Abstract
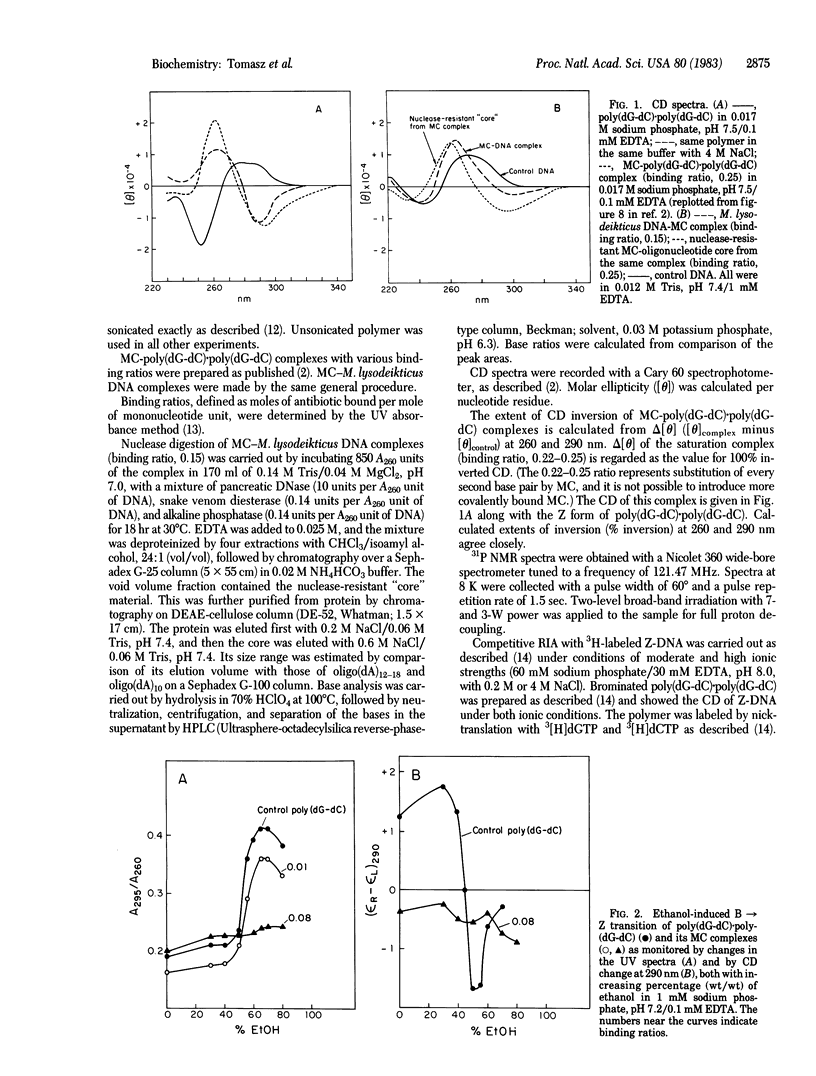
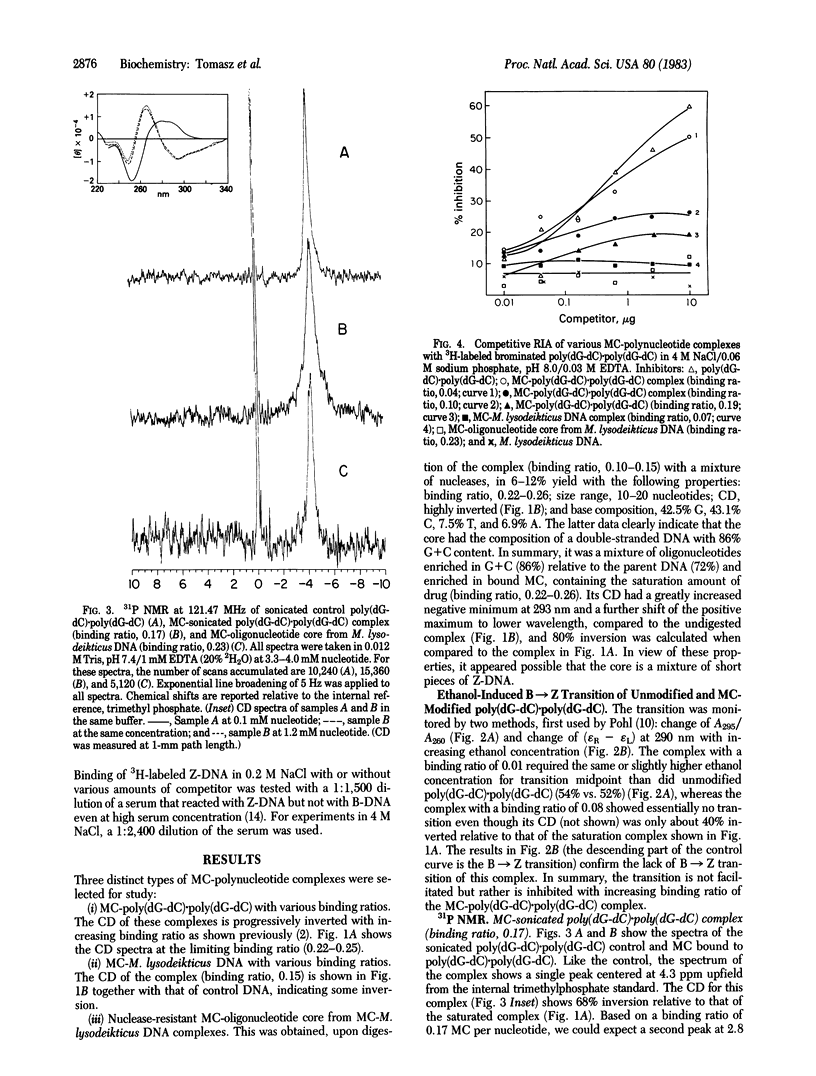
Poly(dG-dC) . poly(dG-dC) and Micrococcus lysodeikticus DNA were modified by exposure to reductively activated mitomycin C, an antitumor antibiotic. The resulting covalent drug-polynucleotide complexes displayed varying degrees of CD inversions, which are strikingly similar to the inverted spectrum observed with Z-DNA. The following criteria have been used to establish, however, that the inverted CD pattern seen in mitomycin C-polynucleotide complexes does not reflect a Z-DNA conformation. (i) The ethanol-induced transition of poly(dG-dC) . poly(dG-dC) from B to Z conformation is not facilitated but rather is inhibited by mitomycin C modification. This may be due to the presence of crosslinks, (ii) Radioimmunoassay indicated no competition for Z-DNA-specific antibody by any of the mitomycin C-modified polynucleotides, (iii) 31P NMR of the complexes yielded a single relatively narrow resonance, which is inconsistent with the dinucleotide repeat characteristic of Z-DNA. Alternative explanations for the inverted CD pattern include a drug-induced left-handed but non-Z conformational change or the superposition of an induced CD onto the CD of B-DNA due to drug-base electronic interactions. These results illustrate the need for caution in interpreting CD changes alone as an indication of Z-DNA conformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Pilet J., Ptak M., Ramstein J., Malfoy B., Leng M. The B reversible Z transition of poly(dI-br5dC).poly(dI-br5dC). A quantitative description of the Z form dynamic structure. Nucleic Acids Res. 1982 May 25;10(10):3261–3277. doi: 10.1093/nar/10.10.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. J., Tomasz M. Altered physiochemical properties of the deoxyribonucleic acid-mitomycin C complex. Evidence for the conformational change in deoxyribonucleic acid. Biochemistry. 1982 Jun 8;21(12):3006–3013. doi: 10.1021/bi00541a031. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown J. W., Begleiter A., Johnson D., Morgan A. R. Studies related to antitumor antibiotics. Part V. Reactions of mitomycin C with DNA examined by ethidium fluorescence assay. Can J Biochem. 1976 Feb;54(2):110–119. doi: 10.1139/o76-018. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Nelson J. H., Grunberger D., Cantor C. R., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. IV. Circular dichroism and proton magnetic resonance studies of oligonucleotides modified with N-2-acetylaminofluorene. J Mol Biol. 1971 Dec 14;62(2):331–346. doi: 10.1016/0022-2836(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Hao W. M., Wogan G. N., Rich A. Salt-induced conversion of B-DNA to Z-DNA inhibited by aflatoxin B1. Science. 1983 Mar 25;219(4591):1434–1436. doi: 10.1126/science.6402818. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz M., Mercado C. M., Olson J., Chatterjie N. The mode of interaction of mitomycin C with deoxyribonucleic acid and other polynucleotides in vitro. Biochemistry. 1974 Nov 19;13(24):4878–4887. doi: 10.1021/bi00721a002. [DOI] [PubMed] [Google Scholar]
- Ushay H. M., Santella R. M., Caradonna J. P., Grunberger D., Lippard S. J. Binding of [(dien)PtCl] Cl to poly(dG-dC)-poly(dG-dC) facilitates the B goes to Z conformational transition. Nucleic Acids Res. 1982 Jun 11;10(11):3573–3588. doi: 10.1093/nar/10.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Crothers D. M. Solution structural studies of the A and Z forms of DNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6808–6811. doi: 10.1073/pnas.78.11.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Crothers D. M. Solution structural studies of the A and Z forms of DNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6808–6811. doi: 10.1073/pnas.78.11.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zimmerman S. B. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]



