Abstract
Objective
To investigate the correlation between maternal leptin levels and 100 gram oral glucose test (OGTT) results as well as the correlation between leptin levels and the development of gestational diabetes mellitus (GDM) and glucose intolerance during pregnancy.
Material and Method: 104 subjects with gestational weeks ranging from 24 to 32 weeks who had increased 50 gr OGTT values (>140) were included in this study. After the screening test, 100 gr OGTT was administered to the subjects. Sixty cases were selected from these subjects; twenty patients with one abnormal test result were identified as “glucose intolerant” group (Group 1), 20 patients with two abnormal test values were diagnosed with GDM (Group 2) and 20 patients with normal test results constituted the control group. The serum leptin levels of the groups were measured with enzyme linked immunosorbent assay (ELISA).
Results
The serum leptin level was 8.4±5.1 ng/ml for group 1, 9.1±5.3 ng/ml for group 2 and 6.3±4.6 ng/ml for the control group. Although serum leptin levels for group 1 and 2 was observed to be higher than the control group, the result was not statistically significant (p>0.05). This result did not change after adjusting for body mass index (BMI).
Conclusion
There is no statistically significant difference between leptin levels among three groups.
Keywords: Gestational Diabetes Mellitus, Oral Glucose Tolerance Test, Leptin
Özet
Amaç
Gebelikte maternal leptin seviyelerinin 100 gr oral glukoz tolerans testi sonuçları ve gestasyonel diyabet veya glukoz intoleransı gelişimi ile korelasyonunun araştırılması.
Gereç ve Yöntemler
Gebelik haftası 24–32 haftalar arasında olup 50 gr yükleme testi >140 mg/dl olan 104 vaka çalışmaya dahil edildi. tarama sonrası 100 gr testi uygulandı. Altmış vaka seçildi ve tek değeri bozuk olan 20 hasta “glukoz intolerans” grubu (Grup 1) olarak adlandırıldı. İki değeri yüksek olan hasta GDM tanısı aldı (Grup 2). Sonucu normal olan 20 hasta ise kontrol grubu olarak sınıflandırıldı. serum leptin düzeyleri ELISA ile ölçüldü.
Bulgular
Serum leptin seviyeleri grup 1 için 8.4±5.1 ng/ml, grup 2 için 9.1±5.3 ng/m ve kontrol grubu için 6.3±4.6 ng/ml idi. Grup 1 ve 2 deki serum leptin düzeyleri kontrol grubuna göre yüksek olsa da bu istatistiksel olarak anlamlı değildi (p>0.05). Sonuçlar vücut kitle indeksi ayarlaması yapıldıktan sonra da değişmedi.
Sonuç
Her üç grup arasında serum leptin seviyeleri açısından anlamlı fark saptanmadı.
Introduction
Leptin is a polypeptide hormone that is secreted by adipose tissue cells, a product of the obesity (ob) gene, and is related to energy regulation (1). Research results indicate that the serum leptin level increases during pregnancy, independent of the body mass index (BMI). It is claimed that there is a link between the development of gestational diabetes mellitus (GDM) and leptin concentration. It is suggested that the measurement of leptin along with the assessment of other risk factors could help in identifying women at risk of developing GDM (2). The measurement of leptin level is interesting since GDM is a common metabolic complication of pregnancy and is related to type 2 diabetes and obesity (2).
It is estimated that the incidence of perinatal morbidity such as macrosomia increases in patients who have a single value abnormality in 100 gram OGTT (3). Researchers suggest that these patients may be treated like GDM patients (4). It is found that insulin resistance is related to increased plasma leptin levels independent of the BMI (5). Plasma leptin concentration is also expected to be high in patients with glucose intolerance.
The aim of this study is to investigate the correlation between maternal leptin levels and 100 gr OGTT results as well as the correlation between leptin levels and development of GDM and glucose intolerance during pregnancy.
Material and Method
One hundred and four subjects at gestational weeks ranging from 24 to 32 with 50 gr OGTT values above 140 mg/dl were included in this study. The study was approved by the Ethics Committee of the hospital and informed consent was obtained from all patients. The histories of the patients were obtained, maternal age, gravidity and parity were recorded. Heights and weights of the patients at the time of glucose tolerance testing were measured and used to calculate the BMI as body weight (kg)/(body height (m))2. All the patients were administered the 100 gr OGTT. In this test, serum fasting glucose levels and glucose values in the first, second and third hour which were above 95, 180, 155, 140 mg/dl respectively were treated as abnormal values. Out of these 104 subjects; 20 patients were randomly selected from women with one abnormal test value and were designated as the “glucose intolerant” group (Group 1), 20 patients with two abnormal test results were diagnosed as GDM and designated as group 2 and 20 patients were randomly selected from women with normal test results as the control group. The serum leptin levels of these patients were measured with enzyme linked immunosorbent assay (ELISA). The blood samples for measurement of 100 gr OGTT and leptin were taken between 8–11 am after 10–12 hours of fasting. The blood samples were centrifuged at 5000 turns/minute for 5 minutes and serum samples were stored at −70 ºC. The serum leptin levels were analyzed by enzyme linked immunosorbent assay (ELISA) (Biosource Company, catalog number KAP 2281).
The resultant data was expressed as mean ± SD. Kruskal– Wallis Test and Mann-Whitney Test were used as statistical tests. Data from cross-tables were analyzed using Chi-square test. Spearman correlation test and linear regression analysis were used for the evaluation of the effects of different factors on leptin levels. SPSS for Windows version 13.0 was used for statistical analysis and p<0.05 was accepted as statistically significant.
Results
The clinical characteristics of the groups are shown in Table 1. There was no statistically significant difference in maternal ages, gestational weeks and parity between the groups.
Table 1.
Demographic characteristics of patients included in study
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| BMI (kg/m2) | 29.1 ± 3.6 | 29.0 ± 3.2 | 26.2 ± 3.6 |
| Maternal age (years) | 29.4 ± 4.3 | 29.6 ± 5.8 | 27.5 ± 4.2 |
| Gestational age at diagnosis(weeks) | 28.2 ± 2.5 | 27.8 ± 2.5 | 26.9 ± 1.8 |
| Number of pregnancies | 2.8 ± 1.5 | 2.8 ± 1.4 | 2.4 ± 1.4 |
| Number of deliveries | 1.3 ± 0.1 | 1.3 ± 1.0 | 0.8 ± 0.8 |
Values are mean ±S.D. or n
A history of diabetes mellitus (DM) in their previous pregnancies was reported in 2 patients (10%) in group 1 and 4 patients (20%) in group 2. The patients in the control group had no history of DM. The BMI in the control group was statistically lower than group 1 (p=0.032) and 2 (p=0.036) (Table 1).
The serum leptin level was 8.4±5.1 ng/ml for group 1, 9.1±5.3 ng/ml for group 2, and 6.3±4.6 ng/ml for the control group. Although serum leptin levels for group 1 and 2 were higher than the control group, this was not statistically significant (p>0.05). The result did not change after adjusting for BMI. There was also no statistically significant correlation between BMI and maternal leptin levels (p=0.644). There was a positive but weak correlation between blood glucose and leptin levels (r=0.276, p=0.032) (Figure 1).
Figure 1.
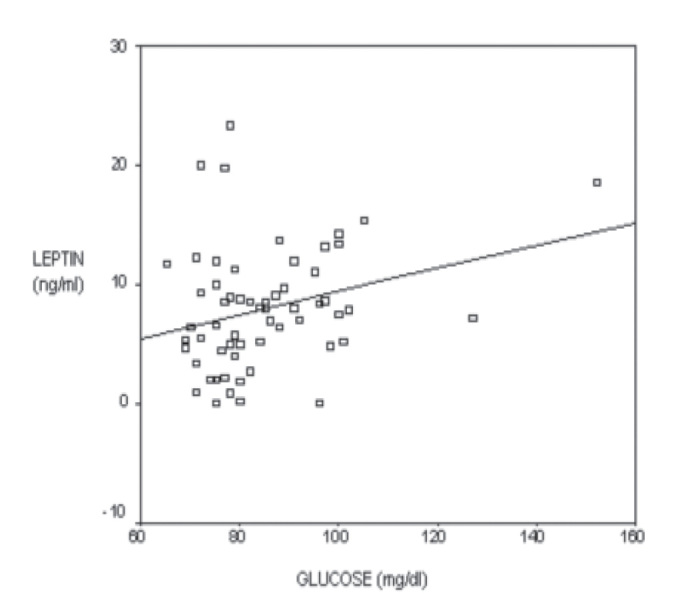
Relationship of blood glucose levels and leptin levels. There is a positive but weak correlation between maternal blood glucose level and leptin level (r=0.276, p=0.032)
Discussion
In the present study, although serum leptin levels of subjects who were glucose intolerant or diagnosed with GDM were observed to be higher than subjects with normal blood glucose levels, the difference was not statistically significant. This result did not change after adjusting for BMI. Although the data is limited by the small sample size, it is suggested that this is likely a result of effects of other confounding factors in addition to insulin resistance on leptin levels. It is estimated that insulin resistance is related to increased plasma leptin levels independent of the BMI, but leptin production is not only dependent on the plasma insulin (5). Besides insulin; maternal weight gain, excessive energy, cytokines, estrogen, retinoic acid, corticosteroids and IL-1 regulate leptin have an effect (6). Lee et al. suggested that adiponectin but not leptin was associated with impaired glucose tolerance after adjusting for potential confounding factors. Low levels of adiponectin may increase the risk of glucose intolerance and type 2 diabetes (7).
In the literature it is claimed that glucose homeostasis is provided by increased insulin secretion and this is accompanied by decreased sensitivity to insulin (8). Yılmaz et al. showed that women with GDM had significantly higher levels of serum insulin and leptin concentrations compared to women with normal blood glucose levels. They did not find any correlation between serum leptin levels and insulin resistance but they found a negative correlation between leptin levels and insulin sensitivity. They suggested that leptin may contribute to the development of GDM by decreasing insulin sensitivity but not increasing insulin resistance (9). German et al. suggested that hormones such as insulin and leptin act in the hypothalamus to regulate energy balance and glucose metabolism. They showed that hypothalamic leptin action increased peripheral insulin sensitivity which primarily affects the liver by enhancing suppression of hepatic glucose production, with no change of insulin stimulated glucose utilization (10).
Wiznitzer et. al reported that there is a statistically significant positive correlation between plasma leptin levels and neonatal birthweight (11). Insulin resistance, hyperinsulinism and hyperleptinemia exist in infants of diabetic mothers and the trend of higher leptin levels in infants of diabetic mothers than in infants of non-diabetic mothers shows that leptin could be related to insulin resistance in these infants. Also glucose levels were found to be lower in large-for-gestational-age infants of both diabetic and non-diabetic mothers (12).
Ergin et al. found similarities in terms of patient characteristics between GDM patients and patients with single value abnormality as a result of OGTT test. Also, taking fasting insulin levels and insulin resistance as 2 separate criteria of analysis, patients with a single value abnormality were found to be indistinguishable from patients with GDM; both groups were significantly different from the normal oral glucose tolerance test group. It was observed that that mean insulin secretion increased from normal oral glucose tolerance test to impaired glucose tolerance and GDM (4). It was suggested that a single abnormal test value in an oral glucose tolerance test should be regarded as a pathologic finding and patients with glucose intolerance might also be treated as GDM patients (4).
Some studies in the literature suggest that plasma leptin levels in diabetic subjects are not different from non-diabetic ones and they are related to adiposity (13, 14). McGregor et al. found no difference in leptin levels between diabetic and non-diabetic subjects (13). In obese subjects, the defective leptin receptors in pancreatic β cells alter the regulation of this adipoinsular axis, leading to hyperinsulinemia and diabetes (15). Leptin is also found to be significantly high in obese subjects due to leptin resistance. The most important factor affecting the leptin level is found to be the amount of adipose tissue (16, 17).
In this study, no statistically significant correlation between maternal leptin level and BMI was observed. The maternal leptin level increases in the first and second trimester of pregnancy and it peaks around 22–27 gestational weeks (18). Leptin level correlates strongly and positively with maternal body weight and BMI in the first and second trimester of pregnancy but such a relationship cannot be revealed in the third trimester of pregnancy (19). The BMI in pregnancy is also an imprecise measure of the amount of fat stores and relates fetal weight as well as placental size, amniotic fluid and maternal fluid expansion (20).
In the literature, it is estimated that GDM patients are older and more obese than normal subjects (21, 22). The measurements of body fat percentage calculated from skinfold thickness were higher in GDM group compared to those of subjects with normal glucose levels (9). It is also shown that 60–80% of GDM patients are obese (23). In this study the BMI of patients with GDM and glucose intolerance were significantly higher than the patients with normal glucose levels, suggesting that obesity is a risk factor for the development of GDM. Also the development of GDM in the previous pregnancies and a family history of GDM are risk factors. For the early diagnosis and treatment of GDM, these risk factors should also be taken into consideration and, if a single value abnormality is detected in 100 gram OGTT in high risk patients, the physician needs to be alert for the development of GDM.
Finally, since there are controversial conclusions drawn from different studies about leptin, and the pathophysiology of hyperleptinemia is not exactly understood to this date, further studies are necessary to better evaluate the mechanism and exact relationship between leptin and insulin.
References
- 1.Flier JS. Leptin expression and action:new experimental paradigms. Proc Nat Acad Sci USA. 1997;94:4242–5. doi: 10.1073/pnas.94.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maghbooli Z, Hossein-Nezhad A, Rahmani M, Shofaei AR, Larijani B. Relationship between leptin concentration and leptin resistance. Horm Metab Res. 2007;39:903–7. doi: 10.1055/s-2007-992812. [DOI] [PubMed] [Google Scholar]
- 3.Obstetrics Maternal-Fetal Medicine. Perinatology. 2001:589–617. [Google Scholar]
- 4.Ergin T, Lembet A, Duran H, Kuscu E, Bagis T, Saygılı E, Batıoğlu S. Does. insulin secretion in patients with one abnormal glucose tolerance test value mimic gestational diabetes mellitus? Am J Obstet Gynecol. 2002;186:204–9. doi: 10.1067/mob.2002.119634. [DOI] [PubMed] [Google Scholar]
- 5.Saad MF, Khan A, Sharma A, Michael A, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–9. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- 6.Licinio J, Negrao AB, Matzoros C, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone and estradiol in healthy women. Proc Natl Acad Sci USA. 1998;95:2541–6. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CY, Lee CH, Tsai S, Hwang CT, Wu NT, Tai SY, Lin FF, Chao NC, Chang CJ. Association between serum leptin and adiponectin levels with risk of insulin resistance and impaired glucose tolerance in non-diabetic women. Kaohsiung J Med Sci. 2009;25:116–25. doi: 10.1016/S1607-551X(09)70050-6. [DOI] [PubMed] [Google Scholar]
- 8.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–72. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz O, Kucuk M, Ilgin A, Dagdelen M. Assesment of insulin sensitivity/resistance and their relations with leptin concentrations and anthropometric measures in a pregnant population with and without gestational diabetes mellitus. J Diabetes Complications. 2009 Mar 6; doi: 10.1016/j.jdiacomp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 10.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via neurocircuit involving the vagus nerve. Endocrinology. 2009 Jul 2; doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiznitzer A, Furman B, Zuili I, Shany S, Reece EA, Mazor M. Cord leptin level and fetal macrosomia. Obstet Gynecol. 2000;96:707–13. doi: 10.1016/s0029-7844(00)00992-3. [DOI] [PubMed] [Google Scholar]
- 12.Vela-Huerta MM, San Vicente-Santoscoy EU, Guizar-Mendoza JM, Amador-Licona N, Aldana-Valenzuela C, Hernnández J. Leptin, insulin, and glucose serum levels in large-for-gestational-age infants of diabetic and non-diabetic mothers. J Pediatr Endocrinol Metab. 2008;21:17–22. doi: 10.1515/jpem.2008.21.1.17. [DOI] [PubMed] [Google Scholar]
- 13.McGregor GP, Desega JF, Ehlenz K, Fischer A, Heese F, Hegele A, Lammer C, Peiser C, Lang RE. Radioimmunological measurement of leptin in plasma of obese and diabetic human subjects. Endocrinology. 1996;137:1501–4. doi: 10.1210/endo.137.4.8625930. [DOI] [PubMed] [Google Scholar]
- 14.Montzoros CS, Moschas SJ. Leptin: in search of role(s) in human physiology and pathophysiology. Clin Endocrinol. 1998;49:551–67. doi: 10.1046/j.1365-2265.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 15.Seufert J, Kleffer TJ, Leech AC, Holz GG, Moritz W, Ricardi C, Habener J. Leptin suppression of insulin secretion and gene expression in human pancreatic islets:implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab. 1999;84:670–6. doi: 10.1210/jcem.84.2.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive leptin concentrations in normal weight and obese humans. New England Journal of Medicine. 1996;324:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 17.Maffei N, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight reduced subjects. Nature Medicine. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 18.Tamura T, Goldenberg RL, Johnston KE, Cliver S. Serum leptin concentrations during pregnancy and their relationship to fetal growth. Obstet Gynecol. 1998;91:389–95. doi: 10.1016/s0029-7844(97)00670-4. [DOI] [PubMed] [Google Scholar]
- 19.Tamas P, Sulyok E, Szabo I, Viser M, Ertl T, Rascher W, Blum WF. Changes of maternal serum leptin levels during pregnancy. Gynecol Obstet Invest. 1998;46:169–71. doi: 10.1159/000010026. [DOI] [PubMed] [Google Scholar]
- 20.King JC, Butte NF, Bronstein MN, Kopp LE, Lindquist SA. Energy metabolism during pregnancy:influence of maternal energy status. Am J Clin Nutr. 1994;59:439–58. doi: 10.1093/ajcn/59.2.439S. [DOI] [PubMed] [Google Scholar]
- 21.Kühl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21:19–26. [PubMed] [Google Scholar]
- 22.MD, Stern MP, Mitchell BD, Abashawl A, Langer O. Relationship between glucose levels and insulin secretion during a glucose challange test. Am J Obstet Gynecol. 1990;163:1818–22. doi: 10.1016/0002-9378(90)90756-w. [DOI] [PubMed] [Google Scholar]
- 23.McFarland MB, Langer O, Conway DL, Berkus MD. Dietary therapy for gestational diabetes ; how long is long enough? Obstet Gynecol. 1999;93:978–85. doi: 10.1016/s0029-7844(98)00547-x. [DOI] [PubMed] [Google Scholar]


