Abstract
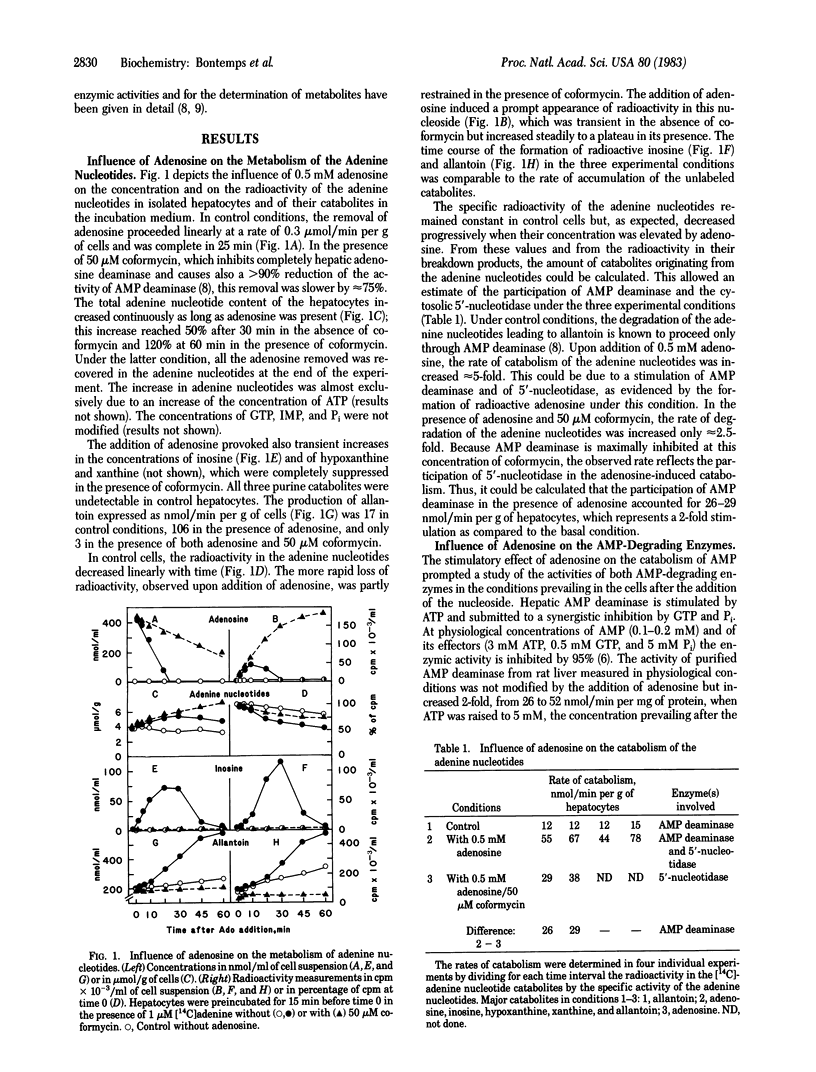
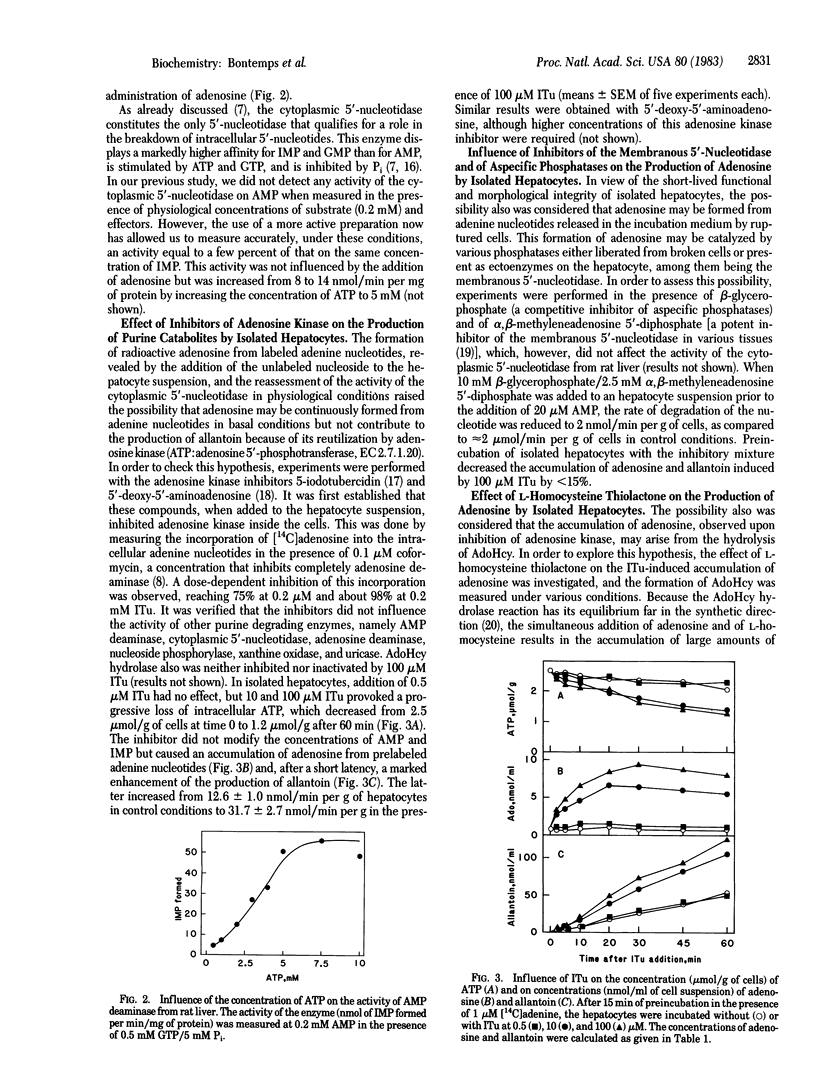
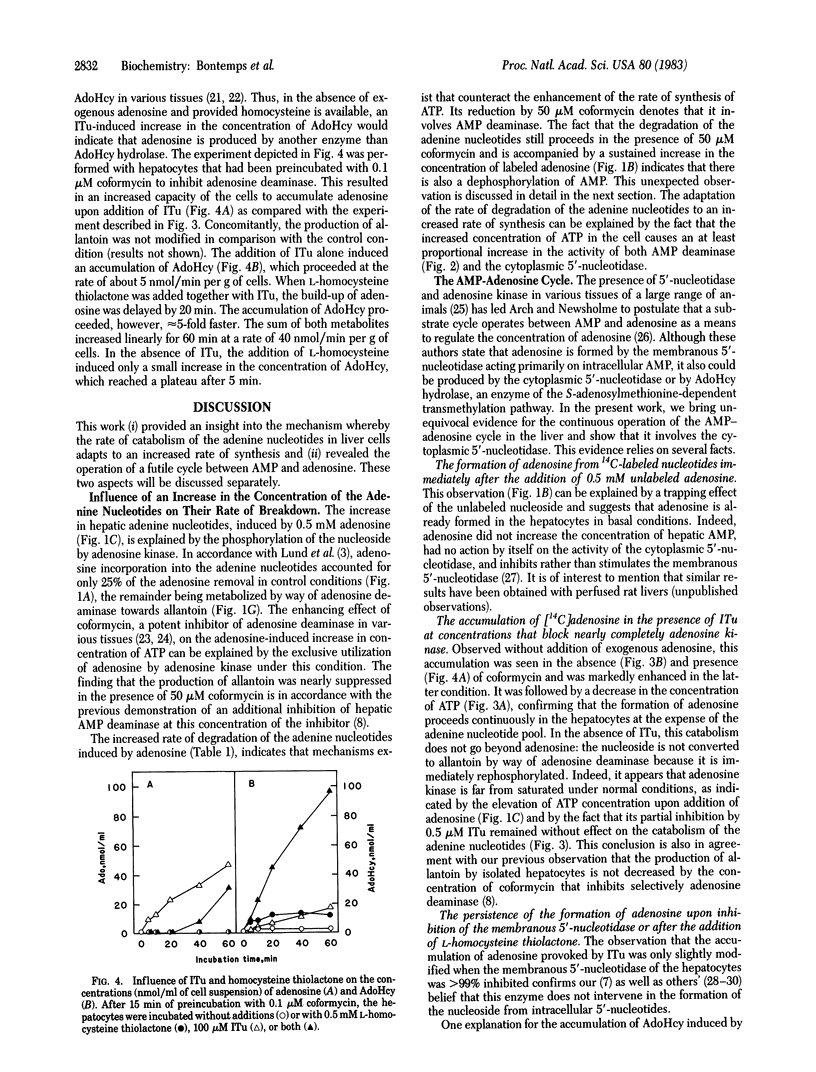
The effect of adenosine on the metabolism of prelabeled adenine nucleotides was investigated in isolated hepatocytes. Adenosine caused an approximately equal to 2-fold increase in the ATP content of the cells. This effect was in part counteracted by an increased rate of adenine nucleotide catabolism that could be explained by a stimulation of both AMP deaminase (AMP aminohydrolase, EC 3.5.4.6) and the cytoplasmic 5'-nucleotidase (5'-ribonucleotide phosphohydrolase, EC 3.1.3.5) because of the increased concentration of ATP. The unexpected finding that labeled adenosine was formed immediately after the addition of the unlabeled nucleoside could be explained by the trapping effect of adenosine. An accumulation of labeled adenosine was observed also in the presence of 5-iodotubercidin, a potent inhibitor of adenosine kinase (ATP:adenosine 5'-phosphotransferase, EC 2.7.1.20). Under these conditions, there was a decrease in the concentration of ATP in the cell and a 2- to 3-fold increase in the rate of formation of allantoin. This formation of adenosine was only slightly decreased by inhibition of the membranous 5'-nucleotidase; it led to the accumulation of S-adenosylhomocysteine in the presence of coformycin and an excess of L-homocysteine. It was concluded that, under basal conditions, the cytoplasmic 5'-nucleotidase present in the liver cell continuously produces adenosine, which is immediately reconverted into AMP by adenosine kinase, without giving rise to allantoin. This futile cycle between AMP and adenosine amounts to at least 20 nmol/min per g of liver and, thus, exceeds the basic rate of allantoin formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Brox L. W., Henderson J. F. The "adenosine cycle" is not a significant route of purine metabolism im mammalian cells. Can J Biochem. 1976 Feb;54(2):200–202. doi: 10.1139/o76-031. [DOI] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. Preparation and properties of 5'-nucleotidase from smooth muscle of small intestine. J Biol Chem. 1970 Dec 10;245(23):6274–6280. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Wasson D. B. The potential importance of soluble deoxynucleotidase activity in mediating deoxyadenosine toxicity in human lymphoblasts. J Immunol. 1981 Jan;126(1):348–352. [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Piña E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1441–1445. doi: 10.1016/s0006-291x(72)80138-4. [DOI] [PubMed] [Google Scholar]
- Chan T. S., Ishii K., Long C., Green H. Purine excretion by mammalian cells deficient in adenosine kinase. J Cell Physiol. 1973 Jun;81(3):315–322. doi: 10.1002/jcp.1040810304. [DOI] [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Palella T. D., Thompson D., Herring C. Adenosine metabolism: modification by S-adenosylhomocysteine and 5'-methylthioadenosine. Arch Biochem Biophys. 1982 Apr 15;215(1):302–308. doi: 10.1016/0003-9861(82)90308-3. [DOI] [PubMed] [Google Scholar]
- Helland S., Ueland P. M. Inactivation of S-adenosylhomocysteine hydrolase by 9-beta-D-arabinofuranosyladenine in intact cells. Cancer Res. 1982 Mar;42(3):1130–1136. [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Paul B., Chan M. C., Lau K. F. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2. 1972 Nov;3(1):71–85. [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine biosynthesis in human lymphoblasts. Coordinate control of proximal (rate-determining) steps and the inosinic acid branch point. J Biol Chem. 1976 Dec 10;251(23):7348–7354. [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R., Usami C., Nishino T., Tsushima K. Kinetic properties of cytosol 5'-nucleotidase from chicken liver. Biochim Biophys Acta. 1978 Sep 11;526(1):154–162. doi: 10.1016/0005-2744(78)90300-5. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Hershfield M. S. S-adenosylhomocysteine toxicity in normal and adenosine kinase-deficient lymphoblasts of human origin. Proc Natl Acad Sci U S A. 1979 May;76(5):2450–2454. doi: 10.1073/pnas.76.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax C. A., Henderson J. F. Adenosine formation and metabolism during adenosine triphosphate catabolism in Ehrlich ascites tumor cells. Cancer Res. 1973 Nov;33(11):2825–2829. [PubMed] [Google Scholar]
- Lund P., Cornell N. W., Krebs H. A. Effect of adenosine on the adenine nucleotide content and metabolism of hepatocytes. Biochem J. 1975 Dec;152(3):593–599. doi: 10.1042/bj1520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J. C., Lavoinne A., Giroz M., Matray F. The influence of adenosine on intermediary metabolism of isolated hepatocytes. Biochimie. 1979;61(11-12):1273–1282. doi: 10.1016/s0300-9084(80)80286-0. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H., Koszalka G. W., Rideout J. L., Beacham L. M., 3rd, Chao E. Y., Haggerty J. J., Krenitsky T. A., Elion G. B. Adenosine kinase from rabbit liver. II. Substrate and inhibitor specificity. J Biol Chem. 1979 Apr 10;254(7):2346–2352. [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981 Nov 15;200(2):399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. Role of adenosine deaminase, ecto-(5'-nucleotidase) and ecto-(non-specific phosphatase) in cyanide-induced adenosine monophosphate catabolism in rat polymorphonuclear leucocytes. Biochem J. 1980 Mar 15;186(3):907–918. doi: 10.1042/bj1860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibel D. K., Rovetto M. J. Separation of myocardial purine nucleosides and nucleotides by one-dimensional thin-layer chromatography. J Chromatogr. 1978 Nov 21;161:406–409. doi: 10.1016/s0021-9673(01)85263-8. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., BRENNER B. M. 5'-Nucleotidase of rat liver microsomes. J Biol Chem. 1960 Feb;235:471–474. [PubMed] [Google Scholar]
- Sawa T., Fukagawa Y., Homma I., Takeuchi T., Umezawa H. Mode of inhibition of coformycin on adenosine deaminase. J Antibiot (Tokyo) 1967 Jul;20(4):227–231. [PubMed] [Google Scholar]
- Schrader J., Schütz W., Bardenheuer H. Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J. 1981 Apr 15;196(1):65–70. doi: 10.1042/bj1960065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Henderson J. F. Alternative pathways of deoxyadenosine and adenosine metabolism. J Biol Chem. 1973 Aug 25;248(16):5899–5904. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. The pathway of adenine nucleotide catabolism and its control in isolated rat hepatocytes subjected to anoxia. Biochem J. 1982 Jan 15;202(1):117–123. doi: 10.1042/bj2020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening J., Nowack J., Decker K. The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta. 1975 Jun 12;392(2):299–309. doi: 10.1016/0304-4165(75)90011-2. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


