Abstract
Joubert syndrome (JBTS) is an autosomal recessive disorder characterized by intellectual disability, hypotonia, ataxia, tachypnea/apnea, and abnormal eye movements. A pathognomonic midbrain-hindbrain malformation seen on cranial magnetic resonance imaging (MRI), which consists of hypoplasia of the midline cerebellar vermis that resembles the cross-section through a molar tooth, has been described previously. The molar tooth sign is defined by a peculiar appearance resembling a molar tooth secondary to an abnormally deep interpeduncular fossa and enlarged superior cerebellar peduncles on axial images at the pontomesencephalic level. The term Joubert Syndrome and Related Disorders (JSRD) has recently been adopted to describe all disorders presenting the “molar tooth sign” (MTS) on brain imaging. JSRD is characterized by lack of decussation of the superior cerebellar peduncles, central pontine tracts and corticospinal tracts suggesting defective axon guidance. Prenatal sonographic findings in fetuses with JSRD are relatively nonspecific and include increased nuchal translucency, enlarged cisterna magna, cerebellar vermian agenesis, occipital encephalocele, ventriculomegaly and polydactyly. We report a case of JSRD detected prenatally at 23 weeks of gestation. The fetus in the present case had a normal karyotype. Sonographic features of the fetus included polydactyly, partial vermian hypoplasia, dilated 4th ventricle and mild ventriculomegaly which were also confirmed by prenatal MRI. MTS was demonstrated in a postnatal MRI after pregnancy termination.
Keywords: Joubert syndrome, prenatal diagnosis, ultrasonography, polydactyly, cerebellar vermian agenenesis
Özet
Joubert sendromu entellektüel bozukluk, hipotoni, ataksi, takipne/apne ve anormal göz hareketleri ile karakterize otozomal resesif bir hastalıktır. Önceki çalışmalarda kraniyal manyetik rezonans görüntüleme ile hastalığa ait patognomonik bir görüntü saptanmıştır. Bu görüntü molar diş görünümü olarak ifade edilmiştir. Molar diş görüntüsü anormal derin yerleşimli interpedünküler fossa ve genişlemiş superior serebellar pedünkülllere bağlı olarak pontomezensefalik seviyedeki aksial kesitlerde ortaya çıkmaktadır. Joubert sendromu ve ilişkili bozukluklar son dönemde molar diş görünümünün olduğu tüm bozukluklar için kullanılmaktadır. Joubert sendromu ve ilişkili bozukluklar, superior serebellar pedünküllerde, santral pontin yollarda ve kortikospinal yollarda çaprazlaşmanın olmaması ile karakterize bir grup hastalıktır. Bu hastalığa ait prenatal sonografik bulgular görece non spesifiktir. Başlıca bulgular arasında artmış nukal saydamlık, genişlemiş sisterna magna, serebellar vermian agenezi oksipital ensefalosel ve polidaktili vardır. Biz, 23. haftada sonografik olarak polidaktili, vermian agenezi, dilate 4. ventrikül ve hafif ventrikülomegali saptanan normal karyotipli bir Joubert sendromu olgusu sunmaktayız. Bu olguda molar diş işareti doğum sonrası manyetik rezonans görüntüleme ile saptanmıştır.
Introduction
Ciliopathies are a group of disorders characterized by defective primary ciliary function (1). Exploring the role of primary cilia in humans has enabled categorization of certain syndromes under the umbrella term “ciliopathy”. Joubert Syndrome (JBTS) is a ciliopathy which was initially described by the triad of cerebellar vermis hypoplasia, oculomotor apraxia and intermittent hyperventilation (2). The prevalence of Joubert Syndrome and Related Disorders JSRD has been estimated as approximately 1:100,000 (3). Molar tooth sign (MTS) is a pathognomonic magnetic resonance imaging (MRI) finding of JBTS. MTS refers to hypoplasia of the midline cerebellar vermis that resembles the cross-section through a molar tooth. JSRD, like many of the disorders considered as ciliopathies, shows considerable heterogeneity in clinical features and molecular basis (4). JSRD defines a group of disorders that encompasses classic JBTS as well as other features such as central nervous system anomalies, ocular coloboma, retinal dystrophy, renal disease and hepatic fibrosis (5). The description of MTS has greatly facilitated both prenatal and postnatal diagnosis of this syndrome. We report a case of JSRD detected prenatally at 23 weeks of gestation based on demonstration of the MTS and postaxial polydactyly.
Case
A 27-year-old woman, was referred to our perinatology department for routine second trimester genetic sonogram at 19 weeks of gestation according to her last menstrual period. She was gravida 1 and she denied consanguinity with her husband and had no history of teratogen exposure during or immediately preceding pregnancy.
On ultrasound examination, a singleton viable intrauterine pregnancy was detected. The fetus was female and fetal biometric measurements were consistent with 19 weeks gestation. At this gestational age hypoplasia of cerebellar vermis, mild ventriculomegaly, postaxial polydactyly in both hands as well as left foot were detected (Figure 1). Amniocentesis revealed a 46 XX karyotype. The patient was scheduled for control sonography 2 weeks later with which the previous findings were confirmed. Fetal MRI was performed at 22nd gestational week and also confirmed ventriculomegaly and hypoplasia of cerebellar vermis (Figure 2). Dilatation of the 4th ventricle was also demonstrated by MRI. A presumptive diagnosis of ciliopathy was then made. MRI and sonographic examination of the fetus did not reveal any coexisting abnormalities. Based on these findings parental counseling was made and the family opted for pregnancy termination at the 23rd gestational week. Gross examination after pregnancy termination revealed a female fetus weighing 700 grams with postaxial polydactyly in both hands and left foot. Postabortal MRI examination of the fetus revealed the molar tooth sign owing to a deep interpeduncular fossa, elongated, thick and mal-oriented superior cerebellar peduncles combined with cerebellar vermis hypoplasia (Figure 3). A diagnosis of JSRD was made based on demonstration of MTS and postaxial polydactyly.
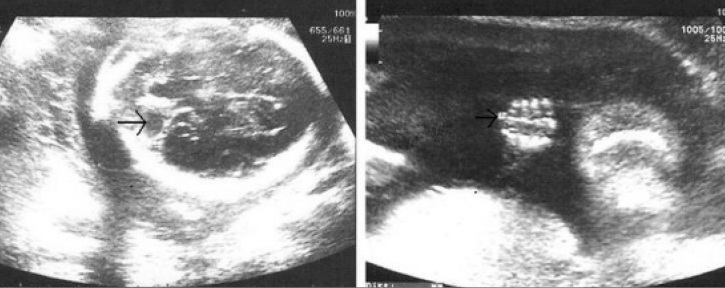
Figure 1.
Prenatal sonographic view of postaxial polydactyly (right) and cerebellar vermian hypoplasia (left)
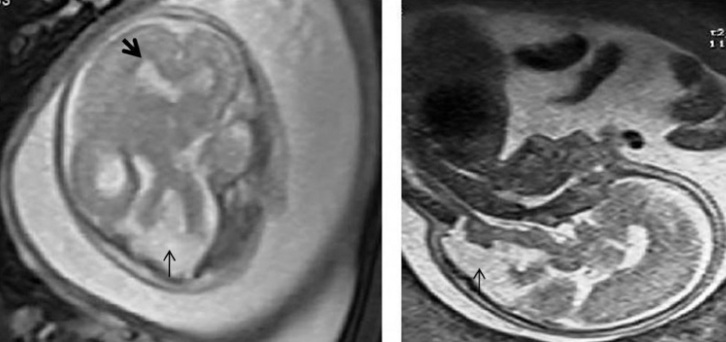
Figure 2.
Axial (left) and sagittal (right) prenatal MR images demonstrating hypoplastic cerebellar vermis and ventriculomegaly
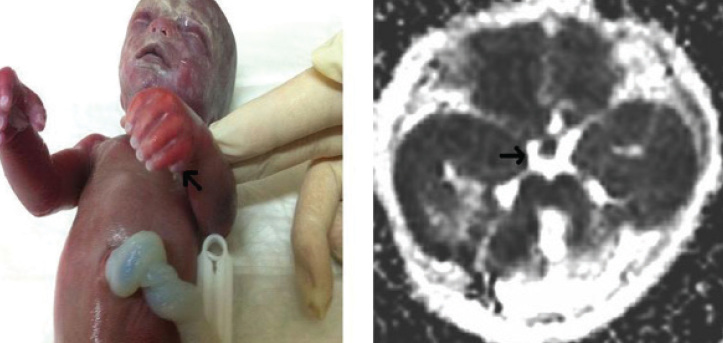
Figure 3.
Polydactyly in both hands demonstrated in the photograph of fetus following abortion (left). Axial postnatal MR image demonstrating the “ molar tooth appearance” (right)
Discussion
Primary cilia are sophisticated organelles found in almost all vertebrate cell types. Primary cilia were first observed in the epithelium of renal tubules and thyroid gland more than a century ago (6). Cells of many other organs and tissues in the body (e.g. kidney, liver, pancreas, brain, and oviduct) display primary cilia on their surface (7–9). It has been recently found that primary cilia perform fundamental tasks in developmental processes. These organelles are thought to serve as sensory units that detect and transmit signals from the surrounding environment to the cell body in order to regulate embryonic development (6). The term ‘ciliopathy’ describes a class of rare human genetic diseases whose etiologies lie in defective cilia. Joubert syndrome belongs to the group of disorders which are collectively considered as ciliopathies (1). JS was initially described by the triad of cerebellar vermis hypoplasia, oculomotor apraxia (jerky eye movements with inability to visually fixate) and intermittent hyperventilation (2). A pathognomonic midbrain-hindbrain malformation seen on cranial MRI was described in 1997. This consists of hypoplasia of the midline cerebellar vermis that resembles the cross-section through a molar tooth (4). Contemporary diagnostic criteria of JBTS include characteristic brainstem malformation and demonstration of MTS (10). The term JSRD has been recently adopted to describe all disorders presenting the MTS on brain imaging (11). JSRD are genetically heterogeneous, and all known genes encode proteins localized at or near the primary cilium. Ten causative genes have been identified to date. JSRD has an estimated prevalence of 1 in 100.000 live births (3, 12). Only a few cases have been described prenatally. The neurological features of JSRD include hypotonia, ataxia, psychomotor delay, irregular breathing pattern and oculomotor apraxia and are variably associated with multi-organ involvement, mainly retinal dystrophy, nephronophthisis (NPH) and congenital liver fibrosis (5).
Prenatal sonographic findings in fetuses with JSRD are relatively nonspecific and include increased nuchal translucency, enlarged cisterna magna, cerebellar vermian agenesis, occipital encephalocele, ventriculomegaly, hypoplastic phallus, renal cysts, and polydactyly (13). Molar tooth sign associated with JSRD has previously been demonstrated by fetal MRI in three cases. Saleem and associates were able to identify the molar tooth sign as early as 22 weeks of gestation (14). MTS was identified at 27 weeks of gestation in two other cases (13, 15). MTS was demonstrated in two cases before the 24th gestational week by sonography alone in a recent study (16). In the present case we were able to identify the molar tooth sign following pregnancy termination at 23 weeks. Increased nuchal translucency (NT) has also been reported to be associated with JSRD (17). Increased NT, together with other extracranial findings such as polydactyly, may aid earlier diagnosis especially when a prior family history is present. However, it is not possible to demonstrate Vermian hypoplasia before the 18th gestational week (18).
Due to the marked heterogeneity in this group of disorders and the relatively high frequency of associated medical conditions, it is difficult to make generalizations about outcomes. Cognitive impairment in JSRD is highly variable, with many children exhibiting moderately severe disability (19). Moreover behavioral problems, typically impulsivity, perseveration, and temper tantrums, appear to be relatively common, particularly with increasing age (20). Close survelliance for retinal, renal and hepatic involvement is also necessary since the prognosis largely depends on the presence of these conditions (21).
Given the fact that these group of disorders are genetically heterogeneous and not all the causative genes are identified, the molecular diagnosis of JSRD is mainly for research purposes. It has been reported that currently identified genes account for an estimated 50% of causative mutations in JSRD (22). In addition, clinical features can vary between affected siblings within the same family. Also, many genotype-phenotype correlations that may require molecular diagnosis are age-dependent and may not be present in infants or young children (22). Other conditions to consider in the differential diagnosis of Joubert syndrome are those with cerebellar vermis hypoplasia or dysgenesis without the molar tooth sign on MRI. These include: Dandy-Walker malformation, X-linked cerebellar hypoplasia, congenital disorders of glycosylation, cranio-cerebello-cardiac syndrome, the pontocerebellar hypoplasias/atrophies, oral-facial-digital syndromes II and III and Meckel-Gruber syndrome (23).
Footnotes
Conflict of interest
No conflict of interest was declared by the authors.
References
- 1.D’Angelo A, Franco B. The primary cilium in different tissues-lessons from patients and animal models. Pediatr Nephrol. 2011;26:655–62. doi: 10.1007/s00467-010-1650-7. [DOI] [PubMed] [Google Scholar]
- 2.Joubert M, Eisenring JJ, Andermann F. Familial dysgenesis of the vermis, a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology. 1968;18:302–3. [PubMed] [Google Scholar]
- 3.Yachnis AT, Rorke LB. Neuropathology of Joubert syndrome. J ChildNeurol. 1999;14:655–9. doi: 10.1177/088307389901401006. [DOI] [PubMed] [Google Scholar]
- 4.Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, et al. “Joubert syndrome” revisited, key ocular motor signs with magnetic resonance imaging correlation, J”. Child Neurol. 1997;12:423–30. doi: 10.1177/088307389701200703. [DOI] [PubMed] [Google Scholar]
- 5.Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies, An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 6.Zaghloul NA, Brugmann SA. The emerging face of primary cilia. Genesis. 2011 Feb;49:231–46. doi: 10.1002/dvg.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–73. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiology. 2008;111:39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 10.Valente EM, Brancati F, Dallapiccola B. Genotypes and phenotypes of Joubert syndrome and related disorders. Eur J Med Genet. 2008;51:1–23. doi: 10.1016/j.ejmg.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, et al. Molar tooth sign of the midbrainhindbrain junction, occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125A:125–34. doi: 10.1002/ajmg.a.20437. [DOI] [PubMed] [Google Scholar]
- 12.Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) Eur J Hum Genet. 2007;15:511–21. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- 13.Chen CP, Su YN, Huang JK, Liu YP, Tsai FJ, Yang CK, et al. Fetal magnetic resonance imaging demonstration of central nervous system abnormalities and polydactyly associated with Joubert syndrome. Taiwan J Obstet Gynecol. 2010;49:243–6. doi: 10.1016/S1028-4559(10)60055-1. [DOI] [PubMed] [Google Scholar]
- 14.Saleem SN, Zaki MS. Role of MR imaging in prenatal diagnosis of pregnancies at risk for Joubert syndrome and related cerebellar disorders. Am J Neuroradiol. 2010;31:424–9. doi: 10.3174/ajnr.A1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluss J, Blaser S, Chitayat D, Akoury H, Glanc P, Skidmore M, et al. Molar tooth sign in fetal brain magnetic resonance imaging leading to the prenatal diagnosis of Joubert syndrome and related disorders. J Child Neurol. 2006;21:320–4. doi: 10.1177/08830738060210041001. [DOI] [PubMed] [Google Scholar]
- 16.Pugash D, Oh T, Godwin K, Robinson AJ, Byrne A, Van Allen MI, et al. The sonographic “molar tooth sign” in the diagnosis of Joubert syndrome. Ultrasound Obstet Gynecol. 2011 Mar;38:598–602. doi: 10.1002/uog.8979. [DOI] [PubMed] [Google Scholar]
- 17.Reynders CS, Pauker SP, Benacerraf BR. First trimester isolated fetal nuchal lucency: significance and outcome. J Ultrasound Med. 1997;16:101–5. doi: 10.7863/jum.1997.16.2.101. [DOI] [PubMed] [Google Scholar]
- 18.Bromley B, Nadel AS, Pauker S, Estroff JA, Benacerraf BR. Closure of the cerebellar vermis: evaluation with second trimester US. Radiology. 1994;193:761–3. doi: 10.1148/radiology.193.3.7972820. [DOI] [PubMed] [Google Scholar]
- 19.Steinlin M, Schmid M, Landau K, Boltshauser E. Follow-Up in Children with Joubert Syndrome. Neuropediatr. 1997;28:204–11. doi: 10.1055/s-2007-973701. [DOI] [PubMed] [Google Scholar]
- 20.Hodgkins PR, Harris CM, Shawkat FS, Thompson DA, Chong K, Timms C, et al. Joubert syndrome: long-term follow-up. Dev Med Child Neurol. 2004;46:694–9. doi: 10.1017/s0012162204001161. [DOI] [PubMed] [Google Scholar]
- 21.Brancati F, Dallapiccola B, Valente EM. Joubert Syndrome and related disorders. Orphanet J Rare Dis. 2010;5:20. doi: 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valente EM, Brancati F, Dallapiccola B. Genotypes and phenotypes of Joubert syndrome and related disorders. Eur J Med Genet. 2008;51:1–23. doi: 10.1016/j.ejmg.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Parisi M, Glass I. Joubert Syndrome. 2003 Jul 9 [updated 2007 Mar 8] In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. Gene Reviews [Internet] Seattle (WA): University of Washington, Seattle; 1993. [PubMed] [Google Scholar]