatment.
Figure 2.

Expression of signaling molecules on breast cancer cells in presence or absence of γδ T cells. Breast cancer cells were cocultured with γδ T cells for 24 hr at 1:5 ratios. Total proteins were isolated from cancer cells after gentle removal of γδ T cells, and Western blot was performed for various signaling molecules stated in SkBr7 (left panel) and BrCa-MZ01 (right panel) cells. Each experiment was performed at least three times using γδ T cells from three different donors.
Another, tumor suppressor and stress inducible gene, p53, is also upregulated to inhibit cancer cell growth.32,33 Additionally, higher expression of p53 and its downstream signaling pathway proteins such as p21, CDK2/4 and Cyclin D1, is correlated with cell cycle arrest.34 Therefore, we analyzed the levels of p53 and its downstream signaling pathway molecules.
Interestingly, we did not see any significant difference in p53, CDK2/4 levels at later time points in both the cell lines. However, a significant increase in CDK4 was observed in earlier time points in both the cell lines (Fig. 2). These data indicate that γδ T cell-mediated cell death is not due to upregulation of these tumor suppressor pathways and does not affect the cell cycle.
γδ T cells induce breast tumor cell death in vivo
As in vitro studies showed differences in cell viability in breast tumor cells on coculture with γδ T cells, we wanted to investigate if similar effects were seen in vivo xenograft model using NOD/SCID mice. To perform in vivo studies sensitive (SkBr7) and resistant (BrCa-MZ01), tumor cells were coinjected with γδ T cells to determine any changes in tumor burden. We observed a positive staining for the anti-CD3 in tumors coinjected with tumor cells and γδ T cells only, confirming γδ T cell survival after 4 weeks of injection (Supporting Information Fig. S2). We also found smaller tumor size in mice coinjected with SkBr7 and γδ T cells in both ratios (1:15 or 1:30) compared to the SkBr7 cell alone-injected mice (Figs. 4a and 4b). No tumor was detected in mice injected with γδ T cells only, confirming that γδ T cells do not have potential to develop tumor. Interestingly, tumor size was also smaller in mice coinjected with BrCa-MZ01 and γδ T cells in both ratios compared to BrCa-MZ01 cell alone-injected mice (Figs. 3c and 3d). At this point, it is not clear why BrCa-MZ01 cells showed the differences between in vitro and in vivo cytotoxicity on γδ T cell induction, but we anticipate that tumor microenvironment and in vivo immune response might influence the tumor cell viability. Nonetheless, these results show that indeed, ex vivo expanded γδ T cells have potent antitumor activities that could reduce the growth of breast tumor cells in vivo.
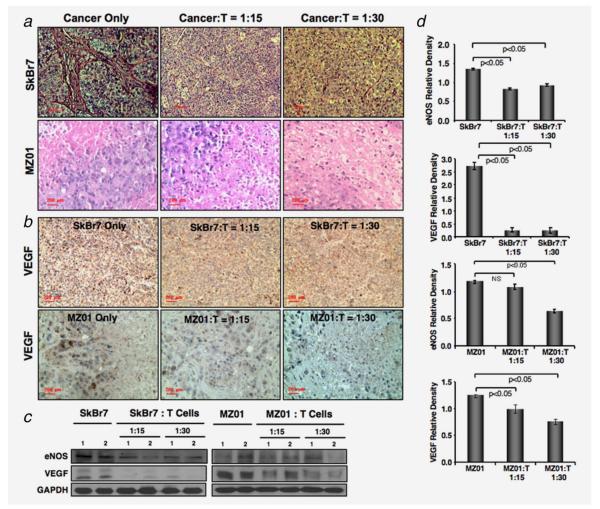
Figure 4.
Immunopathology of xenograft tumors. (a) Lower number of microvessels was observed in SkBr7 or BrCa-MZ01 cell-mediated tumors, which were coinjected with γδ T cells (with a ratio of Cancer:T = 1:15 or 1:30), compared to SkBr7 or BrCa-MZ01 cells alone. The size of the cells was smaller in BrCa-MZ01-mediated tumors, which were coinjected with γδ T cells compared to BrCa-MZ01 cells alone (lower panels). (b) Angiogenesis was evaluated in xenografts of breast cancer cells (SkBr7 or BrCa-MZ01) coinjected with γδ T cells by using anti VEGF Ab. Decreased levels of VEGF expression were observed in both cancer cell xenografts after coinjection of γδ T cells at either concentration, compared to cancer cells alone. (c) Level of angiogenesis-related signaling molecules (VEGF and eNOS) was evaluated using western blot. (d) Western blot protein expression levels of VEGF and eNOS molecules were measured by densitometric scanning using UN-SCAN-IT software (Silk Scientific) and graphically presented. Each experiment was performed three times. NS = nonsignificant. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
In vivo antitumorigenic effects of γδ T cells. Human breast cancer cells such as (a) SkBr7 or, (c) BrCa-MZ01 were coinjected subcutaneously with γδ T cells (with a ratio of Cancer:T = 1:15 or 1:30), cancer cells alone or γδ T cells alone (30 million/mouse) in immunocompromised NOD/SCID male mice, and images were presented after 4 weeks of tumor growth. (b and d) After 4 weeks of xenotransplant tumor volume was measured. Reduced tumor size and weight were observed in the mice, which received coinjection of γδ T cells compared to breast cancer cell alone. No tumors were detected in animals xenotransplanted with γδ T cells alone. n = 3–5 mice per group and used γδ T cells from three different donors. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
γδ T cells inhibit angiogenesis in vivo
To assess the mechanisms, tumor tissues harvested from all groups of animals were subjected to H&E staining for morphological evaluation. We observed increased blood vessels and angiogenesis in the mice injected with only SkBr7 cells. However, remarkably reduced blood vessels were found in the tumors of mice coinjected with γδ T cells and SkBr7 cells (Fig. 4a, upper panel). Interestingly, tumors of BrCa-MZ01 cells do not have any prominent blood vessels, but they have a combination of proliferating cells and necrotic tissue within the tumor. We did not see any remarkable morphological differences in angiogenesis process with or without γδ T cells injection (Fig. 4a, lower panel). As VEGF is known to mediate angiogenesis, we evaluated VEGF level in tumors. We observed that tumor cells coinjected with γδ T cells markedly reduced the VEGF expression level compared to the mice injected with tumor cells alone, derived from either SkBr7 or BrCa-MZ01 tumors (Figs. 4b and 4c). This finding provides an evidence of mechanisms, that the γδ T cells limit breast tumor growth in vivo by inhibiting angiogenic-signaling pathways. Protein level of VEGF was also downregulated in both SkBr7 and BrCa-MZ01 tumors coinjected with γδ T cells compared to the tumor alone group (Figs. 4c and 4d). It was shown that VEGF regulates expression of endothelial nitric oxide synthase (eNOS), which promote angiogenesis.35 Expression of eNOS was also downregulated in tumors coinjected with γδ T cells (Figs. 4c and 4d). Taken together, our results show that γδ T cells downregulate VEGF and eNOS to limit blood supply, and negatively influence angiogenesis and tumor growth.
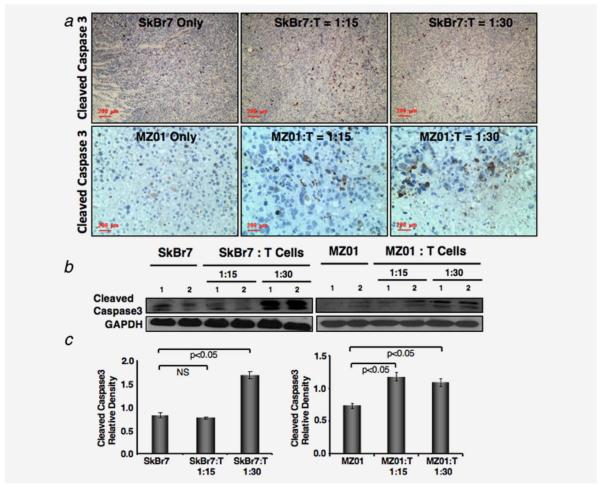
γδ T cells enhances apoptosis in vivo
We further examined whether apoptosis contributed to the reduction in tumor size. We assessed the expression level of apoptotic marker cleaved Caspase 3, in tumor tissues of mice coinjected with γδ T cells or injected with tumor cells only. Tumors from both SkBr7 and BrCa-MZ01 cells, coinjected with γδ T cells showed a higher number of apoptotic cells compared to the mice injected with tumor cells only (Fig. 5a). The levels of cleaved Caspase 3 were also confirmed by western blot analysis (Figs. 5b and 5d). These results showed that γδ T cells promote the tumor cells death via induction of apoptosis.
Figure 5.
Increased apoptosis in tumors coinjected with γδ T cells. (a) Immunohistochemical analysis of xenografts of breast cancer cells (SkBr7 or BrCa-MZ01) coinjected with γδ T cells was performed using antiapoptotic marker (cleaved Caspase 3). Increased levels of cleaved Caspase 3 staining were observed in both xenografts after coinjection of γδ T cells at either concentration, compared to cancer cells alone. (b) Cleaved Caspase 3 level was analyzed by western blot from the xenografts. Cleaved Caspase 3 level was increased in tumors of mice that received breast cancer cells coinjected with γδ T cells, compared to cancer cells alone. (c) Western blot protein expression level of cleaved Caspase 3 molecule was measured by densitometric scanning using UN-SCAN-IT software (Silk Scientific) and graphically presented. Each experiment was performed three times. NS = nonsignificant. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
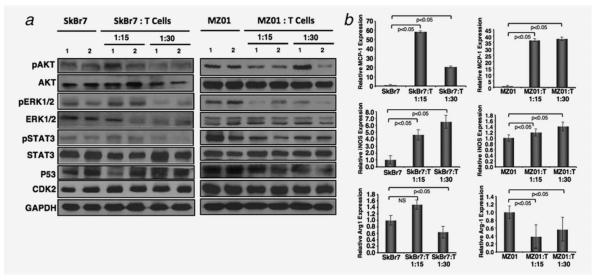
In vivo changes in signaling molecules in tumor with γδ T cell induction
As constitutive activation of proliferative pathways confers chemo-resistance to various current therapies in breast tumor, we evaluated the status of these pathways in tumors coinjected with γδ T cells compared to tumor alone. AKT, ERK and STAT3 phosphorylation were downregulated in tumors coinjected with γδ T cells (Fig. 6a). Quantitation of phospho and total protein levels were determined by densitometry scanning (Supporting Information Fig. S3). Similar to the in vitro study, we did not see any significant changes in p53 and CDK2 levels, further confirming our results that cell death is not mediated through this pathway.
Figure 6.
Decreased levels of survival genes in xenograft tumors coinjected with γδ T cells. (a) Western blot analysis of survival signaling molecules such as AKT, ERK, STAT3 was performed in tumors of SkBr7 (left panel), and BrCa-MZ01 (right panel) of mice that received breast cancer cells along with γδ T cells or cancer cells alone. (b) Effect of γδ T cells on the TAM in tumor xenografts. Significant increase in the expression of macrophage chemotactic protein1 (MCP1) in tumors coinjected with γδ T cells was observed compared to tumors alone. Significant increase in the expression of inducible nitric oxide synthase (iNOS), signature of M2 tumor suppressive macrophages in tumors coinjected with γδ T cells was observed compared to tumors alone. Significant decrease in the expression of Arginase1 (Arg1), signature of M1 tumor-promoting macrophages in tumors coinjected with γδ T cells was observed compared to tumors alone. Each experiment was performed three times. NS = nonsignificant.
In vivo micro-environmental changes in tumor with γδ T cells
Adoptive transfer of immune cells such as cytolytic T cells into tumor harboring animals increased the infiltration of macrophages within the tumor,36 causing shrinkage of tumor. Increased level of MCP1 mediates infiltration of macrophages to tumors and was linked to better prognosis in breast cancer patients.37 However, some reports showed no link between prognosis and MCP1 level.38,39 We observed a positive staining of F4/80+ macrophages in tumors of both SkBr7 and BrCa-MZ01 tumors coinjected with γδ T cells compared to injection of tumor cells only (data not shown). A significant increase in mRNA level of MCP1 was found in tumor extracts of coinjected mice compared to mice harboring tumor cells only (Fig. 6b). Additionally, the level of M1 marker, iNOS, revealed a significant increase in mice coinjected with γδ T cells compared to those injected with tumor cells alone. Conversely, M2 specific markers for TAM, arginase1 (Arg1), level was decreased significantly in tumors coinjected with tumor cell and γδ T cells compared to tumor cells alone (Fig. 6b). These results clearly indicate that γδ T cells modulate the tumor microenvironment to increase the immune surveillance via macrophage infiltration and may also increase the antigen presentation to γδ T cells, thus enhancing cytotoxic lysis of cancer cells.40 Additionally, γδ T cells may secrete chemotactic cytokines that may enhance the recruitment of immune cells to the borders of the tumors.
Discussion
Despite studies of the complex biology, signaling and interactions of the breast tumor and immune cell, the mechanisms of tumor cell-response to innate immune cells remain elusive. Numerous efforts are underway to illustrate the mechanistic basis of innate immune response in tumor cells, especially in the context of γδ T cells, which were well known to provide the first line of defense against various bacterial and viral infections. The tumoricidal potential of γδ T cells was derived from the observation of preferential expansion and infiltration of γδ T cells in certain tumors.28 Also, the presence of lymphocytic infiltrates was related to better prognosis in breast cancer patients.41 Our results showed that γδ T cells induce about ~80% cell death in most of the breast cancer cell lines tested (Figs. 1a and 1b) and ~40%–50% cell death in tumors of SkBr7 and BrCa-MZ01 cell lines (Fig. 3), which is significant tumor shrinkage. Herein, we identified the molecular mechanisms of γδ T cell-mediated cell death. We identified the signaling pathways activated or inactivated on γδ T cell induction, so that we could use this knowledge to further target pro-survival signaling molecules along with γδ T cells to increase the therapeutic efficacy. We and others demonstrated that Vγ2Vδ2 T cells are involved in the tumoricidal effects partly via recognition of MICA/B molecules expressed on tumor cells through NKG2D receptors present on γδ T cells.8,23,28 However, our work with breast tumor cell lines showed that the SkBr7 cell line expresses MICA/B, while BrCa-MZ01 does not, and thus SkBr7 cells are susceptible and BrCa-MZ01 cells are resistant to the Vγ2Vδ2 T cell-mediated cytotoxic lysis. However, expression of MICA has not been always clinically correlated with better prognosis of malignant disease. Sustained expression of MICA results in downregulation of NKG2D, leading to escape from γδ T or NK cell-mediated cytotoxicity, thus posing functional impairment to immunosurveillance.42,43 Therefore, we hypothesize that there might be other pathways which could play a role in antineoplastic effect of γδ T cells on tumors.
The analysis of signaling pathway molecules showed defects in AKT and ERK phosphorylation in SkBr7 sensitive cell line, indicating that activation of these pro-survival molecules and their downstream signaling is not functional in the presence of γδ T cell induction. As these phosphorylation levels are not reduced in BrCa-MZ01 resistant cell line, we anticipate that activation of AKT and ERK signaling might result in resistance, and therefore, AKT and ERK pathway molecules would be potential targets to enhance the therapeutic sensitivity. The inhibitors of these pathways are readily available for overcoming this resistance. Interestingly, our in vivo results suggest that in the context of tumor microenvironment, the tumors generated from BrCa-MZ01 cells did not show resistance; in fact, these tumors showed similar sensitivity levels as the SkBr7 tumors.
We showed that MICA downregulation in ovarian cancer cell line (A2780) after exposure to γδ T cells caused increase in ERK signaling and proliferation.8,25 At this point, it is not clear if MICA upregulation in SkBr7 cells is directly related to defects in AKT and ERK signaling which needs further investigation. Interestingly, the cell death of these tumors is not mediated by p53-dependent apoptotic pathway, as we did not see any change in p53 level. We also investigated if the γδ T cell mediated cell death is due to an effect on the cell cycle progression, which would be reflected by the levels of cell cycle proteins expressed in specific stage of the cell cycle. We observed that SkBr7 sensitive cells did not seem to modulate levels of CDK2; however, CDK4 levels were significantly increased at earlier time points, but no change was observed in the later time points (24 hr), indicating that the cell death is not mediated due to a cell cycle defect.
Unexpectedly, we observed that, BrCa-MZ01 cell line, which was highly resistant in vitro to cytotoxic lysis by γδ T cells, became susceptible in vivo. This data support the possibility that in vivo tumor microenvironment must be influencing the tumor cell death when γδ T cells are coinjected. Therefore, we analyzed some parameters of tumor microenvironment such as angiogenesis and macrophage induction. Angiogenesis is the hallmark of invasion for neoplasms irrespective of the origin of tumor type. Of the numerous angiogenic factors, VEGF has been shown to be the crucial rate-limiting step in the pathological angiogenesis during tumor progression. It is well known that VEGF upregulation is linked with increased proliferation.44 Indeed, we observed a significant reduction in VEGF staining in both SkBr7 and BrCa-MZ01 tumors after γδ T cell induction. Furthermore, VEGF downstream eNOS level was also reduced in both of these tumors on γδ T cell induction. Xenograft models of SkBr7 cell line in immunocompromised mice showed that increased VEGF expression caused increased expression in survival signaling molecules (AKT and ERK) as well as reduced expression of Caspase 3 cleavage in tumors.45 Thus, VEGF not only plays a pivotal role in tumor angiogenesis but also endows protection against apoptosis. Consistent with this, we predict that downregulation of AKT and ERK signaling molecules might be directly related to VEGF and eNOS downregulation. We observed an increased infiltration of macrophages as evidenced by the positive staining of F4/80+ cells in tumor after coinjection of γδ T cells with either SkBr7 or BrCa-MZ01 cells as compared to SkBr7 or BrCa-MZ01 cells alone. We found that the difference in the increased numbers of the macrophages in the coinjected tumors was due to an increase in the RNA levels of MCP1, compared to the tumor alone. This could be due to the possible modulation of tumor microenvironment by γδ T cells that enhance the migration of macrophages to the tumor borders. It was reported that the breast cancer patients expressing higher levels of MCP1 had better prognosis of disease-free survival compared to those that had low levels of MCP1.37 However, these results were contrary to other studies where higher levels of MCP1was linked to poor prognosis.46,47 Thus, the possibility of ex vivo transfer of γδ T cells to the tumors may stimulate the host’s immune system and hence render immunity against tumor. Thereafter, we wanted to test if the population of macrophages present in the tumor microenvironment were tumor suppressive or tumor promoting. Indeed, our results showed that a decrease in the Arg1 expression, signature of M2, tumor-promoting macrophages in tumor coinjected with γδ T cells compared to control. Concomitantly, an increase in the expression of iNOS was seen in tumor coinjected with γδ T cells compared to tumor cell alone. These data correlate with others, where it was shown that tumor microenvironment and in vivo physiological context has a great impact in tumor cell killing as opposed to the cell death in vitro.48
Taken together, our study identified that γδ T cell mediates cell death by reducing the activation of pro-survival molecules and increasing the levels of apoptotic molecules. Moreover, we revealed that tumor microenvironment plays a critical role in tumor cell lysis. Although current experimental approach does not re-capitulate exact clinical breast cancer patient situation, it provides a strong proof-of-concept of antitumoricidal properties of γδ T cells in vivo.
Both in vitro and in vivo results indicate that AKT and ERK signaling pathways might be the dominant signaling pathway affected on γδ T cells induction. Several studies implicated that pretreatment with AKT and ERK inhibitors overcome the resistance to therapy combined with radiation.49 Therefore, one of the reasonable strategies would be to use γδ T cells and to specifically target AKT and ERK pathway molecules in tumor cells to induce cancer cell killing. In immunotherapy, immune system is enhanced or suppressed to maximize the body’s ability to remove the disease without using any drug or radiation. As immunotherapies are more targeted in eliminating the tumorigenic cells while sparing the normal cells, immune-based therapies may be more appropriate biological therapy compared to chemo- and radiotherapy.50
Currently available therapies cannot treat effectively triple negative breast cancer patients. Considering our results, we believe that immunotherapy using expanded γδ T cells could be used as one of regimen for effective treatment of triple negative breast cancer patients. Moreover, personalized health care using patient’s own blood-derived expansion of γδ T cells could also be considered for clinical application.
Supplementary Material
What’s new?
Cancer growth is delayed in patients treated via the adoptive transfer of γδ T cells. The molecular mechanisms of this effect aren’t clearly understood. In this study, the authors examined the signaling pathways by which the Vγ2Vδ2subset of T cells inhibit breast-cancer cells. They found that these T cells down-regulated survival signals, while up-regulating apoptosis-related molecules, both in vitro and in vivo. The γδ T cells also caused disruption of the tumor microvasculature and increased infiltration of tumor associated macrophages (TAMs). These findings may lead to targeted therapies that combine both small molecules and immune cells.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: K01 AR054114 (NIAMS), SBIR R44 HL092706-01 (NHLBI), R21 CA143787 (NCI); Grant sponsors: Pelotonia Idea Award (OSUCCC), The Ohio State University start-up fund
Abbreviations
- Akt
serine/threonine kinase, protein kinase B
- Arg1
arginase1
- Bcl2
B-cell CLL/lymphoma 2
- CD
cluster of differentiation
- CD206
mannose receptor
- Ct
threshold cycle
- DiOC18
(3) 3,3′-dioctadecyloxacarbocyanine
- eNOS
endothelial nitric oxide synthase
- ErbB
epidermal growth factor receptor family member
- Erk1/2
extracellular signal-regulated kinases 1/2
- ER+
estrogen receptor positive
- ER−
estrogen receptor negative
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- γδ T
gamma delta T
- H&E
hematoxylin and eosin
- HER2
human epidermal growth factor receptor 2
- HER2+
herceptin 2 positive
- ICAM-1
intercellular adhesion molecule 1
- IL2
interleukin 2
- iNOS
inducible nitric oxide synthase
- IU
international units
- LFA1
lymphocyte function associated antigen-1
- MCP1
monocyte chemoattractant protein-1
- MHC
major histocompatibility complex
- MICA/B
MHC class-I A/B
- M1
classically activated macrophages
- M2
alternatively activated macrophages
- MTT
3–4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide
- Neu
neuro/glioblastoma derived oncogene homolog
- NKG2D
natural killer gamma 2 delta
- NOD/SCID
nonobese diabetes/severe combined immuno-deficient
- PARP
poly (ADP-ribose) polymerases
- PBMC
peripheral blood mononuclear cells
- PR+
progesterone receptor positive
- PTEN
phosphatase and tensin homolog
- p53
protein 53
- qPCR
quantitative polymerase chain reaction
- rIL-2
recombinant IL-2
- 18s RNA
18S ribosomal RNA
- TAMs
tumor associated macrophages
- TCR: αβ
T cell receptor alpha/beta
- VEGF
vascular endothelial growth factor
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Mohamed H, Chillar R, et al. Clinical significance of Akt and HER2/neu overexpression in African-American and Latina women with breast cancer. Breast Cancer Res. 2008;10:R3. doi: 10.1186/bcr1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taneja P, Maglic D, Kai F, et al. Classical and novel prognostic markers for breast cancer and their clinical significance. Clin Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morita CT, Parker CM, Brenner MB, et al. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–88. [PubMed] [Google Scholar]
- 5.Alam SM, Clark JS, Leech V, et al. T cell receptor gamma/delta expression on lymphocyte populations of breast cancer patients. Immunol Lett. 1992;31:279–83. doi: 10.1016/0165-2478(92)90127-a. [DOI] [PubMed] [Google Scholar]
- 6.Bank I, Book M, Huszar M, et al. V delta 2+ gamma delta T lymphocytes are cytotoxic to the MCF 7 breast carcinoma cell line and can be detected among the T cells that infiltrate breast tumors. Clin Immunol Immunopathol. 1993;67:17–24. doi: 10.1006/clin.1993.1040. [DOI] [PubMed] [Google Scholar]
- 7.Chargui J, Combaret V, Scaglione V, et al. Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother. 2010;33:591–8. doi: 10.1097/CJI.0b013e3181dda207. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Aggarwal R, Kanji S, et al. Human ovarian tumor cells escape gammadelta T cell recognition partly by down regulating surface expression of MICA and limiting cell cycle related molecules. PLoS One. 2011;6:e23348. doi: 10.1371/journal.pone.0023348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary A, Davodeau F, Moreau A, et al. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–40. [PubMed] [Google Scholar]
- 10.Maeurer MJ, Martin D, Walter W, et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–96. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 12.Raspollini MR, Castiglione F, Rossi Degl’innocenti D, et al. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol. 2005;16:590–6. doi: 10.1093/annonc/mdi112. [DOI] [PubMed] [Google Scholar]
- 13.Gomes AQ, Martins DS, Silva-Santos B. Targeting gammadelta T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–7. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 14.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 15.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein M, Keshav S, Harris N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottazzi B, Walter S, Govoni D, et al. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol. 1992;148:1280–5. [PubMed] [Google Scholar]
- 19.Walter S, Bottazzi B, Govoni D, et al. Macrophage infiltration and growth of sarcoma clones expressing different amounts of monocyte chemotactic protein/JE. Int J Cancer. 1991;49:431–5. doi: 10.1002/ijc.2910490321. [DOI] [PubMed] [Google Scholar]
- 20.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 21.Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–35. [PubMed] [Google Scholar]
- 22.Samuel S, Fan F, Dang LH, et al. Intracrine vascular endothelial growth factor signaling in survival and chemoresistance of human colorectal cancer cells. Oncogene. 2011;30:1205–12. doi: 10.1038/onc.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das H, Groh V, Kuijl C, et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 24.Das H, Sugita M, Brenner MB. Mechanisms of Vdelta1 gammadelta T cell activation by microbial components. J Immunol. 2004;172:6578–86. doi: 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- 25.Das H, Wang L, Kamath A, et al. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–8. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 26.Das H, George JC, Joseph M, et al. Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PLoS One. 2009;4:e7325. doi: 10.1371/journal.pone.0007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friese MA, Platten M, Lutz SZ, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 30.Vanky F, Wang P, Patarroyo M, et al. Expression of the adhesion molecule ICAM-1 and major histocompatibility complex class I antigens on human tumor cells is required for their interaction with autologous lymphocytes in vitro. Cancer Immunol Immunother. 1990;31:19–27. doi: 10.1007/BF01742491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaeble W, Kerlin M, Meyer zum Buschenfelde KH, et al. De novo expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in pancreas cancer. Int J Cancer. 1993;53:328–33. doi: 10.1002/ijc.2910530226. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Kon N, Jiang L, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 34.Sykes SM, Mellert HS, Holbert MA, et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–51. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loibl S, Strank C, von Minckwitz G, et al. Immunohistochemical evaluation of endothelial nitric oxide synthase expression in primary breast cancer. Breast. 2005;14:230–5. doi: 10.1016/j.breast.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Brown CE, Vishwanath RP, Aguilar B, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–41. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 37.Dehqanzada ZA, Storrer CE, Hueman MT, et al. Correlations between serum monocyte chemotactic protein-1 levels, clinical prognostic factors, and HER-2/neu vaccine-related immunity in breast cancer patients. Clin Cancer Res. 2006;12:478–86. doi: 10.1158/1078-0432.CCR-05-1425. [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 39.Valkovic T, Fuckar D, Stifter S, et al. Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol. 2005;131:453–8. doi: 10.1007/s00432-004-0667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Bottazzi B, Colotta F, et al. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–70. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 41.Pupa SM, Bufalino R, Invernizzi AM, et al. Macrophage infiltrate and prognosis in c-erbB-2-overexpressing breast carcinomas. J Clin Oncol. 1996;14:85–94. doi: 10.1200/JCO.1996.14.1.85. [DOI] [PubMed] [Google Scholar]
- 42.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 43.Oppenheim DE, Roberts SJ, Clarke SL, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 44.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–7. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 45.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 46.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 47.Valkovic T, Lucin K, Krstulja M, et al. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract. 1998;194:335–40. doi: 10.1016/S0344-0338(98)80057-5. [DOI] [PubMed] [Google Scholar]
- 48.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.No M, Choi EJ, Kim IA. Targeting HER2 signaling pathway for radiosensitization: alternative strategy for therapeutic resistance. Cancer Biol Ther. 2009;8:2351–61. doi: 10.4161/cbt.8.24.10131. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.