Abstract
Claudins are a family of proteins and the most important component of the tight junction. They constitute a paracellular barrier that controls the flow of molecules in the intercellular space of an epithelium. Although it seems that claudin should be down regulated in cancer cell, some claudins are, in fact highly elevated in various human cancers, including ovarian cancer. Whereas the functional significance of claudin overexpression in ovarian carcinoma is unclear, these proteins are important for migration, invasion, and survival of ovarian cancer cells. They clearly represent a general pathway in tumorigenesis, are a novel marker for ovarian cancer and may become a target for therapy or diagnosis of this disease.
Keywords: Tight junction, occludin, claudin, ovarian cancer, Clostridium perfringens enterotoxin
Özet
Klaudinler bir protein ailesidir ve konneksonların en önemli üyeleridir. Moleküllerin epitelin hücrelerarası boşluğuna geçmesini kontrol eden bir engel oluştururlar. Klaudinlerin kanser olgularında azalması beklenirken bazı klaudinler ise over kanserini de içeren farklı insan kanserlerinde yüksek oranlarda artmıştır. Bu proteinlerin over kanserlerinde artmış bulunmasının fonksiyonel önemi bilinmemekle birlikte bu proteinler migrasyon, invazyon ve over kanserlerinin hayatta kalaması için önemlidir. Tümör oluşumunda genel bir yolu temsil ettiklerinden over kanserleri için yeni bir belirteçtirler ve bu hastalığın tanı ve tedavisinde yeni bir hedef olmaya adaydırlar.
Introduction
Claudins are 20–27 kDa transmembrane proteins spanning the cellular membrane 4 times, with the N-terminal end and the C-terminal end both located in the cytoplasm, and two extracellular loops which show the highest degree of conservation. The first extracellular loop consists on average of 53 amino acids and the second one, being slightly smaller, of 24 amino acids. The N-terminal end is usually very short (4–10 amino acids), the C-terminal end varies in length from 21 to 63 and is necessary for the localisation of these proteins in the tight junctions. It is suspected that the cysteines of individual or separate claudins form disulfide bonds. All human claudins (with the exception of Claudin 12) have domains that let them bind to PDZ domains of scaffold proteins.
Work from several groups has now confirmed the over expression of these proteins in ovarian cancer. To find the subtypes based on a specific marker is a lucrative idea in diagnosis and specific managements including targeting and prognosis, such as ER, PR and HER neu in breast cancer, cyclin D and some translocations in leukemias. Ovarian cancer is a killer disease of unknown aetiology. It is detected late and has no good treatment. So, effort is concentrated on the use of claudin for diagnosis and target of therapy in ovarian cancer. It is worthwhile to study claudin in detail to know its significance in ovarian cancer.
Tight junction
Specialized intercellular junctions, known as desmosomes and terminal bars were recognized as local modifications of the surface of adjacent yet separate cells, rather than as intercellular bridges (1,2) with an effect on cell-to-cell adhesion and epithelial permeability.
In the nineties, mammalian intercellular junctions were described and were categorized into four types: adherens junctions (AJ), desmosomes (DS), gap junctions (GJ), and tight junctions (TJ) (3). The major integral membrane proteins in AJ are glycoprotein, cadherins. The desmosomal integral membrane proteins are called desmogleins and desmocollins, similar in amino acid sequence to cadherins, and they fall into the cadherin superfamily. The integral membrane protein in GJ is a dense aggregation of multimeric channels, each of which consists of six identical proteins named connexins. TJ is an element of the epithelial and endothelial junctional complex. It seals cells to create the primary barrier to the diffusion of solutes through the paracellular pathway. It also works as a boundary between the apical and basolateral plasma membrane domains to create the polarization of epithelial and endothelial cells. It remains controversial whether the particles in the strands of TJ are predominantly lipidic in nature. However, detergent stability of TJ strands as visualized by negative staining and freeze fracture proves that these elements are not composed solely of lipids
Tight junctions, together with adherens junctions and desmosomes, form the apical junctional complex in epithelial and endothelial cellular sheets. Adherens junctions and desmosomes are responsible for the mechanical adhesion between adjacent cells, whereas tight junctions are essential for the tight sealing of the cellular sheets, thus controlling paracellular ion flux and therefore maintaining tissue homeostasis. Tight junctions also play a crucial role in the maintenance of cell polarity by forming a fence that prevents lateral diffusion of membrane proteins and lipids, thereby maintaining the differential composition of the apical and basolateral domains.
Occludin
In the late nineties, ZO-1, a tight junction-associated protein, was derived from chick liver. This protein was not extractable from plasma membranes without detergent, suggesting that it is an integral membrane protein. When its cDNA was cloned and sequenced a new 504-amino acid, 55.9 kDa polypeptide with a hydrophilicity plot very similar to that of connexin was found. A search of the data base identified no proteins with significant homology to this membrane protein. Furuse et al. (3) designated this integral membrane protein localizing at tight junctions as “occludin.”
Claudin
Identification of claudin was regarded as the Holy Grail in this field. Although successive studies emphasized the importance of occludin in the structure and functions of TJs, it gradually became clear that the molecular architecture of TJ strands is more complex than expected. Especially, the fact that the occludin-deficient visceral endoderm cells still bear a well developed network of TJ strands indicated that membrane proteins or lipid molecules as yet unidentified may constitute TJ strands (4). Another two distinct peptide sequences of 211 and 230 amino acids of about 22-kD were obtained in a similar experiment on chick liver. Hydrophilicity analysis suggested that both bore four transmembrane domains (Fig. 1), although they did not show any sequence similarity to occludin. Immunofluorescence and immunoelectron microscopy revealed that both proteins were targeted to and incorporated into the TJ strand itself. Furuse et al. (3) designated them as “claudin-1” and “claudin-2”, respectively (12). Gradually in humans, 23 members of the family have been described.
Figure 1.
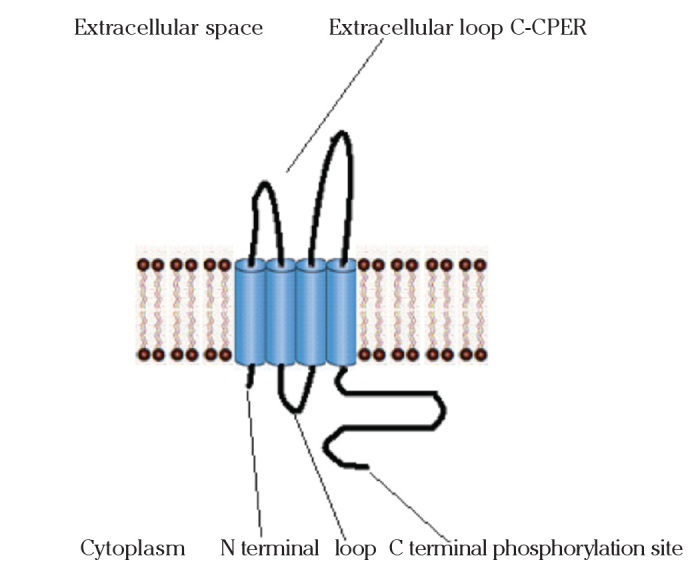
Claudins with four transmembrane domains. The extracellular loops target for therapy. The C-terminal is PDZ binding domain
Claudin Expression in Cancer
Decreased tight junction protein expression in cancer is consistent with the generally accepted idea that tumorigenesis is accompanied by a disruption of tight junctions, a process that may play an important role in the loss of cohesion, invasiveness, and lack of differentiation observed in cancer cells. Down-regulation of both occludin (in gastrointestinal tumors) and claudins (in breast cancer, gastric cancer as well as in colon cancer) is noticed in cancer. Claudin-7 is down-regulated in invasive breast cancer (and in head and neck cancer). Likewise, expression of claudin-4 in pancreatic cancer cells reduces invasiveness of these cells. Phosphorylation of tight junction proteins, including claudins, may also disrupt tight junction function in cancer5. Claudins down regulated are listed in Table 1.
Table 1.
Down regulated claudins in various cancers
Paradox
Many claudins, such as claudin-3 and claudin-4, are typically up-regulated in many cancers, suggesting that these proteins may have a positive effect on tumorigenesis. Recent work has shown that, in ovarian cells, expression of claudin-3 and claudin-4 may lead to an increase in invasion, motility, and cell survival, all characteristics important for metastasis. This was observed in advanced ovarian cancer but not in ovarian cystadenomas. Therefore, the functions of claudins may be highly tissue specific and may depend on the exact molecular circuitry of the cell. In addition, claudin-3 and claudin-4 have also been reported to be expressed in other cancers, such as breast, prostate, and pancreatic cancers. The overwhelming majority of studies published thus far report an overexpression of many claudins in various cancers (Table 2).
Table 2.
Up regulated claudins in various cancers
| Cancer | Claudin gene | Expression | References |
|---|---|---|---|
| Breast | CLDN3 | Up | 13 |
| CLDN4 | Up | 13 | |
| Hepatocellular carcinoma | CLDN10 | Up | 14 |
| Squamous cell carcinoma | CLDN1 | Up | 15 |
| CLDN4 | Up | 15 | |
| Ovarian | CLDN3 | Up | 16,17, 18, 19 |
| CLDN4 | Up | 16, 18, 19 | |
| CLDN16 | Up | 20 | |
| Pancreatic | CLDN4 | Up | 21, 22,23,24 |
| Pancreatic (intraductal papillary mucinous neoplasms) | CLDN4 | Up | 25 |
| CLDN18.2 | Up | ||
| Prostate | CLDN3 | Up | 26 |
| CLDN4 | Up | 26 | |
| Thyroid papillary cancer | CLDN10 | Up | 27 |
| Endometrial Cancer | CLDN4 | Up | 28 |
| Oesophageal tumour | CLDN18.2 | Up | 29 |
| non-small cell lung cancer: Squamous cell carcinoma | CLDN1 | Up | 30 |
| Adenocarcinoma | CLDN4 | Up | 30 |
| CLDN5 | Up |
Thus, recent reports suggest that we may lack a full vision of the functional complexity of claudins and their possible functional connection to a larger protein family. Database searching reveals a large number of proteins with structural similarities to claudins but whose functional similarities remain largely unexplored. There was a lack of consistent nomenclature. Before their naming in 1998, three of the orthodox claudins had already been cloned and given other names and were characterized by nonbarrier functions.
All are not barrier-forming claudins, neither is it known if they have involvement in apoptosis and proliferation. Several orthodox claudins are not restricted to tight junctions; claudin-7, for example, is on the basolateral surface of cells in the kidney tubule epithelium. Many other claudins have large pools of protein on the lateral surface distinct from the barrier-forming TJ strands. The role and organization of extrajunctional claudin remain unclear. Even more enigmatic is the inclusion in the pfam0082 family of some subunits of voltage dependent calcium channels. Cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) can modulate claudin. Moreover, claudins play a significant role in some autoimmune and hereditary diseases such as inflammatory bowel disease, hereditary deafness, multiple sclerosis, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), diabetic retinopathy etc (31). It seems that not only tissue specificity but inflammatory and immunological conditions prevail, and variation of the functions of claudins may be responsible for a contradictory expression of claudins in many cancer.
Claudin and Ovarian Cancer
To date, research on claudin in ovarian cancer has been both on a basic and clinical level. Basic research involing cell line or animal experiments include a) detection of expression of many claudins b) exploring the mechanism of gene expression, methylation status, epigenetic study, influence of gonadotrophin and metabolic pathways like phospholyration etc. In clinical research this effort is mainly limited to observation of tissue expressions of claudin in snap frozen or archival specimen for diagnosis and targeting by Clostridium perfringens enterotoxin, lipidoid siRNA etc.
1. Important basic researches
Serial analysis of gene expression (SAGE) was used to generate global gene expression profiles from various ovarian cell lines and tissues, including primary cancers and ovarian surface epithelia cells, in archival material and cystadenoma cells (16) This showed upregulation of claudin-3 and claudin-4 gene and this was further validated through immunohistochemical analysis. It was only in the previous year that SAGE had detected the tag for the Claudin-7 gene 12 times in the tumor cell lines for the first time, whereas no Claudin-7 tags were found in the normal breast cultures (32). The earlier group tried to account for the significance of its upregulation. Although CLDN4 overexpression is well established, the mechanisms responsible for this abnormal regulation remain unknown. In a study, they delineated a small region of the CLDN4 promoter critical for its expression. This region contains two Sp1 sites, both of which are required for promoter activity. However, because of the ubiquitous expression of Sp1, these sites, although necessary, are not sufficient to explain the patterns of CLDN4 gene expression in various ovarian tissues. CLDN4 promoter is further controlled by epigenetic modifications of the Sp1-containing critical promoter region. Cells that overexpress CLDN4 exhibit low DNA methylation and high histone H3 acetylation of the critical CLDN4 promoter region, and the reverse is observed in cells that do not express CLDN4. Moreover, the CLDN4-negative cells can be induced to express CLDN4 through treatment with demethylating and/or acetylating agents. Because CLDN4 is elevated in a large fraction of ovarian cancer, they opined that the mechanism leading to deregulation may represent a general pathway in ovarian tumorigenesis and may lead to novel strategies for therapy and an overall better understanding of the biology of this disease (33).
In a recent phophorylation study, analyses using PKC inhibitors, siRNA and immunofluorescence have shown that PKC-epsilon, an isoform typically expressed in ovarian cancer cells, may be important in the TPA-mediated claudin-4 phosphorylation and weakening of the TJs (34). In CLDN3 promoter it was possible to identify a minimal region containing a Sp1 site crucial for its activity (35). In addition, this group found that the CLDN3 promoter is regulated through epigenetic processes as in CLDN4. Interestingly, in vitro binding experiments, as well as chip assays show that Sp1 binds the unmethylated promoter much more efficiently, providing a mechanism for CLDN3 silencing in non-expressing cells. siRNA-mediated knockdown of Sp1 led to a significant decrease of CLDN3 expression at both the mRNA and protein levels, demonstrating a crucial role for this transcription factor in the regulation of CLDN3 (36).
Animal experiments from Canada showed that, in the absence of FSH-R signaling, claudin-3, claudin-4, and claudin-11 are selectively upregulated, whereas claudin-1 decreases in ovarian surface epithelium. In vitro experiments using a mouse ovarian surface epithelial cell line derived from wild-type females reveal a direct hormonal influence on claudin proteins. Although recent studies suggest that cell junction proteins are differentially expressed in ovarian tumors in women, the etiology of such changes remains unclear. They suggest an altered hormonal environment resulting from FSH-R loss as a cause of early changes in tight junction proteins that predispose the ovary to late-onset tumors that occur with aging (36).
2. Clinical research
a) Diagnosing ovarian cancer, tissue marker
The result of the SAGE study on ovarian cancer was established in a detailed analysis of CLDN3 and CLDN4 expression in a panel of ovarian tumors of various subtypes and cell lines. RNA was obtained from a panel of 39 microdissected epithelial ovarian tumors of various histological subtypes for real-time reverse transcription-PCR analysis. In addition, a total of 70 cases of ovarian carcinomas, ovarian cysts, and normal ovarian epithelium from a tissue array were analyzed and validated by immunohistochemistry (21).
Affymetrix human genome arrays (U95 series) was utilized to analyze differences in gene expression of 41,441 known genes and expressed sequence tags between five pools of normal ovarian surface epithelial cells (OSE) and 42 epithelial ovarian cancers of different stages, grades, and histotypes (17). The 3-fold up-regulated genes were analyzed using recursive descent plot analysis (RDPA), and the combination of elevated claudin 3 gene (CLDN3) and elevated VEGF distinguished the cancers from normal OSE. A combination of CLDN3, of CA125, and MUC1 (mucin-1 transmembrane) stained 99.4% of 158 cancers, and all of the tumors were detected with a combination of CLDN3, CA125, MUC1, and VEGF. Thus, a limited number of markers in combination might identify >99% of epithelial ovarian cancers despite the heterogeneity of the disease.
Total RNA was extracted and transcription profiling performed in another experiment (37) using the Eos Hu03, a customized Affymetrix GeneChip oligonucleotide microarray (Affymetrix, Santa Clara, CA). The expression patterns of three integral membrane proteins, discoidin domain receptor 1 (DDR1), claudin 3 (CLDN3), and epithelial cell adhesion molecule, all of which are involved in cell adhesion, were evaluated in a cohort of 158 primary EOC using immunohistochemistry identifying DDR1 and CLDN3 as new biomarkers of EOC. Two other DNA microarray affymetrix study results corroborated claudin 3 and 4 gene overexpression in ovarian cancer. They used Hierarchical clustering of the expression data and Youden’s misclassification index for each gene marker via pairwise tissue comparisons respectively (18, 19).
No new claudins were implicated with ovarian cancer in 2005. The Professor Bast Jr. group, in search of potential markers that complement expression of CA125 in epithelial ovarian cancer, included claudin 3 with at least ten other markers at the level of tissue expression (38).
In 2006 it was substantiated by both Swedish and Italian groups that both claudin 3 and 4, even though they differ in expression during ovarian malignant transformation, might be used as novel markers for ovarian tumours (39,40). In another experiment, strong expression of claudins 1, 4, and 7 was seen in benign and malignant epithelial ovarian tumors. Expression of claudin 5, reported to be mainly present in endothelial cells, was seen in ovarian epithelial tumors, but with a significantly lower frequency than claudins 1, 4, and 7. On the contrary, sexcord stromal tumors and cysts, such as fibromas/thecomas, Sertoli-Leydig cell tumors, granulosa cell tumors, and follicular and luteinized cysts were mainly negative for claudins 1, 4, 5, and 7 (41). Ovarian/primary peritoneal serous carcinoma (OC/ PPC) and diffuse peritoneal malignant mesothelioma (DMPM) are highly aggressive tumors. Claudins 3, 4, and 6 overexpression was noticed in them (42). Claudin 4 was among few proteins whose expression profiles correlated with cisplatin resistance in ovarian cancer cells. Several proteins may be involved in modulating response to cisplatin and have a potential as markers of treatment response or treatment targets (43).
Expression of claudin-3 or claudin-7 is specific for adenocarcinoma and rules out the diagnosis of cells as mesothelial and the absence of claudin-1 expression excludes ovarian carcinoma as the possible origin of metastatic adenocarcinoma. Claudins may, therefore, be of diagnostic value in biopsy and effusion cytology (44).
Kaplan-Meier survival analysis showed that serous ovarian adenocarcinoma patients with high CLDN3 expression had a substantially shorter survival (P=0.027). Multivariate analysis demonstrated that CLDN3 overexpression is an independent negative prognostic factor (45). Expression for all cell-junction proteins with a typical honeycomb-staining pattern in the serous adenocarcinomas and not Clear-cell and endometrioid adenocarcinomas prove that Serous adenocarcinomas form functional TJs in vitro (46).
CLDN-7 transcript was found significantly overexpressed in both primary and metastatic EOCs compared to normal human ovarian surface epithelium cell lines (fold change=111.4, P<0.001) by reverse transcription-polymerase chain reaction, regardless of the histologic type, the grade of differentiation, and the pathologic stage of the disease (47). Moreover, a strong immunoreactivity for CLDN-7 was detected in EOC cells present in ascites fluids, whereas ascites-derived inflammatory cells, histiocytes, and reactive mesothelial cells were negative. With the exception of claudin-4, claudins are upregulated in OC effusions compared with solid tumors, in agreement with previous data for cadherins and integrins in this cancer type, suggesting a prosurvival role for these surface molecules. Claudin-3 and claudin-7 expression in effusions independently predicts poor survival in OC (48).
Increased expression of claudin-3 and claudin-4 may contribute to the aggressive phenotype of endometrial cancer of serous papillary or clear-cell histology also and suggests their potential utility as diagnostic biomarkers and possible targets for therapeutic intervention (28).
b) Targeting claudin for therapy
Clostridium perfringens enterotoxin receptor
The peptide toxin Clostridium perfringens enterotoxin (CPE) has been shown to bind to claudin-3 and −4, but not to claudin-1 or −2 (49). Claudin-1/claudin-3 chimeric molecules showed that the second extracellular loop of claudin-3 conferred CPE sensitivity on L fibroblasts. The second extracellular loop is the site through which claudin-3 interacts with CPE on the cell surface.
Claudins 3 and 4 have been described as the low- and high-affinity receptors, respectively, for the cytotoxic Clostridium perfringens enterotoxin (CPE) (50). Their sensitivity to CPE treatment was seen in vitro when 100% (17 of 17) of the primary ovarian tumors tested overexpressed one or both CPE receptors by quantitative reverse transcription-PCR. All ovarian tumors showed a dose-dependent cytotoxic effect to CPE in vitro. All primary ovarian tumors tested died within 24 hours of exposure to 3.3 microg/mL CPE in vitro. CPE therapy in SCID mouse xenografts in a highly relevant clinical model of chemotherapy-resistant freshly explanted human ovarian cancer (i.e., OVA-1) showed that multiple i.p. administration of sublethal doses of CPE every 3 days significantly inhibited tumor growth in 100% of mice harboring 1 week established OVA-1. Claudin-4 overexpression in epithelial ovarian cancer (EOC) does not correlate with survival or other clinical endpoints and was found to be associated with hypomethylation. Claudin-4 overexpression correlates with Rb and C-CPE. Treatment of EOC cells with C-CPE significantly decreased Rb in a dose- and claudin-4-dependent noncytotoxic manner. Thus, C-CPE treatment of EOC cells may lead to altered TJ function induced cytotoxicity, (51) and hence is a therapeutic measure.
Pan-cancer target
CLDN18.2 is retained on malignant transformation and is expressed in a significant proportion of primary gastric cancers and the metastases thereof. In addition, its orthotopic expression was found in pancreatic, esophageal, ovarian, and lung tumors, correlating with distinct histologic subtypes. The activation of CLDN18.2 depends on the binding of the transcription factor cyclic AMP-responsive element binding protein to its unmethylated consensus site. Most importantly, it was possible to raise monoclonal antibodies that bind to CLDN18.2. Its highly restricted expression pattern in normal tissues, its frequent ectopic activation in a diversity of human cancers, and the ability to specifically target this molecule at the cell surface of tumor cells, qualify CLDN18.2 as a novel, highly attractive pan-cancer target for the antibody therapy of epithelial tumors including ovarian cancer (52).
Claudin-3 gene silencing with siRNA
In a recent US study (53), intratumoral injection of lipidoid/CLDN3 siRNA into OVCAR-3 xenografts resulted in dramatic silencing of CLDN3, significant reduction in cell proliferation, reduction in tumor growth, and a significant increase in the number of apoptotic cells. Intraperitoneal injection of lipidoid-formulated CLDN3 siRNA resulted in a substantial reduction in tumor burden in MISIIR/TAg transgenic mice and mice bearing tumors derived from mouse ovarian surface epithelial cells. It has been reported that CLDN3 is expressed at very low levels in several normal tissues in humans including the lungs, kidneys, breast, uterus, pancreas, and thyroid. Colon, small bowel, and prostate are the only normal tissues that show appreciable expression. An i.p. administration of CLDN3 siRNA formulations may reduce the concern of adverse effects of silencing CLDN3 in healthy tissues that reside outside the peritoneum. Lipidoid-formulated CLDN3 siRNA has potential as a therapeutic agent for ovarian cancer.
Disruption of TJs
The C terminus of claudin-3 was seen to be an excellent substrate for cAMP-dependent protein kinase (PKA) at threonine 192. Overexpression of the protein containing a T192D mutation, mimicking the phosphorylated state, resulted in a decrease in TJ strength in ovarian cancer cell line OVCA433. This may provide a mechanism for the disruption of TJs in this cancer causing cytotoxicity (54). Another report showed that the claudin-expressing ovarian epithelial cells were found to have increased matrix metalloproteinase-2 (MMP-2) activity indicating that claudin-mediated increased invasion might be mediated through the activation of MMP proteins. However, siRNA inactivation of claudins in ovarian cancer cell lines did not have a significant effect on the high endogenous MMP-2 activity present in these cells, showing that malignant cells have alternative or additional pathways to fully activate MMP-2 (55).
Claudin crosstalk
Gene expression mediated by the promoter of claudin-2 may be regulated by factors involved in Wnt signaling (56). Moreover, a functional crosstalk between Wnt signaling and transcriptional activation related to caudal-related homeobox (Cdx) proteins could be demonstrated in the regulation of claudin-2 promoter-mediated gene expression.
Although formed by different molecules, tight junctions (TJs) and adherens junctions (AJs) are functionally and structurally linked, but the signalling pathways behind this interaction are unknown. A cell-specific mechanism of crosstalk between these two types of structure was shown when endothelial VE-cadherin at AJs upregulated the gene encoding the TJ adhesive protein claudin-5 (57). This effect required the release of the inhibitory activity of forkhead box factor FoxO1 and the Tcf-4-beta-catenin transcriptional repressor complex. Vascular endothelial (VE)-cadherin acts by inducing the phosphorylation of FoxO1 through Akt activation and by limiting the translocation of beta-catenin to the nucleus. These results offer a molecular basis for the link between AJs and TJs and explain why VE-cadherin inhibition may cause a marked increase in permeability. These findings might have bearing in ovarian cancer.
Conclusion
Unusual expression patterns of claudins suggest an utility for detection, diagnosis, and treatment of drug-resistant cancers. Hopefully, new experiments will pave the way for proper utilization of knowledge already gathered to the benefit of advancement in management of many diseases, including ovarian cancer. However, clinical trials will be required to establish these potentials. Until then, the interesting nature of the subject, and need for basic and clinical research on claudins cannot be overemphasized as claudin is likely to remain valuable for providing important insights into normal and neoplastic cellular physiology. The paradox of the findings has to be solved, and diagnosis and targeting has to be established in future.
Acknowledgement
The author gratefully acknowledges the advice of Professor Eberhardt Nieschlag, Head of the Department of Andrology, University of Münster, Germany who rekindled the interest in this subject while the author was there on a study visit jointly sponsored by DFG and INSA.
Footnotes
Conflict of interest
None declared
References
- 1.Fawcett DW. Intercellular bridges. Exp Cell Research. 1961;(suppl 8):174. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. Links. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and −2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin PJ. Claudin Proteins in Human Cancer: Promising New Targets for Diagnosis and Therapy. Cancer Res. 2005;65:9603–6. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 7.Kramer F, White K, Kubbies M, Swisshelm K, Weber BH. Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Hum Genet. 2000;107:249–56. doi: 10.1007/s004390000375. [DOI] [PubMed] [Google Scholar]
- 8.Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–33. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 9.Tokes AM, Kulka J, Paku S, et al. Claudin-1, −3 and −4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7:R296–305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soini Y. Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma. Hum Pathol. 2004;35:1531–6. doi: 10.1016/j.humpath.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511–8. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 12.Lee SK, Moon J, Park SW, Song SY, Chung JB, Kang JK. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep. 2005;13:193–9. [PubMed] [Google Scholar]
- 13.Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, Mc-Bryan J, Jirstrom K, Landberg G, Martin F, Duffy MJ, Gallagher WM. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 2009;124:2088–97. doi: 10.1002/ijc.24159. [DOI] [PubMed] [Google Scholar]
- 14.Cheung ST, Leung KL, Ip YC, et al. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res. 2005;11:551–6. [PubMed] [Google Scholar]
- 15.Morita K, Tsukita S, Miyachi Y. Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen’s disease. Br J Dermatol. 2004;151:328–34. doi: 10.1111/j.1365-2133.2004.06029.x. [DOI] [PubMed] [Google Scholar]
- 16.Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- 17.Lu KH, Patterson AP, Wang L, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291–300. doi: 10.1158/1078-0432.CCR-03-0409. [DOI] [PubMed] [Google Scholar]
- 18.Hibbs K, Skubitz KM, Pambuccian SE, et al. Differential gene expression in ovarian carcinoma: identification of potential biomarkers. Am J Pathol. 2004;165:397–414. doi: 10.1016/S0002-9440(10)63306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Bignotti E, Anfossi S, Gokden M, Dunn D, Roman JJ, O’Brien TJ, Tian E, Cannon MJ, Shaughnessy J, Jr, Pecorelli S. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer. 2004;112:14–25. doi: 10.1002/ijc.20408. [DOI] [PubMed] [Google Scholar]
- 20.Rangel LBA, Sherman-Baust CA, Wernyj RP, Schwartz DR, Cho KR, Morin PJ. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene. 2003;22:7225–32. doi: 10.1038/sj.onc.1207008. [DOI] [PubMed] [Google Scholar]
- 21.Gress TM, Muller-Pillasch F, Geng M, et al. A pancreatic cancer-specific expression profile. Oncogene. 1996;13:1819–30. [PubMed] [Google Scholar]
- 22.Michl P, Buchholz M, Rolke M, et al. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678–84. doi: 10.1053/gast.2001.27124. [DOI] [PubMed] [Google Scholar]
- 23.Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol. 2004;121:226–30. doi: 10.1309/K144-PHVD-DUPD-D401. [DOI] [PubMed] [Google Scholar]
- 24.Terris B, Blaveri E, Crnogorac-Jurcevic T, et al. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–54. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato N, Fukushima N, Maitra A, et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903–14. doi: 10.1016/S0002-9440(10)63178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61:7878–81. [PubMed] [Google Scholar]
- 27.Aldred MA, Huang Y, Liyanarachchi S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–9. doi: 10.1200/JCO.2004.08.127. [DOI] [PubMed] [Google Scholar]
- 28.Konecny GE, Agarwal R, Keeney GA, Winterhoff B, Jones MB, Mariani A, Riehle D, Neuper C, Dowdy SC, Wang HJ, Morin PJ, Podratz KC. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol Oncol. 2008;109:263–9. doi: 10.1016/j.ygyno.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–34. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 30.Junga Ji Han, Junga Chan Kwon, Choia Hyun Joo, Junb Kyoung Hwa, Yooa Jinyoung, Kanga Seok Jin, Leea Kyo Young. Diagnostic utility of expression of claudins in non-small cell lung cancer: Different expression profiles in squamous cell carcinomas and adenocarcinomas. Pathology - Research and Practice Article. doi: 10.1016/j.prp.2008.12.015. in Press. [DOI] [PubMed] [Google Scholar]
- 31.Förster Carola. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nacht Mariana, Ferguson2 Anne T, Zhang Wen, Petroziello Joseph M, Cook Brian P, Gao Yu Hong, Maguire Sharon, Riley Deborah, Coppola George, Landes Gregory M, Madden Stephen L, Sukumar3 Saraswati. Combining Serial Analysis of Gene Expression and Array Technologies to Identify Genes Differentially Expressed in Breast Cancer1. Cancer Res. 1999;59:5464–70. [PubMed] [Google Scholar]
- 33.Honda H, Pazin MJ, Ji H, Wernyj RP, Morin PJ. Crucial roles of Sp1 and epigenetic modifications in the regulation of the CLDN4 promoter in ovarian cancer cells. J Biol Chem. 2006;281:21433–44. doi: 10.1074/jbc.M603767200. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza T, Indig FE, Morin PJ. Phosphorylation of claudin-4 by PK-Cepsilon regulates tight junction barrier function in ovarian cancer cells. Exp Cell Res. 2007;313:3364–75. doi: 10.1016/j.yexcr.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda H, Pazin MJ, D’Souza T, Ji H, Morin PJ. Regulation of the CLDN3 gene in ovarian cancer cells. Cancer Biol Ther. 2007;6:1733–42. doi: 10.4161/cbt.6.11.4832. [DOI] [PubMed] [Google Scholar]
- 36.Aravindakshan J, Chen X, Sairam MR. Differential expression of claudin family proteins in mouse ovarian serous papillary epithelial adenoma in aging FSH receptor-deficient mutants. Neoplasia. 2006;8:984–94. doi: 10.1593/neo.06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, Heinzelmann M, Kalish LH, Bali A, Kench JG, Edwards LS, Vanden Bergh PM, Hacker NF, Sutherland RL, O’Brien PM. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10:4427–36. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 38.Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, Hellstrom I, Mok SC, Liu J, Bast RC., Jr Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–77. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Brännström M, Janson PO, Sundfeldt K. Differences in expression patterns of the tight junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface epithelium as compared to epithelia in inclusion cysts and epithelial ovarian tumours. Int J Cancer. 2006;118:1884–91. doi: 10.1002/ijc.21506. [DOI] [PubMed] [Google Scholar]
- 40.Bignotti E, Tassi RA, Calza S, Ravaggi A, Romani C, Rossi E, Falchetti M, Odicino FE, Pecorelli S, Santin AD. Differential gene expression profiles between tumor biopsies and short-term primary cultures of ovarian serous carcinomas: identification of novel molecular biomarkers for early diagnosis and therapy. Gynecol Oncol. 2006;103:405–16. doi: 10.1016/j.ygyno.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 41.Soini Y, Talvensaari-Mattila A. Expression of claudins 1, 4, 5, and 7 in ovarian tumors of diverse types. Int J Gynecol Pathol. 2006;25:330–5. doi: 10.1097/01.pgp.0000215298.38114.cc. [DOI] [PubMed] [Google Scholar]
- 42.Davidson B, Zhang Z, Kleinberg L, Li M, Flørenes VA, Wang TL, Shih IeM. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–50. doi: 10.1158/1078-0432.CCR-06-1059. [DOI] [PubMed] [Google Scholar]
- 43.Stewart JJ, White JT, Yan X, Collins S, Drescher CW, Urban ND, Hood L, Lin B. Proteins associated with Cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Mol Cell Proteomics. 2006;5:433–43. doi: 10.1074/mcp.M500140-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Kleinberg L, Holth A, Fridman E, Schwartz I, Shih IeM, Davidson B. The diagnostic role of claudins in serous effusions. Am J Clin Pathol. 2007;127:928–37. doi: 10.1309/V025QRN3R9CJGNPX. [DOI] [PubMed] [Google Scholar]
- 45.Choi YL, Kim J, Kwon MJ, Choi JS, Kim TJ, Bae DS, Koh SS, In YH, Park YW, Kim SH, Ahn G, Shin YK. Expression profile of tight junction protein claudin 3 and claudin 4 in ovarian serous adenocarcinoma with prognostic correlation. Histol Histopathol. 2007;22:1185–95. doi: 10.14670/HH-22.1185. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Sundfeldt K. Tight junction formation in epithelial ovarian adenocarcinoma. Acta Obstet Gynecol Scand. 2007;86:1011–9. doi: 10.1080/00016340701463889. [DOI] [PubMed] [Google Scholar]
- 47.Tassi RA, Bignotti E, Falchetti M, Ravanini M, Calza S, Ravaggi A, Bandiera E, Facchetti F, Pecorelli S, Santin AD. Claudin-7 expression in human epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:1262–71. doi: 10.1111/j.1525-1438.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 48.Kleinberg L, Holth A, Trope CG, Reich R, Davidson B. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Hum Pathol. 2008;39:747–57. doi: 10.1016/j.humpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–61. doi: 10.1016/s0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 50.Santin AD, Cané S, Bellone S, Palmieri M, Siegel ER, Thomas M, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Treatment of chemotherapy- resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer Res. 2005;65:4334–42. doi: 10.1158/0008-5472.CAN-04-3472. [DOI] [PubMed] [Google Scholar]
- 51.Litkouhi B, Kwong J, Lo CM, Smedley JG, 3rd, McClane BA, Aponte M, Gao Z, Sarno JL, Hinners J, Welch WR, Berkowitz RS, Mok SC, Garner EI. Claudin-4 overexpression in epithelial ovarian cancer is associated with hypomethylation and is a potential target for modulation of tight junction barrier function using a C-terminal fragment of Clostridium perfringens enterotoxin. Neoplasia. 2007;9:304–14. doi: 10.1593/neo.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–34. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 53.Huang YH, Bao Y, Peng W, Goldberg M, Love K, Bumcrot DA, Cole G, Langer R, Anderson DG, Sawicki JA. Claudin-3 gene silencing with siRNA suppresses ovarian tumor growth and metastasis. Proc Natl Acad Sci U S A. 2009 Feb 10; doi: 10.1073/pnas.0813348106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–40. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378–85. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- 56.Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun. 2004;314:1001–7. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- 57.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Bio. 2008;10:923–34. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]


