Abstract
We have formulated hydrophobic curcurmin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] into stable nanoparticle suspensions (nano-curcumin) to overcome its relatively low bioavailability, high rate of metabolism and rapid elimination and clearance from the body. Employing the curcumin nanoformulations as the platform, we discovered that curcumin has the potential to alleviate morphine tolerance. The two types of stable polymeric nanoparticles - poly(lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol)-b-poly(lactic acid) (PEG-b-PLA) - and the hybrid of the two were generated using flash nanoprecipitation integrated with spray drying. The optimized formulations have high drug loading (>45%), small particles size with narrow distribution, and controlled surface properties. Mice behavioral studies (tail-flick and hot-plate tests) were conducted to verify the effects of nano-curcumin on attenuating morphine tolerance. Significant analgesia was observed in mice during both tail-flick and hot-plate tests using orally administrated nano-curcumin following subcutaneous injections of morphine. However, unformulated curcumin at the same dose showed no effect. Compared with PEGylated nano-curcumin, negatively charged PLGA nanoparticles showed better functionality.
Keywords: polymeric nanoparticles, bioavailability, oral delivery, drug tolerance, pain management
1. Introduction
Opioids, such as morphine, are widely used in clinical management of acute and chronic pains.1-3 Drug tolerance (a decreased effect of a drug with repeated administration) is one of the major issues associated with opioids, which greatly limit their clinical applications.4
Curcuma longa is a yellow-colored traditional herb which has been used for thousands of years in Asia for food flavoring, preservation, coloring material, as well as medicinal treatment. Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the active constituent of Curcuma longa.5 It was recently found to have a wide spectrum of pharmacological activities, including antioxidant, cancer chemopreventive, anti-inflammatory, neuro-protective and antinociceptive actions.5, 6 In recent years, a preliminary report suggested that chronic dose of large amount of curcumin (100 mg/kg) might attenuate morphine tolerance.7 This opens a new window for curcumin to be potentially used to alleviate morphine tolerance in patients with chronic pain and on long-term opioid therapy. However, the applications of curcumin have been hindered by its poor aqueous solubility, relatively low bioavailability, high rate of metabolism and rapid elimination and clearance from the body.6 Although curcumin has been shown to be safe in many animal and human studies even at relatively high dosages, recent studies indicate that some of the effects (such as inhibition of proteasomal function and potentiation of Huntington toxicity) of high dose curcumin are clearly toxic and undesirable beyond its use in cancer therapy.8, 9 Biphasic effect of curcumin on morphine tolerance and body weight loss on mice study were also observed.10
Nano-sizing is recently of great interest to increase hydrophobic drug solubility and therefore bioavailability, because of the large surface area of the nanoparticles and enhanced solubility based on the curvature of the particles.11 Among various types of nano-materials, polymeric nanoparticles have the advantages of relatively high biocompatibility, stability, and flexibility of conjugating ligands on their surface for targeted drug delivery.12, 13 Although many methods have been developed to generate polymeric nanoparticles, limited drug loading (<15%) and insufficient size and surface control have been major problems despite the ideal hydrophobic interaction between the drug and the hydrophobic block of the copolymers. Moreover, producing polymeric nanoparticles encapsulating hydrophobic drugs in a scalable and reproducible manner is challenging, although it is the key for nanoparticles to be practically applied as novel medical formulations. We have developed a continuous and scalable process to reproducibly generate stable nanoparticles with controlled size distribution and high drug loading.14, 15 The enhanced oral bioavailability of a hydrophobic drug compound was demonstrated in mice and beagle dogs.14, 16
In this study, a similar process was applied and optimized to produce polymeric nanoparticles encapsulating curcumin, which we call nano-curcumin. High drug loading of 47% was achieved in the formulations. Relatively low dose of nano-curcumin (<20 mg/kg) was orally administrated in mice following subcutaneous injections of morphine. Mice behavioral studies were conducted to demonstrate the effects of nano-curcumin on attenuating morphine tolerance. Direct comparison was drawn between negatively charged poly(lactic-co-glycolic acid) (PLGA) nanoparticles with partially and completely PEGylated nanoparticles.
2. Experimental section
2.1. Materials
PLGA (acid terminated; PLA:PGA 50:50 w/w; Mw 7000-17000, Mw/Mn=1.8), curcumin, tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), ethanol, leucine, trehalose, and naloxone were purchased from Sigma-Aldrich (St Louis, MO). Methyl tert-butyl ether (MTBE) (HPLC grade) was purchased from Fisher Scientific (Pittuburgh, PA). Morphine was purchased from Hospira (Lake Forest, IL). Poly(ethylene glycol)-b-poly(lactic acid) (PEG-b-PLA) (Mn 5000-b-6700, Mw/Mn=1.08) was purchased from Polymer Source (Dorval, Canada). Unless otherwise stated, all chemicals were purchased at standard grades and used as received.
2.2. Preparation and size characterization of nanoparticle suspensions
PLGA and PEG-b-PLA nanoparticles encapsulating curcumin were generated by using a multi-inlet vortex mixer (MIVM). Among the four inlet streams, stream 1 was 0.2 wt% polymer and 0.2 wt% curcumin dissolved in THF. The other three inlet streams were Millipore water as an anti-solvent to precipitate the drug compound and polymers. The volumetric flow rate of streams 1 and 2 was 6 mL/min and it was 54 mL/min for streams 3 and 4.
Nanoparticle size distributions were measured by dynamic light scattering (DLS) (Malvern, Zetasizer Nano ZS90, Worcestershire, UK). The particle sizes were reported as the intensity-weighted radius. Viscosity and refractive index of the solvent were set to be 0.933 cP and 1.333, respectively.
The definition of drug loading (DL) and encapsulation efficiency (EE) were the same as previously reported.14 Drug loading of curcumin in nanoparticles was quantified by UV-Vis spectrophotometer measurements at the absorbance wavelength of 435 nm, after the samples were spray dried and then redissolved in DMSO at a solid concentration of 2 mg/mL.
Zeta potentials of the formulations were measured by zeta sizer (Agilent, 7030 Nicomp DLS/ZLS-size and zeta, Santa Clara, CA) at 23 °C. Dielectric constant was 78.5.
2.3. Spray drying of the nanoparticle suspensions
Spray drying of the nanoparticle suspensions was carried out by integrating the MIVM with a spray dryer (LabPlant, SD-05 Spray Dryer, North Yorkshire, UK). We have previously optimized the spray drying conditions for the nanoparticles to be re-suspended in aqueous solutions.14 To prevent the nanoparticles from permanent agglomeration, trehalose (300:1 ratio to the nanoparticles) and leucine (5:1 ratio to the nanoparticles) were added to the nanoparticle suspensions as the excipients during the spray drying process. Ethanol (60 v/v%) was added in order to lower the inlet temperature of the spray dryer, which was set as 75 °C, to compensate the relatively low melting temperature of the polymers. The feed rate of the solutions was 10 mL/min. Spray-dried powders were collected in a glass container at the outlet of the spray dryer.
Nanoparticles were resuspended into sterile Millipore water at vigorous stirring for 10 minutes before dosing animals.
2.4. Measurements of curcumin in vitro release from the nanoparticles
The spray dried PLGA, PEG-b-PLA, and hybrid nanoparticles were resuspended in 0.1 M PBS buffers at pH 2, pH 5 and pH 7.4 at the solid concentration of 10 mg/ml for the measurements of curcumin in vitro release. A two-phase system (Figure 2A) was designed to overcome the solubility limits of curcumin in aqueous solutions. MTBE was added to the aqueous buffers at 1:2 v/v ratio, which could extract the over saturated curcumin from the aqueous solutions. Solutions (500 μL) were taken from the organic MTBE phase at designed time points (0.5, 1, 2, 4, 6, 24 hours and 2, 3, 5, 7, 14, 21 days) and same amount (500 μL) of fresh MTBE was added back to maintain the constant volume of the system. Curcumin in MTBE was quantified using fluorescence plate reader under excitation of 395nm and emission of 475nm, respectively.
Figure 2.
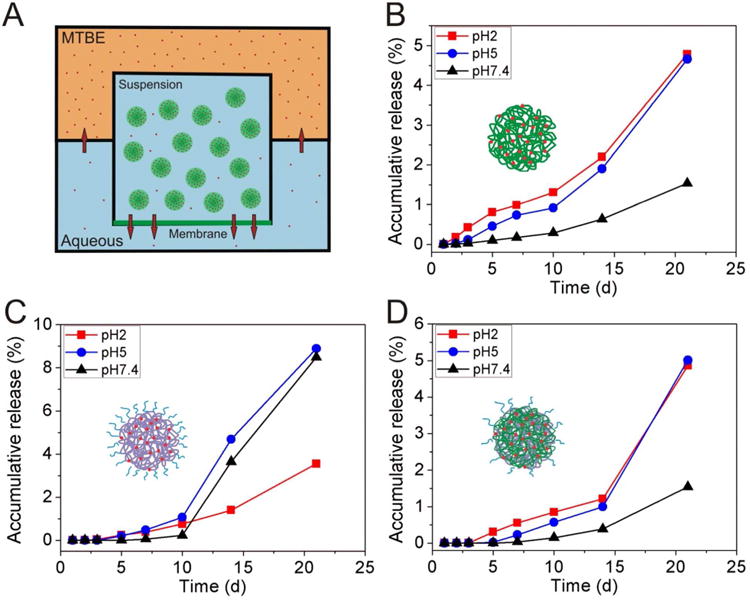
In vitro release of curcumin from nano-curcumin formulations in the MTBE-aqueous two phase system. (A) Schematic drawing of the MTBE-aqueous two phase system. The membrane at the bottom of the inner container was a dialysis membrane with a molecular weight cut-off (MWCO) 6000. (B) Curcumin release from PLGA-curcumin nanoformulation. (C) Curcumin release from PEG-b-PLA-curcumin nanoformulation. (D) Curcumin release from hybrid-curcumin nanoformulation.
2.5. Animals
Male ICR mice (20-25g, Charles River Laboratories, Wilmington, MA) were maintained on a 14/10h light/dark cycle with access to food and water ad libitum before experimental procedures. All experimental procedures were performed with an approval by the Animal Care and Use Committee of the University of Illinois at Chicago and in accordance with the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals.
2.6. Tests for antinociception
2.6.1 Tail-flick test
Basal nociception and morphine-induced antinociception were studied using the 52°C warm-water tail-flick test.17, 18 In brief, mice were held over the water bath and one third of the distal portion of the tail was immersed into the water. The latency to a quick tail-flick response was recorded as a base-line measurement. Any mouse not responding within 5 sec was excluded from further experiment. To prevent tissue damage, a cut-off time of 12 sec was applied. Morphine-induced antinociception was evaluated 30 min after the injection of a testing dose of morphine (10mg/kg s.c), and expressed as the percentage of maximal possible effect (%MPE) according to the following formula,19
2.6.2 Hot-plate test
For assessment of response latencies to thermal stimulus in mice, a Cold/Hot Plate Analgesia Meter (Ugo Basile, Comerio, Italy) was used. In this study, mice were placed on a hot plate that was thermostatically maintained at 55 ± 1 °C. The nociceptive response was evaluated as the latency to the first licking or lifting of the rear paws or escape jumping. Mice were removed from the hot plate immediately after displaying the response and a cut-off time of 30 sec was set to prevent tissue damage. The anti-nociceptive effect was measured using the same percentage of maximal possible effect (%MPE) as above.
2.7. Acute opioid tolerance
To induce acute tolerance, separate groups of three ICR mice (20-25g) were treated with a large dose of morphine (100mg/kg s.c.). Maximal morphine tolerance peaked at approximately 4 to 6 h.17, 20, 21 Mice in the control group received an equal volume of saline. Tolerance to opioids was assessed 4.5h later by monitoring the antinociceptive effect produced by a test dose of morphine (10mg/kg, s.c.). The presence of opioid tolerance was signified by a significant reduction of antinociceptive effect. To determine the effect of nano-curcumin and unformulated curcumin on preventing acute morphine tolerance, nano-curcumin and curcumin were given 15 min before the induction dose of morphine (100 mg/kg s.c.). The tests were replicated independently and conducted by experimenters blinded to the treatments. Data were analyzed by one way ANOVA followed by Turkey post test.
3. Results and discussion
3.1. Size distribution, drug loading, and encapsulation efficiency of the nanoparticles
Nanoparticle formulations have to meet several requirements to be possibly used for clinical applications – (1) all the materials are FDA approved; (2) the process has to be reproducible and scalable; (3) size distribution and surface properties of the nanoparticles have to be well characterized; (4) drug loading of the nanoparticles has to be high enough to contain sufficient active compound at a patient-compliance dose; and (5) high encapsulation rate for economic reasons. We have previously developed an integrated process of flash nanoprecipitation and freeze drying to satisfy aforementioned requirements. The multi-inlet vortex reactor, custom-designed for flash nanoprecipitation, provides rapid micromixing to generate nanoparticles with narrow size distribution.22 In our experiments, all the nanoparticles were generated at high Reynolds number (> 9000) to ensure the homogeneous and flash precipitation.22 PLGA, PEG-b-PLA and hybrid (PLGA to PEG-b-PLA 1:1 molar ratio) nanoparticles encapsulating curcumin were generated to compare the effect of surface properties on drug oral bioavailability and functionality. These polymers are chosen for the study because they are all degradable, biocompatible, and easy to synthesize. Moreover, PLGA and PLA have similar polymer structure with negative charges. PEG brushes were applied to partially or completely shield the surface charges. PEG can also prevent non-specific protein absorption and prolong particle blood circulation time.
The average particle size (before and after spray drying), drug loading, encapsulation efficiency, and zeta potential of the nanoparticles with the three polymeric nanoparticle formulations are reported in Table 1. We consider the size of the particles after spray drying more important, since it represents the particles dosed to animals. After spray drying, the average diameters of all three re-suspended nanoparticles are similar (about 150 nm) with a trend of linearly reduced size when increasing the amount of PEG chains on the surface of the particles, but the difference is within 14% (or 21 nm). Highest drug loading and encapsulation efficiency were found in PEG-b-PLA particles among the three, but the difference is small compared to the PLGA nanoparticles with the lowest drug loading (< 3.5%). More than 90% of the drug encapsulation efficiency was found for all three formulations, and PEG-b-PLA nanoparticles presented the highest encapsulation rate. As we expected that with more PEG chains on the surface, zeta potential of the negatively charged PLGA (or PLA) nanoparticles became closer to be neutral.
Table 1.
Average particle diameter (before and after spray drying), drug loading, encapsulation efficiency, and zeta potential of the three curcumin nanoformulations.
| Polymer | Diameter before spray drying (nm) | Diameter after spray drying (nm) | Drug loading (%) | Encapsulation efficiency (%) | Zeta potential (mV) |
|---|---|---|---|---|---|
| PLGA | 79 | 165.8 | 46.2 | 92.4 | -33.1±0.8 |
| PLGA & PEG-b-PLA | — | 159.9 | 47.1 | 94.2 | -24.7±2.9 |
| PEG-b-PLA | 120 | 144.9 | 47.7 | 95.4 | -2.5±1.8 |
A high inlet temperature of the spray dryer could induce active chain motion and result in permanent agglomeration of the particles, due to the relatively low glass transition temperature of the PLGA. The size of PLGA-curcumin nanoparticles increased from 70 nm to about 165 nm (Figure 1A). Dimers and trimers were formed during the spray drying process. Also the size distribution after spray drying was boarder compared the size distribution before spray drying. Less agglomeration was observed for PEG-b-PLA-curcumin nanoparticles which may be due to the repulsion of PEG brushes (Figure 1A).
Figure 1.
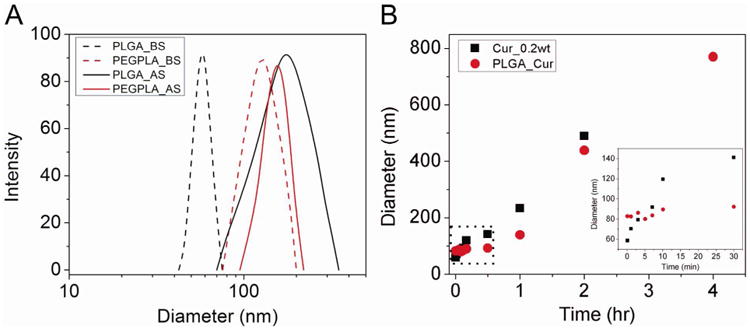
(A) Size distributions of PLGA-curcumin and PEG-b-PLA-curcumin nanoparticles before and after spray drying. Trehalose and leucine were added as excipients. Ethanol (60% v/v) was also added to the inlet stream. Dash lines: before spray drying. Solid lines: after spray drying. Black: PLGA-curcumin nanoparticles. Red: PEG-b-PLA-curcumin nanoparticles. (B) Growth kinetics of curcumin and PLGA curcumin (at 1:1 weight ratio). Size change in the first half hour (dash area) is separately plotted in the right corner. Black: curcumin alone; and red: PLGA curcumin (1:1 weight ratio).
The suspension of PEG-b-PLA nanoparticles is relatively stable, since the long PEG chains (Mw 5000) form brushes on the surface of the nanoparticles, which provide repulsion and energy barrier to prevent particle aggregation. Ostwald ripening based on the diffusive transport of curcumin, which is a slow process, takes most responsibility for the growth of the PEG-b-PLA nanoparticles. Stable suspension of PLGA nanoparticles relied on the charge repulsion, which is much weaker, to reduce particle collision and aggregation. Therefore, the relatively faster growth of the PLGA nanoparticles is our main concern.
The growth kinetics of curcumin alone and PLGA curcumin at 1:1 weight ratio were measured for up to 4 hours, as shown in Figure 1B. Growth of curcumin alone followed a linear fashion with a slope about 3.7 nm/min. The initial size of the nanoparticles was about 60 nm. However, after 30 minutes they grew to be 140 nm and after 2 hours about 500 nm. The PLGA-curcumin nanoparticles follow the growth kinetics of the polymer initially and then later curcumin growth kinetics. For the first 30 minutes, PLGA significantly reduced the growth. Eventually, particle growth kinetics is dominated by the collision rate which is similar to that of the pure curcumin particles. To maintain the stability of the nanoparticles, flash nanoprecipitation was integrated with the spray drying, which eliminates growth media and produces dry-powder within 10 minutes.
3.2. In vitro release of curcumin
Slow release of curcumin from the nanoparticles was observed for up to 21 days. Release slightly depends on pH of the buffer solutions. At lower pH, PLGA and PLA degrade at faster rates. However, curcumin solubility reduces from 5.3 ng/mL at pH 7.4 to less than 1 ng/mL at pH 2, which hinder the transport and therefore the release of curcumin. The counter balanced effects of pH are reported in Figure 2B to 2D.
3.3. Effects of curcumin and curcumin nanoparticles on attenuating morphine tolerance
An acute mouse model of opioid tolerance17, 20, 21 was used to test the effect of curcumin nanoformulations compared to unformulated free curcumin. Morphine tolerance was developed 2 to 6 hours after the administration of 100mg/kg morphine subcutaneous.21 The development of tolerance was evidenced by significant reduction of morphine antinociception after 4 hours (Figure 3, “MS group”). Tail-flick and hot-plate experiments were conducted 30 minutes after the subcutaneous administration of 10mg/kg morphine 4 hours later. Positive control group, dosed with saline initially and 10mg/kg morphine 4 hours later, exhibited significant antinociceptive effect, while the MS groups (as the negative control) showed significantly reduced of antinociception, indicative of the presence of opioid tolerance. All three nanoparticle suspensions of curcumin attenuated morphine tolerance in both tail-flick and hot-plate tests. PLGA-curcumin nanoformulation shows almost 100% analgesia in tail-flick experiment. Even the PEG-b-PLA nanoformulation, which showed the least effect, still had more than 50% analgesia. In hot-plate experiments, PLGA and hybrid (1:1 wt/wt ratio of PLGA and PEG-b-PCL) nanoparticles showed similar effects. Despite the superior physical and chemical properties of the PEG-b-PCL nanoparticle (in terms of particle size distribution, drug loading, and stability, presented in Table 1), PLGA-curcumin nanoparticles presented better efficacy. The main reason is that the negative charges of the PLGA facilitate the uptake and transport of the nanoparticles from the GI tract to circulation and CNS. We have previously demonstrated the significantly enhanced oral bioavailability of hydrophobic drugs carried in PLGA nanoparticles and we expect PLGA-curcumin nanoparticles to be similar.14
Figure 3.
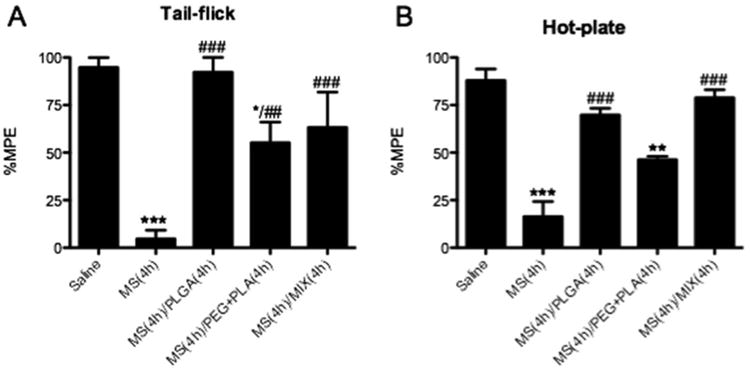
Effect of curcumin nanoformulations on morphine tolerance. (A) Tail-flick assay. (B) Hot plate assay. Separate groups of 3 mice received 500 μL curcumin nanoformulation (20 mg/kg) or saline orally. After 15 min, saline (Saline group) or 100 mg/kg morphine were dosed subcutaneously. MS group received saline, but not curcumin nanoformulation, a priori. Four hours later, 10 mg/kg were administrated subcutaneously to all mice in order to determine morphine-antinociception using the tail-flick assay and hot-plate assay. Data are expressed in mean ± S.E.M. *, p<0.05; **, p<0.01; ***, p<0.001 compared with the saline group; ##, p<0.01; ###, p<0.001 compared with the morphine (MS) group.
Mice also received unformulated free curcumin (20mg/kg), which did not significantly change morphine tolerance, when compared the positive control MS group (Figure 4). Low aqueous solubility and poor oral bioavailability take main responsibility for the low efficacy of free curcumin, which limits curcumin biomedical applications. Nanoformulations protect curcumin from the wide range of pH change in the GI tract and the enzyme along the lumen of the intestine. Meanwhile the surface and size properties of nanoformulations facilitate the permeation through biological membranes, which improve curcumin bioavailability and therefore its antinociceptive effect.
Figure 4.
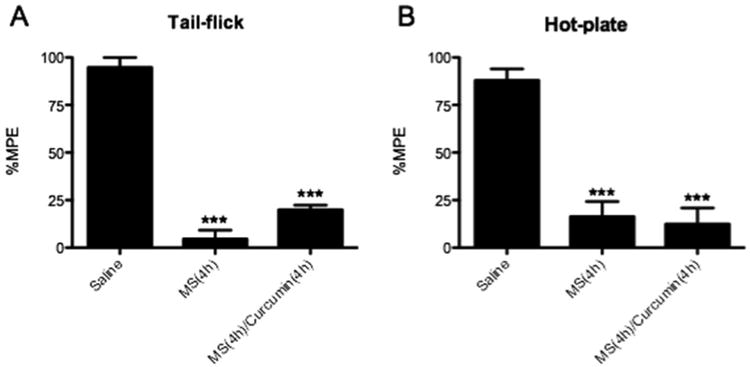
Effect of unformulated free curcumin on morphine tolerance. (A) Tail-flick assay. (B) Hot-plate assay. Groups of 3 mice received 500 μL curcumin (20 mg/kg) or saline orally. After 30 minutes, 100 mg/kg morphine or saline (Saline group) were dosed subcutaneous. Four hours later, morphine (10 mg/kg, s.c.) was administrated to all mice in order to determine antinociception using tail-flick assay and hot-plate assay. Data are expressed in mean ± S.E.M. ***, p<0.001 compared with the saline group.
4. Conclusion
We discovered and reported the effect of nano-curcumin on attenuating morphine tolerance. When nano-curcumin was orally administrated at relatively low doses to mice that are tolerant to opioids, morphine-antinociception was largely restored as determined by both tail-flick and hotplate assays. These data indicate that nano-curcumin diminished opioid tolerance. In contrast, the free curcumin at the same dose (20mg/kg) was ineffective in altering either opioid tolerance. Among the three nanoparticle-curcumin formulations (PLGA-curcumin, PEG-b-PLA-curcumin, and hybrid of PLGA- and PEG-b-PCL-curcumin), PLGA-curcumin presented best efficacy. Although PEG chains help to prolong particle blood circulation time, PEGylated nanoparticles, when orally administrated, may be cleared from GI track quickly and have lower chance, compared to the negatively charged PLGA particles, to pass the multiple biological barriers. At the same dose level, unformulated free curcumin was ineffective, because of the hydrophobic nature of the compound and its low bioavailability. Furthermore, it is essential to control the properties of the nanoparticles in order to improve hydrophobic compound oral bioavailability. The integrated process of flash nanoprecipitation and spray drying has been characterized in our previous studies to generate polymeric nanoparticles with high drug loading, narrow size distribution, well-designed surface charges, and long-term stability. The well-characterized and controlled nanoparticles provide the platform for drug delivery and drug discovery.
Acknowledgments
The authors are grateful to Dr. Yoon Yeo at Purdue University for the access of the spray dryer in her laboratory.
References
- 1.McQuay H. Opioids in pain management. Lancet. 1999;353(9171):2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 2.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429(1-3):79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 3.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348(13):1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 4.Trujillo KA, Akil H. Inhibition of morphine-tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251(4989):85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 5.Sharma RA, Gescher AJ, Steward WP. Curcumin: The story so far. Eur J Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 7.Matsushita Y, Ueda H. Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport. 2009;20(1):63–68. doi: 10.1097/WNR.0b013e328314decb. [DOI] [PubMed] [Google Scholar]
- 8.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikshit P, Goswami A, Mishra A, Nukina N, Jana NR. Curcumin enhances the polyglutamine-expanded truncated N-terminal huntingtin-induced cell death by promoting proteasomal malfunction. Biochem Biophys Res Commun. 2006;342(4):1323–1328. doi: 10.1016/j.bbrc.2006.02.104. [DOI] [PubMed] [Google Scholar]
- 10.Lin JA, Chen JH, Lee YW, Lin CS, Hsieh MH, Chang CC, Wong CS, Chen JJY, Yeh GC, Lin FY, Chen TL. Biphasic Effect of Curcumin on Morphine Tolerance: A Preliminary Evidence from Cytokine/Chemokine Protein Array Analysis. Evidence-Based Complementary Altern Med. 2011:1–11. doi: 10.1093/ecam/neq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Van den Monter G, Augustijns P. Solubility Increases Associated with Crystalline Drug Nanoparticles: Methodologies and Significance. Mol Pharmaceutics. 2010;7(5):1858–1870. doi: 10.1021/mp100209b. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 13.Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed -Nanotechnol Biol Med. 2010;6(6):714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Banerjee AA, Mlynarska P, Hautman M, Hong S, Kapetanovic IM, Lyubimov AV, Liu Y. Enhanced oral bioavailability of a cancer preventive agent (SR13668) by employing polymeric nanoparticles with high drug loading. J Pharm Sci. 2012 doi: 10.1002/jps.23269. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Hong S, Prud'homme RK, Liu Y. Self-assembling process of flash nanoprecipitation in a multi-inlet vortex mixer to produce drug-loaded polymeric nanoparticles. J Nanopart Res. 2011;13(9):4109–4120. [Google Scholar]
- 16.Banerjee AA, Shen H, Hautman M, Anwer J, Hong S, Kapetanovic IM, Liu Y, Lyubimov AV. Polymeric nanoparticles enhance oral bioavailability of the hydrophobic chemopreventive agent (SR13668) in beagle dogs. Curr Pharm Biotechnol. 2013;14:464–469. doi: 10.2174/1389201011314040012. [DOI] [PubMed] [Google Scholar]
- 17.Tang L, Shukla PK, Wang LX, Wang ZJ. Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 2006;317(2):901–909. doi: 10.1124/jpet.105.097733. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of Opioid Tolerance by let-7 Family MicroRNA Targeting the mu Opioid Receptor. J Neurosci. 2010;30(30):10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZJ, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21(5):1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilsky EJ, Bernstein RN, Wang ZJ, Sadee W, Porreca F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J Pharmacol Exp Ther. 1996;277(1):484–490. [PubMed] [Google Scholar]
- 21.Tang L, Shukla PK, Wang ZJ. Disruption of acute opioid dependence by antisense oligodeoxynucleotides targeting neurogranin. Brain Res. 2007;1143:78–82. doi: 10.1016/j.brainres.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Cheng C, Prud'homme RK, Fox RO. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem Eng Sci. 2008;63(11):2829–2842. [Google Scholar]


