Abstract
Avian myelocytomatosis virus MC29 is a highly oncogenic replication-defective retrovirus that predominantly affects hematopoietic cells and causes acute leukemia in vivo and that transforms hematopoietic cells as well as fibroblasts in vitro. The transformation-specific sequence, v-myc, is expressed as part of a fusion protein that contains the viral structural protein p19. By use of monoclonal antibodies against p19 we showed that the v-myc-encoded protein is located in the nucleus of MC29-transformed fibroblasts and that after purification over an immunoaffinity column the protein binds to double-stranded DNA. In this report we describe the analysis of the v-myc gene product from MC29-transformed bone marrow cells. The immunoaffinity column-purified protein from these cells also bound to DNA and was indistinguishable from the purified protein from MC29-transformed fibroblasts. In addition, the v-myc gene products from fibroblasts transformed by three nonconditional mutants of MC29--which transform hematopoietic cells with a markedly decreased efficiency in vivo and in vitro but still transform fibroblasts in vitro, expressing deleted v-myc proteins--were analyzed. In contrast to the wild-type protein, the purified mutant proteins had decreased DNA-binding abilities. Furthermore, a preferential binding of the wild-type protein to poly(dG) . poly(dC) duplexes was observed. Such a specificity was lost with a mutant protein. These results provide evidence that the interaction of the v-myc protein with DNA may be directly involved in transformation of the hematopoietic target cells. Further, the transformation-specific fusion proteins purified from cells transformed by avian erythroblastosis virus, which belongs to a different class of acute leukemia viruses, and by Fujinami sarcoma virus were found not to be DNA-binding proteins, suggesting the existence of different transformation mechanisms.
Full text
PDF

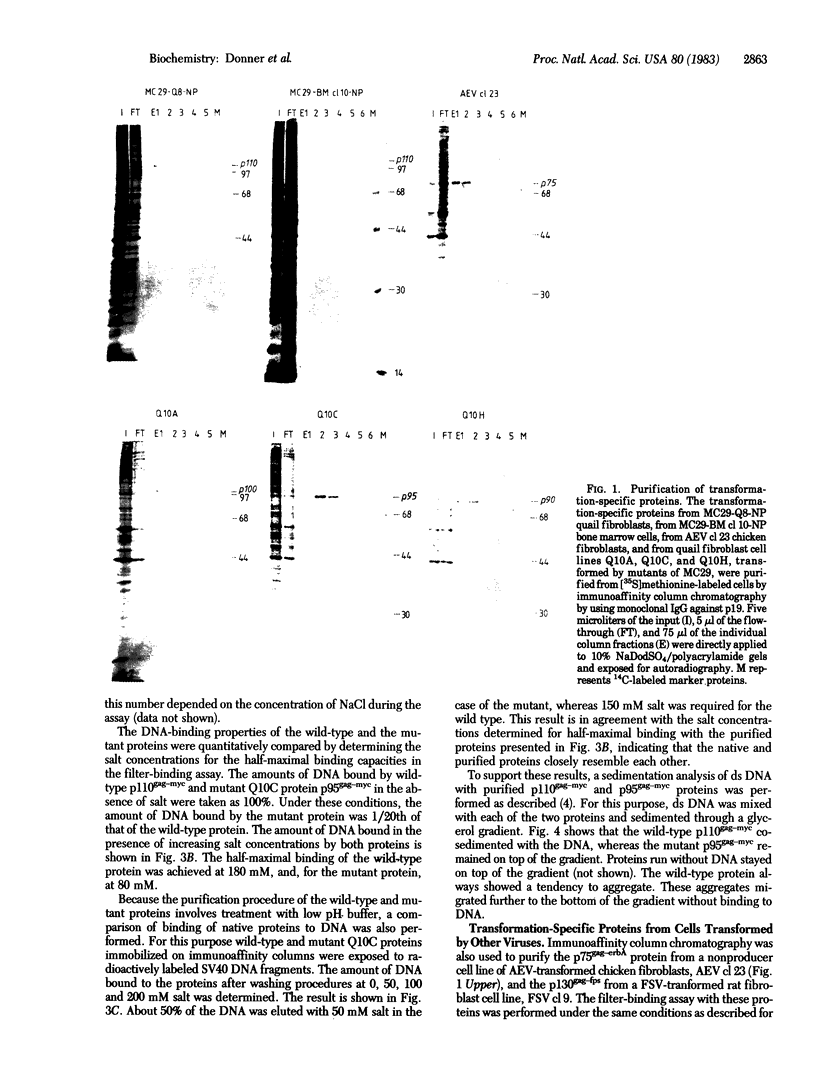
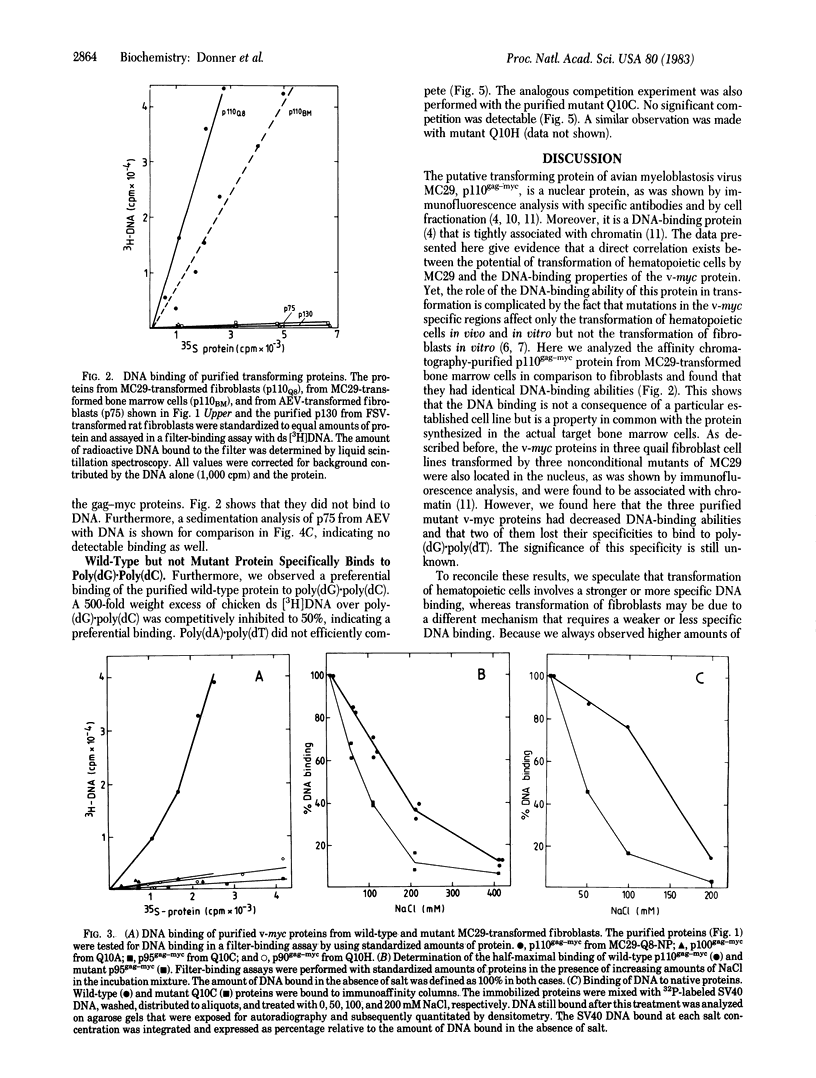
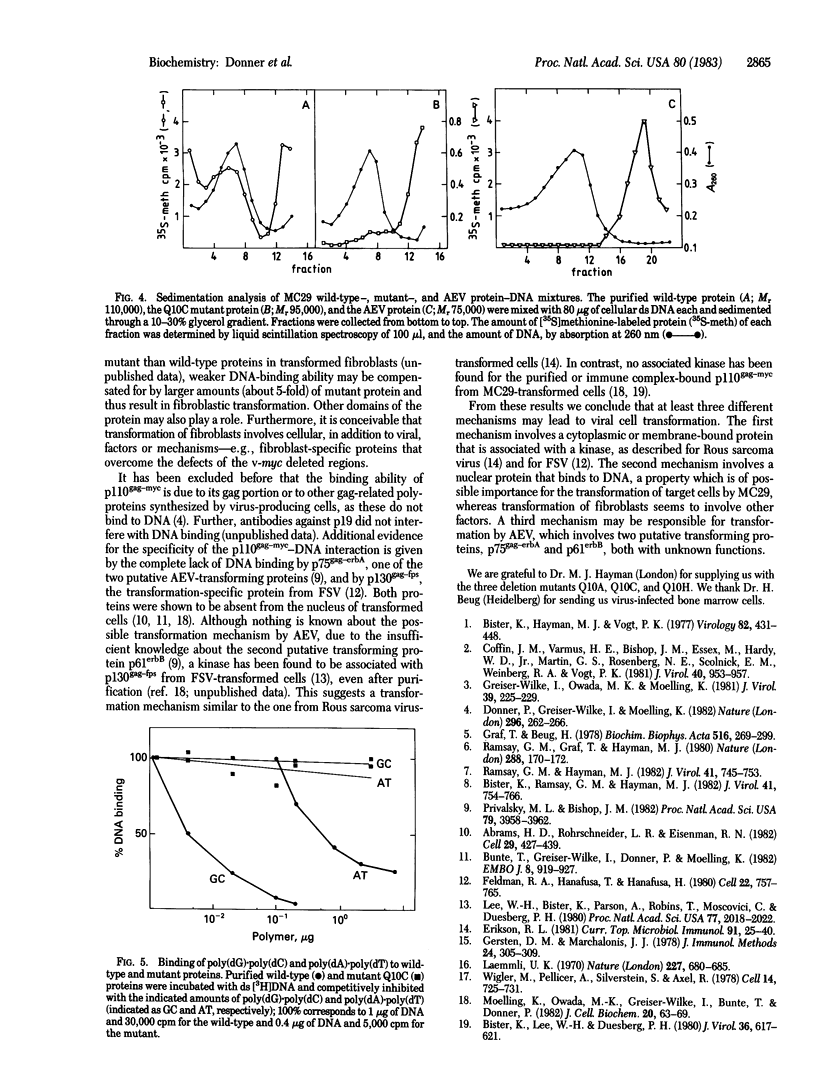
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Lee W. H., Duesberg P. H. Phosphorylation of the nonstructural proteins encoded by three avian acute leukemia viruses and by avian fujinami sarcoma virus. J Virol. 1980 Nov;36(2):617–621. doi: 10.1128/jvi.36.2.617-621.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte T., Greiser-Wilke I., Donner P., Moelling K. Association of gag-myc proteins from avian myelocytomatosis virus wild-type and mutants with chromatin. EMBO J. 1982;1(8):919–927. doi: 10.1002/j.1460-2075.1982.tb01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. The transforming protein of avian sarcoma viruses and its homologue in normal cells. Curr Top Microbiol Immunol. 1981;91:25–40. doi: 10.1007/978-3-642-68058-8_2. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Gersten D. M., Marchalonis J. J. A rapid, novel method for the solid-phase derivatization of IgG antibodies for immune-affinity chromatography. J Immunol Methods. 1978;24(3-4):305–309. doi: 10.1016/0022-1759(78)90133-3. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Owada M. K., Greiser-Wilke I., Bunte T., Donner P. Biochemical characterization of transformation-specific proteins of acute avian leukemia and sarcoma viruses. J Cell Biochem. 1982;20(1):63–69. doi: 10.1002/jcb.240200107. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Proteins specified by avian erythroblastosis virus: coding region localization and identification of a previously undetected erb-B polypeptide. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3958–3962. doi: 10.1073/pnas.79.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Hayman M. J. Isolation and biochemical characterization of partially transformation-defective mutants of avian myelocytomatosis virus strain MC29: localization of the mutation to the myc domain of the 110,000-dalton gag-myc polyprotein. J Virol. 1982 Mar;41(3):745–753. doi: 10.1128/jvi.41.3.745-753.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]