Abstract
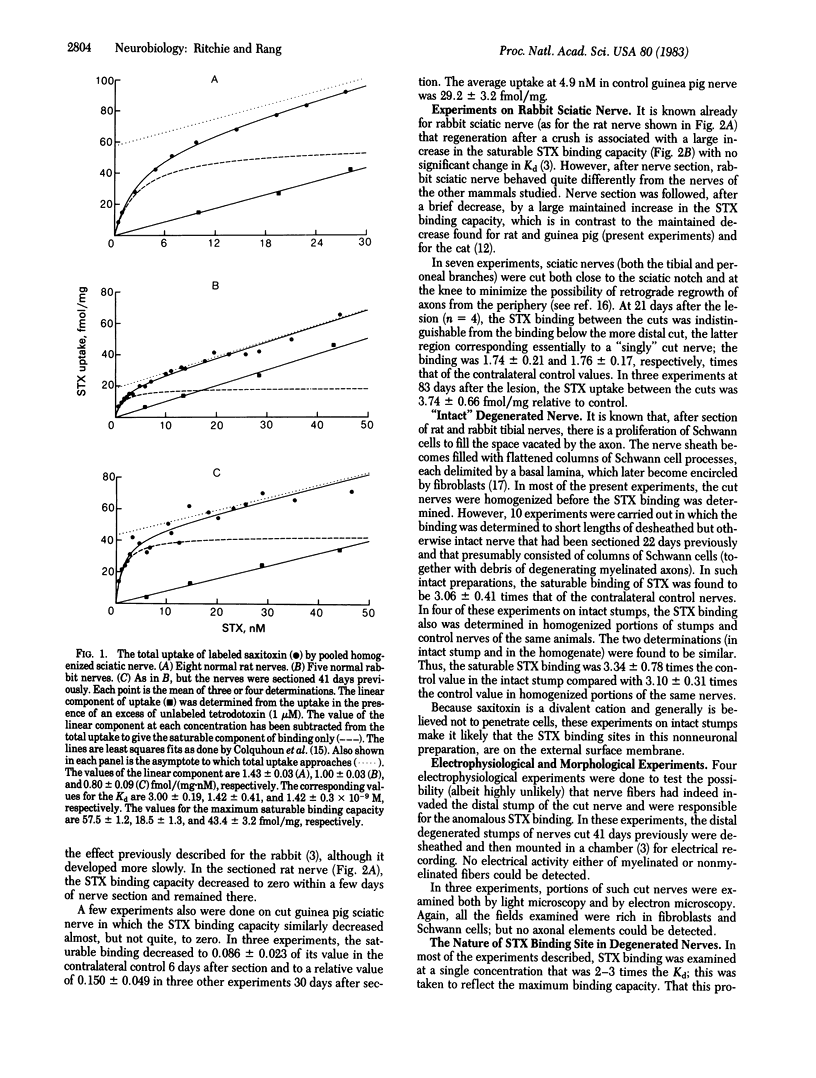
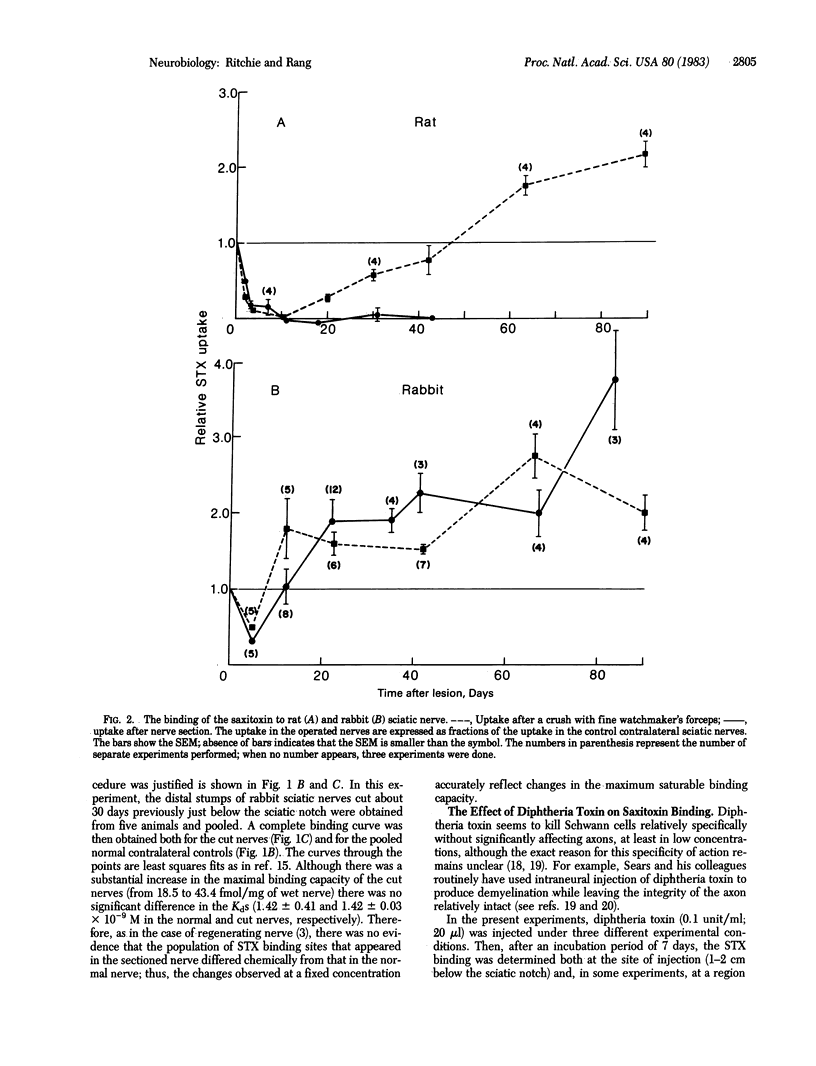
The changes in binding of 3H-labeled saxitoxin (STX) to rabbit sciatic nerve during axonal regeneration (after nerve crush) and during axonal degeneration (after nerve section) were measured and compared with the corresponding changes in the sciatic nerves of other mammals (rat, guinea pig, and cat). In the rabbit and rat, regeneration after nerve crush is associated with a 2- to 4-fold increase in STX binding capacity, consistent with the known corresponding increase in the number of nodes of Ranvier in regenerating nerve. Furthermore, consistent with the disappearance of nodes that occurs with Wallerian degeneration, nerve section leads to a disappearance of all, or most, of the STX binding in rat and guinea pig nerve, similar to that previously found for cat nerve. However, in the rabbit, nerve section leads to a large maintained increase in STX binding. Intraneural injection of diphtheria toxin, which is known to damage Schwann cells and which causes an increase in STX binding in intact nerves, abolishes the binding in cut nerves. It is suggested that the increased binding in cut nerves is to nonneuronal sites situated on the surface membrane of the Schwann cells, which have greatly proliferated in number as axonal degeneration has progressed. The reason for the difference between rabbits and other species and the possibility that the binding sites of rabbit Schwann cells represent functional sodium channels remain to be investigated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRREN J. E., WALL P. D. Age changes in conduction velocity, refractory period, number of fibers, connective tissue space and blood vessels in sciatic nerve of rats. J Comp Neurol. 1956 Feb;104(1):1–16. doi: 10.1002/cne.901040102. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. THE CONDUCTION VELOCITY OF REGENERATED PERIPHERAL NERVE FIBRES. J Physiol. 1964 May;171:164–175. doi: 10.1113/jphysiol.1964.sp007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Asymmetry currents in the mammalian myelinated nerve. J Physiol. 1980 Dec;309:499–519. doi: 10.1113/jphysiol.1980.sp013523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M. Ionic and gating currents in mammalian myelinated nerve. Adv Neurol. 1981;31:313–328. [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HISCOE H. B. Distribution of nodes and incisures in normal and regenerated nerve fibers. Anat Rec. 1947 Dec;99(4):447–475. doi: 10.1002/ar.1090990404. [DOI] [PubMed] [Google Scholar]
- Holmes W., Young J. Z. Nerve regeneration after immediate and delayed suture. J Anat. 1942 Oct;77(Pt 1):63–96.10. [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. M., Cavanagh J. B. Species differences in internode formation following two types of peripheral nerve injury. J Anat. 1969 Sep;105(Pt 2):295–306. [PMC free article] [PubMed] [Google Scholar]
- LUBINSKA L. Demyelination and remyelination in the proximal parts of regenerating nerve fibers. J Comp Neurol. 1961 Dec;117:275–289. doi: 10.1002/cne.901170302. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. Focal experimental demyelination in the central nervous system. Brain. 1970;93(3):575–582. doi: 10.1093/brain/93.3.575. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Westermark B., Glaser L. Tetrodotoxin-sensitive sodium channels in normal human fibroblasts and normal human glia-like cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6425–6429. doi: 10.1073/pnas.76.12.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J Physiol. 1982 Aug;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Jacques Y., Lazdunski M. Identification of a tetrodotoxin-sensitive Na+ channel in a variety in fibroblast lines. Nature. 1980 Jul 10;286(5769):162–164. doi: 10.1038/286162a0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Chiu S. Y. Distribution of sodium and potassium channels in mammalian myelinated nerve. Adv Neurol. 1981;31:329–342. [PubMed] [Google Scholar]
- Ritchie J. M., Rang H. P., Pellegrino R. Sodium and potassium channels in demyelinated and remyelinated mammalian nerve. Nature. 1981 Nov 19;294(5838):257–259. doi: 10.1038/294257a0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A. 1977 Jan;74(1):211–215. doi: 10.1073/pnas.74.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. Sodium and potassium channels in regenerating and developing mammalian myelinated nerves. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):273–287. doi: 10.1098/rspb.1982.0042. [DOI] [PubMed] [Google Scholar]
- Romey G., Jacques Y., Schweitz H., Fosset M., Lazdunski M. The sodium channel in non-impulsive cells. Interaction with specific neurotoxins. Biochim Biophys Acta. 1979 Sep 21;556(2):344–353. doi: 10.1016/0005-2736(79)90053-1. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Bostock H. Conduction failure in demyelination: is it inevitable? Adv Neurol. 1981;31:357–375. [PubMed] [Google Scholar]
- Tang C. M., Strichartz G. R., Orkand R. K. Sodium channels in axons and glial cells of the optic nerve of Necturus maculosa. J Gen Physiol. 1979 Nov;74(5):629–642. doi: 10.1085/jgp.74.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIZOSO A. D., YOUNG J. Z. Internode length and fibre diameter in developing and regenerating nerves. J Anat. 1948 Apr;82(Pt 1-2):110–134. [PubMed] [Google Scholar]
- Villegas J., Sevcik C., Barnola F. V., Villegas R. Grayanotoxin, veratrine, and tetrodotoxin-sensitive sodium pathways in the Schwann cell membrane of squid nerve fibers. J Gen Physiol. 1976 Mar;67(3):369–380. doi: 10.1085/jgp.67.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER H. D., SPIRO D., WAKSMAN B., ADAMS R. D. Phase and electron microscopic studies of experimental demyelination. II. Schwann cell changes in guinea pig sciatic nerves during experimental diphtheritic neuritis. J Neuropathol Exp Neurol. 1961 Jan;20:5–34. doi: 10.1097/00005072-196101000-00002. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978 Oct;7(5):555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- Yates A. J., Bouchard J. P., Wherrett J. R. Relation of axon membrane to myelin membrane in sciatic nerve during development: comparison of morphological and chemical parameters. Brain Res. 1976 Mar 12;104(2):261–271. doi: 10.1016/0006-8993(76)90618-1. [DOI] [PubMed] [Google Scholar]


