Introduction
In 2004 and 2005, Johnson et al. published two very provocative studies (1, 2), in which they claimed that in the adult mouse ovary, neo-oogenesis takes place and originates either from the ovarian surface epithelium (OSE) (1) or from the bone marrow (BM) via circulating blood cells (2). These studies were provocative since they challenged the long-held view that mammals are born with a finite number of eggs that declines with ageing. Consequently, an intensive discussion has developed among experts in the field, some of whom are proponents of neo-oogenesis, while others are opponents (Table 1).
Table 1.
Some articles written by proponents and opponents of neo-oogenesis in the adult ovary
| Experimental studies supporting neo-oogenesis |
| Johnson et al., 2004. Nature 425: 145–50. |
| Bukovsky et al., 2004. Reprod Biol Endocrinol 2: 20. |
| Johnson et al., 2005. Cell 122: 303–15. |
| Bukovsky et al., 2005. Reprod Biol Endocrinol 3: 17. |
| Bukovsky et al., 2005. Reprod Biol Endocrinol 3: 36. |
| Kerr et al., 2006. Reproduction 132: 95–109. |
| Lee et al., 2007. Cell Cycle 6: 2678–84. |
| Lee et al., 2007. J Clin Oncol 25: 3198–204. |
| Bukovsky et al., 2008. Cell Cycle 7: 683–6. |
| Virant-Klun et al., 2008. Differentiation 76: 843–56. |
| Virant-Klun et al., 2009. Stem Cells Dev 18: 137–49. |
| Niikura et al., 2009. Aging 1: 971–8. |
| Zou et al., 2009. Nature Cell Biol 11: 631–6. |
| Pacchiarotti et al., 2010. Differentiation 79: 159–70. |
| Parte et al., 2010. Stem Cells Dev 20: 1451–64. |
| Celik et al., 2009. Int J Gynaecol Obstet 106: 218–22. |
| Reviews, opinions and comments supporting neo-oogenesis |
| Johnson et al., 2005. Cell Cycle 4: e36–e42. |
| Tilly and Johnson 2007. Cell Cycle 6: 879–83. |
| Skaznik-Wikiel etal., 2007. Differentiation 75: 93–9. |
| Abban & Johnson, 2009. Hum Reprod 24: 2974–8. |
| Bukovsky et al., 2009. Birth Defects Res C Embryo Today 87:64–89. |
| Tilly et al., 2009. Biol Reprod 80: 2–12. |
| De Felici 2010. Mol Hum Reprod 16: 632–6. |
| Virant-Klun et al., 2010. Aging 2: 3–6. |
| Virant-Klun et al., 2011. Histol Histopathol. 26: 1071–82. |
| Experimental studies concluding that “there is no neo-oogenesis” |
| Telfer, 2004. Reprod Biol Endocrinol 2: 24. |
| Byskov et al., 2005. Differentiation 73: 438–46. |
| Eggan et al., 2006. Nature 441: 1109–14. |
| Bristol-Gould et al., 2006. Dev Biol 298: 149–54. |
| Liu et al., 2007. Dev Biol 306: 112–20. |
| Veitia et al. Stem Cells. 25: 1334–5. |
| Begum et al., 2008. Hum Reprod 10: 2326–30. |
| Faddy and Gosden 2009. Biol Reprod 81: 231–2. |
| Zhang et al., 2010. Reprod Dom Anim 45: e447–e53. |
| Byskov et al., 2011. Hum Reprod 26: 2129–39. |
| Reviews, opinions and comments concluding that there is no neo-oogenesis |
| Albertini, 2004. Reproduction 127: 513–4. |
| Gosden 2004. Hum Reprod Update 10: 193–5. |
| Greenfeld and Flaws 2004. BioEssays 26: 829–32. |
| Ainsworth, 2005. Nature 436: 609. |
| Hutt and Albertini 2006. J Exp Clin Ass Reprod 3: 6. |
| Notarianni 2011. J Ov Res 4: 1. |
Neo-oogenesis is a very complex issue that has led to many questions. The aim of our lecture is to explain why we believe that spontaneous neo-oogenesis does not take place in the adult ovary by addressing three of these questions.
Is there any evidence for spontaneous neo-oogenesis in adult rodent and human ovaries?
Johnson et al. (1) claimed that, in mice, atresia in the immature follicle pool (i.e. including follicles from the primordial to the preantral stage) is so high that complete exhaustion of the pool would be predicted for young adults. Consequently, according to Johnson et al., only the renewal of oocytes as generated by neo-oogenesis can explain the fact that mice are still fertile after the advanced age of 1 year.
To estimate the rate of this atresia, Johnson et al. (1) scored as atretic those immature follicles exhibiting a “condensed, involuted or fragmented oocyte”, and consequently counted around 2700 healthy follicles and 200 to 400 atretic immature follicles at postnatal day 30 PN in C57/Bl6 mice. Meanwhile, Bykov et al. (3) counted between 1810 and 3280 healthy and 235–480 atretic follicles, i.e. similar numbers to the data of Johnson et al. (1). However, except for 3 atretic primordial follicles, all immature (primordial, primary and preantral) follicles were healthy, which is in agreement with previous published data showing that atresia of immature follicles is very low in rodents and human ovaries. Among the atretic follicles, antral atretic follicles exhibited a healthy looking oocyte, whereas degenerated and fragmented oocytes were only observed in atretic follicles at a late stage of atresia and which were previously antral follicles. Thus, it emerges that Johnson et al. (1), misattributed as atretic immature follicles those 200 to 400 atretic follicles that were already present at least 8 days earlier, as shown by their BrdU labeling. How can we explain such a misinterpretation? Returning to the criteria used to categorize atresia above, whereas condensation of oocytes (Figure 1) constitutes the normal fate of atretic resting follicles, and oocyte degeneration the normal fate of early growing follicles, fragmented oocytes are only seen in antral follicles at a late stage of atresia (Figure 2). When antral follicles undergo atresia, they progressively lose their antrum and shrink to the size of preantral follicles. This is the likely reason why Johnson et al. (1) mistook these follicles as being “immature”.
Figure 1.
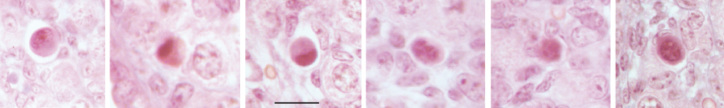
Some examples of atretic resting follicles exhibiting a condensed oocyte in the mouse ovary (bar=20 μm)
Figure 2.
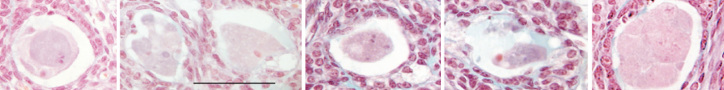
Some examples of involuted and fragmented oocytes from atretic antral follicles at a late stage of atresia in the mouse ovary (bar=80 μm)
Consequently, it cannot be deduced (1) that between postnatal days 30 and 42, 10% to 33% of the immature pool is atretic. These percentages apply to antral follicles that degenerate instead of becoming preovulatory, with the oocyte being one of the last of the constituent cells to disappear. This issue is crucial since the rate of follicle depletion in the postnatal mouse ovary provided by Johnson et al. (1), which was deduced from the percentages of immature atretic follicles at different ages, was calculated based on a follicular clearance rate of between 3 and 18h rather than the more appropriate estimate for antral follicles of more than 8 days. Consequently, the ovarian reserve would not be completely exhausted by young adulthood and adult female mice would not need neo-oogenesis for maintaining a normal ovarian function.
In the rat, meanwhile, Meredith et al. (4) have shown, using BrdU incorporation, that approximately 60% of resting follicles present at a given time are still present 5 months later. Also, in this species, Zhang et al. (5), failed to detect early meiosis-specific proteins at the transcriptional (SCP1, SCP3, SPO11) or translational (SCP1, STRA8) levels in the post natal rat ovary. Together, the long half-life of the resting follicles and the absence of cells in early meiosis argue against the existence of spontaneous neo-oogenesis in the adult rat ovary.
If, as required by the neo-oogenesis concept, oogonia exists in the adult ovary as intermediates between stem cells and oocytes, they will enter meiosis at various times and progress through leptotene, pachytene and zygotene stages to reach diplotene (dictyate oocyte) stage, at which meiosis is blocked. If Johnson’s calculation for follicle renewal were correct (1), around 60 oocytes would be in transit through meiosis every day. Figure 3 shows that it is very easy to discriminate between oocytes in the intermediate (pre-dictyate) stages of meiosis and those that are arrested (dictyate). Despite the hundreds of thousands of primordial follicles that have been analysed for quantification and quality assessment purposes, pre-dictyate-stage meiotic oocytes have never been observed in either primates (at least 250 human and adult macaca ovaries examined by A. Gougeon) or rodent ovaries. Also, Liu et al. (6), failed to observe early meiotic oocytes and proliferating germ cells, or to detect mRNA for early meiosis-specific or oogenesis-associated genes (SPO11, PRDM9, SCP1, TERT and NOBOX), in adult human ovaries. In addition, Byskov et al. (7) observed that, in 82 human ovaries (from the embryonic stage to the age of 32 years), almost all oogonia stained exclusively for SSEA4, NANOG, OCT4 and c-kit, whereas only a small fraction stained for oogonia-specific MAGE-A4. These few oogonia disappeared from the ovary before 2 years of age, leaving only dictyate oocytes that stained for c-kit.
Figure 3.
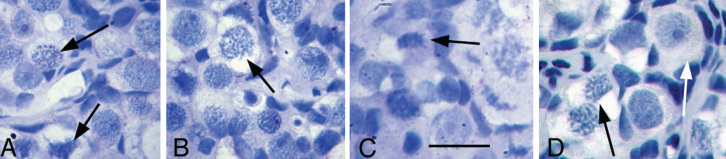
Leptotene, pachytene, zygotene (black arrows) and diplotene (white arrow) stages of meiosis in the foetal monkey (Macaca fascicularis). Notice the difference between the primordial follicle at the diplotene (dictyate) stage and the intermediate stages (Leptotene, pachytene, zygotene) of meiosis (Bar=30 μm)
Consequently, it can be concluded that oogonia, the bona fide female germline stem cells, do not persist to support spontaneous neo-oogenesis in the adult human ovary.
What is the true effect of busulfan on folliculogenesis?
In the study of Johnson et al. (2), strain CB57/Bl6 female mice were treated with a mixture of cyclophosphamide and busufan (Cy/Bu). Two months after the treatment, no follicles were detectable within the ovaries. According to the authors, the spontaneous depletion of the ovarian reserve by atresia of the immature pool was no longer counteracted by neo-oogenesis, owing to the destruction by Bu of premeiotic germ cells present in the ovary at the time of treatment, leading to a complete exhaustion of the ovarian reserve within 3 weeks.
Although logical, this conclusion assumes that Bu is not cytotoxic to the resting and growing follicles already present at the time of treatment. However, Bu treatment disrupts the whole process of folliculogenesis by inducing both atresia and abnormal follicular growth (8); and, as shown by Generoso et al. (9), one injection of Bu has a dose-dependent detrimental effect on fertility via attrition of the oocyte pool. Interestingly, Bu is cytotoxic to follicles, via suppression of c-kit/SCF signaling (10), this system being crucial for activation of resting follicles and for survival of resting and growing follicles.
It can therefore be concluded that, in the study of Johnson et al. (1), Bu would not have inhibited neo-oogenesis, but rather would have destroyed growing follicles and strongly depleted the ovarian reserve. Consequently, all studies using Bu and claiming the existence of neo-oogenesis must be considered with caution.
In addition to the claim contested here that the combination treatment with Cy/Bu depletes the ovary via destruction only of oocytes generated by neo-oogenesis prior to meiosis, Johnson et al. (2) reported that bone marrow transfer (BMT) performed 7 days after the Cy/Bu treatment was able to refurbish the ovary with follicles at all stages of development. They subsequently concluded that neo-oogenesis occurred from germline stem cells present in the bone marrow. However this conclusion was challenged by three studies. Eggan et al. (11) have used parabiosis between a wild-type (WT) mouse, either treated or untreated with Cy/Bu, and a GFP (green fluorescent protein)- transgenic mouse. Six to eight months later the two parabionts were treated with PMSG for superovulation and oocytes harvested. Despite a strong BM chimaerism in both parabionts, the GFP-transgenic mice ovulated only GFP oocytes, whereas the WT-mice only ovulated WT-oocytes. Hence, no transmission of blood-borne oocyte precursors had occurred between parabionts. In another study by Lee et al. (12), using a similar experimental design to that of Johnson et al. (2), mice were treated with Cy/Bu followed by BMT from a GFP transgenic mouse one week later and immediate mating, and none of the offspring produced were GFP positive. Some GFP-positive cells were observed and termed “oocytes”, but were very small in number (1.4%) and apparently only located in small follicles. In a third study, performed by Begum et al. (13), irradiated (and therefore sterilized) WT ovaries were grafted under the kidney capsule of GFP-transgenic recipient mice. One month later, none of the resulting 48 growing oocytes were GFP positive. A similar experiment, consisting of grafting untreated WT-ovaries under the kidney capsule of GFP-transgenic recipient mice, has shown that none of the resulting 819 oocytes, either resting or growing, were GFP positive, 2, 4 and 8 weeks after the grafting procedure. Taken together, the results of these studies failed to show that neo-oogenesis occurs from putative germline stem cells (GSC) that are present in and circulated from the bone marrow.
Are de novo oocytes really generated by the ovarian surface epithelium?
The most recent papers supporting the concept of neo-oogenesis in the postnatal ovary postulate that GSC originate in the ovarian surface epithelium (OSE). Many alternative explanations concerning the presence of putative GSC within the OSE have been recently reported (15), and some of them are presented below.
Johnson et al. (1), as well as Zou et al. (14), reported the presence of cells positively stained for both BrdU and mouse vasa homolog (Mvh) in the OSE. They concluded that replicative GSC are present in the OSE. However, on the one hand BrdU may stain the mitochondrial DNA during its process of replication and/or DNA repair (15); and on the other hand Mvh, which is a germinal cell marker, can stain primordial follicles that are known to be located in the OSE. Consequently, the BrdU/Mvh stained cells present in the OSE are not GSC, but most likely primordial oocytes during their physiologic extrusion process.
Virant-Klun et al. (16), described small round cells, above and below the postmenopausal OSE, which they considered to be GSC. However, they may correspond instead to the small immune cells previously described in this location by Motta et al. (17). Zou et al., (14) claimed to have successfully isolated and purified GSC from disaggregated postnatal and adult mouse ovaries via Mvh-specific binding to an anti-Mvh antibody. However, they did not consider the possibility that their lines come from quiescent oogonia, which are known to be present in the postnatal mouse ovary up to day 7 (15). In addition, the presence of mitotic oogonia in the adult ovary is difficult to reconcile with the absence of the stage specific embryonic antigen-1, as oogonia proliferating in vivo are demonstrably positive for this marker.
Conclusion
This review highlights crucial issues in the debate over the existence, or otherwise, of female germline stem cells in the mammalian ovary. Further experimentation is needed to fully disprove the concepts that the mammalian ovary contains cells with stem cell-like characteristics that can be provoked to enter a differentiation process to oocytes, at least in vitro; and that in vitro conditions may allow the conversion of OSE cells into a multipotent stem cell-like phenotype. However, we claim that, in normal in vivo conditions, neo-oogenesis does not take place in the adult mammalian ovary.
References
- 1.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 2.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Eggs forever? Differentiation. 2005;73:438–46. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Meredith S, Dudenhoeffer G, Jackson K. Classification of small type B/C follicles as primordial follicles in mature rats. J Reprod Fertil. 2000;119:43–8. doi: 10.1530/jrf.0.1190043. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Lv LX, Xing WJ. Early meiotic-specific protein expression in post-natal rat ovaries. Reprod Domest Anim. 2010;45:447–53. doi: 10.1111/j.1439-0531.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306:112–20. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Byskov AG, Høyer PE, Yding Andersen C, Kristensen SG, Jespersen A, Møllgård K. No evidence for the presence of oogonia in the human ovary after their final clearance during the first two years of life. Hum Reprod. 2011;26:2129–39. doi: 10.1093/humrep/der145. [DOI] [PubMed] [Google Scholar]
- 8.Burkl W, Schiechl H. The growth of follicles in the rat ovary under the influence of busulphan and endoxan. Cell Tissue Res. 1978;186:351–9. doi: 10.1007/BF00225543. [DOI] [PubMed] [Google Scholar]
- 9.Generoso WM, Stout SK, Huff SW. Effects of alkylating chemicals on reproductive capacity of adult female mice. Mutat Res. 1971;13:171–84. doi: 10.1016/0027-5107(71)90010-8. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, Yeo SM, et al. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53- independent manner. FEBS Lett. 2004;575:41–51. doi: 10.1016/j.febslet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–14. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-J, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 13.Begum S, Papaioannou VE, Gosden RG. The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum Reprod. 2008;23:2326–30. doi: 10.1093/humrep/den249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou K, Yuan Z, Luo H, Sun K, Zhou L, Xiang J, et al. Production of offspring from a germinal stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 15.Notarianni E. Reinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserve. J Ovarian Res. 2011;4:1. doi: 10.1186/1757-2215-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rülicke T, et al. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009;18:137–49. doi: 10.1089/scd.2007.0238. [DOI] [PubMed] [Google Scholar]
- 17.Motta PM, Heyn R, Makabe S. Three-dimensional microanatomical dynamics of the ovary in postreproductive aged women. Fertil Steril. 2002;78:360–70. doi: 10.1016/s0015-0282(02)03216-8. [DOI] [PubMed] [Google Scholar]


