Several key steps essential for the reduction of vaginal prolapse in patients undergoing laparoscopic hysterectomy are reported.
Keywords: Multimodal concept, Laparoscopy, Hysterectomy, Supracervical hysterectomy, Pectopexy
Abstract
Background and Objectives:
Today, laparoscopic intrafascial hysterectomy and laparoscopic supracervical hysterectomy are well-accepted techniques. With our multimodal concept of laparoscopic hysterectomy for benign indications, preservation of the pelvic floor as well as reconstruction of pelvic floor structures and pre-existing prolapse situations can be achieved.
Methods:
The multimodal concept consists of 3 steps:
- Intrafascial hysterectomy with preservation of existing structures
- Technique 1: Primary uterine artery ligation
- Technique 2: Classic intrafascial hysterectomy
A technique for the stable fixation of the vaginal or cervical stump
A new method of pectopexy to correct a pre-existing descensus situation
Results and Conclustion:
This well-balanced concept can be used by advanced endoscopic gynecologic surgeons as well as by novices in our field.
INTRODUCTION
Hysterectomy is one of the most frequently performed surgeries within the discipline of gynecologic surgery. International gynecologic societies recommend vaginal hysterectomy as the most acceptable technique; however, over the past 20 years, operative laparoscopic methods have gained in standing and they play an increasingly more important role than the classic approaches of abdominal and vaginal hysterectomy.
Today, the most frequent indications for hysterectomy are symptomatic multifibroid uterus, infertility, and therapy-resistant bleeding abnormalities. These constitute up to 60% of indications for hysterectomies (or even more).1 Over the past 10 years, alternative therapeutic strategies have been developed for both indications in Germany,2 as well as other countries.3 Conservative operative management and the invention of ulipristal acetate have led to a decrease in the number of hysterectomies. Hysterectomy rates depend not only on the indication but also on the age group and significantly on the centers where the patients are treated.4,5
Despite these trends, however, the number of pelvic floor corrections worldwide has increased tremendously in recent years. This is the consequence of demographic change. In 2000, 34.8 million women (12.7%) in the United States were aged [me]65 years. By 2030, this number will have risen to 70.3 million (20%). In Germany these tendencies are similar.6 In 2011, women aged >65 years accounted for 20% of the total population. This percentage will rise to 35% by 2060. In other European countries—the United Kingdom, France, the Netherlands, and Sweden—this development will be less striking.6 The rate of pelvic organ prolapse repair in women aged >65 years is 30% to 50%,7 and in the group aged >80 years, it is still 11%.8
Information on the rate of post-hysterectomy prolapse varies. The cumulative risk is described as 1% three years after hysterectomy and up to 15% fifteen years later. The risk is 5.5 times higher if hysterectomy was performed because of a descensus situation. Other investigations found an incidence of up to 46%.8–11 Besides the risk of a pre-existing descensus, vaginal deliveries and age are discussed as reasons for the risk doubling per life decade.12 Of course, the rate of descent also depends on the applied surgical technique and descensus prevention.
When the advantages of endoscopic surgery (shorter hospital stay, faster recovery, improved cosmesis, fewer infections) are taken into account, the question arises whether, during laparoscopic intrafascial hysterectomy (or total laparoscopic hysterectomy [TLH]), by preserving the existing structures of the pelvic floor or reconstructing them, it is possible to reduce the risk of post-hysterectomy prolapse. In the case of pre-existing defects, TLH can provide sufficient fixation of the pelvic floor in harmless cases to minimize the increased risk of a post-hysterectomy prolapse. In severe situations simultaneous pectopexy can correct pre-existing situations.
Our multimodal concept for laparoscopic intrafascial hysterectomy (or TLH) and laparoscopic supracervical hysterectomy (LASH) is aimed at reducing the risk of post-hysterectomy prolapse:
- Intrafascial hysterectomy with preservation of existing structures
- Technique 1: Primary uterine artery ligation
- Technique 2: Classic intrafascial hysterectomy
A technique for stable fixation of the vaginal or cervical stump
A new method of pectopexy (NOē Pectopexy) to correct a pre-existing descensus situation.
DEVELOPMENT OF LAPAROSCOPIC HYSTERECTOMY TECHNIQUES AND INSTRUMENTS
After the initial publication of Harry Reich,13 the trend toward laparoscopic hysterectomy developed slowly in the ensuing years.14 Different variants evolved, such as laparoscopy-assisted vaginal hysterectomy (LAVH) and LASH, as well as TLH and laparoscopic intrafascial hysterectomy. TLH had a steep learning curve and, in the beginning, a relatively high complication rate.3 The development of new instruments and constant training improved the situation. The introduction of the intrauterine manipulator helped to develop the classic intrafascial concept, which today is the aim of every gynecologic surgeon performing TLH. It was Hohl who took up the concept of the classic intrafascial hysterectomy from Kurt Semm's classic intrafascial-supracervical hysterectomy (CISH) technique15 and developed it further with the introduction of his manipulator (Figure 1). Hohl and Hauser16 call the method total atraumatic intrafascial laparoscopic hysterectomy (TAIL). Most of the available manipulators have achieved a high acceptance rate because they are easy to handle, reusable, and durable. The uterus can be moved in all directions, and the elliptical, long tip of the manipulator eases the vaginal and paravaginal tissue intra-abdominally. The manipulator can be pushed straight toward the operation field, especially while cutting the uterus off the vagina with a monopolar hook, targeting on the manipulator tip.
Figure 1.

Hohl uterine manipulator.
Most manipulators have a ceramic cap that creates a flat surface on which to work. Consequently, there is very little or virtually no need for bladder dissection because of the manipulator's cap. It is of interest that after cesarean section, application of the manipulator for bladder dissection is useful and safe. The application of the manipulator imitates the classic movements during abdominal hysterectomy so that the ureters are kept out of the operation field. The cap allows intrafascial hysterectomy, which preserves the ligaments and avoids vaginal shortening. The smooth cutting edge is useful during vaginal occlusion.
The following points should be considered:
Monopolar electricity can be used on the ceramic cap. Ultrasonography destroys this apparatus. This has to be considered when one is using ultrasonographic dissection during hysterectomy.
Intrafascial hysterectomy causes a marked reduction in the size of the opening to the vagina because of the preservation of the circular ligaments. For this reason, larger-sized uteri more frequently have to undergo morcellation or reduction to smaller-sized pieces.
Ultimately, the advantages of the application of a uterus manipulator outweigh any disadvantages.16
The other instruments can be selected as disposable or reusable instruments. Disposable tissue-sealing instruments are faster and save frequent instrument changes; however, the instruments are costly, and the fusion of the tissue layers results in a less clear overview of the anatomic structures. Bipolar forceps are essential; extracorporeal sutures (No. 1.0 polydioxanone [PDS]) are helpful, although intracorporeal sutures (Vicryl; Ethicon, Somerville, New Jersey) are sufficient for vaginal closure. The monopolar hook allows comfortable removal of the uterus from the manipulator but can be replaced by bipolar forceps and scissors. In the case of large uteri, the tenaculum can lift out the uterus and a morcellator avoids troublesome comminution of the uterus.
1: INTRAFASCIAL HYSTERECTOMY WITH PRESERVATION OF EXISTING STRUCTURES
Operative Steps of TLH
Step 1.
The operation starts with the placement of the Hohl uterine manipulator (Figure 1).17 The patient is placed in the Trendelenburg position to allow easy vaginal access for insertion of the uterine manipulator. In addition, a transurethral pelvic catheter (Foley catheter) is inserted. The arms of the patient are positioned alongside the body to give ample room for the first and second surgeons to operate. The first surgeon stands on the left side of the patient; the second surgeon stands on the right side. An assistant sits between the legs of the patient and can manipulate the uterus with the uterine manipulator.
The operation begins by positioning the Veress cannula, which is later replaced by a 10-mm optic trocar. After introduction of the High Definition Television video optic, an exploration of the entire abdominal cavity, focusing on the minor pelvis, is performed. The bladder, rectum, pelvic vessels, and both ureters are identified and displayed.
Step 2: technique 1—primary uterine artery ligation.
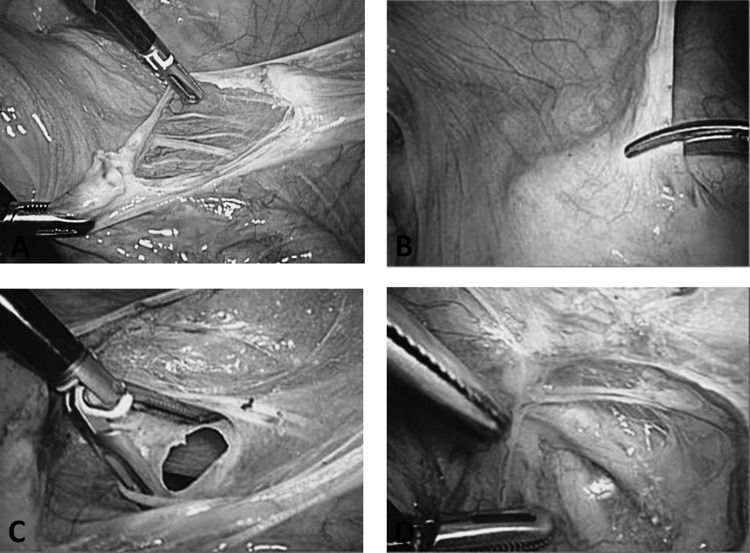
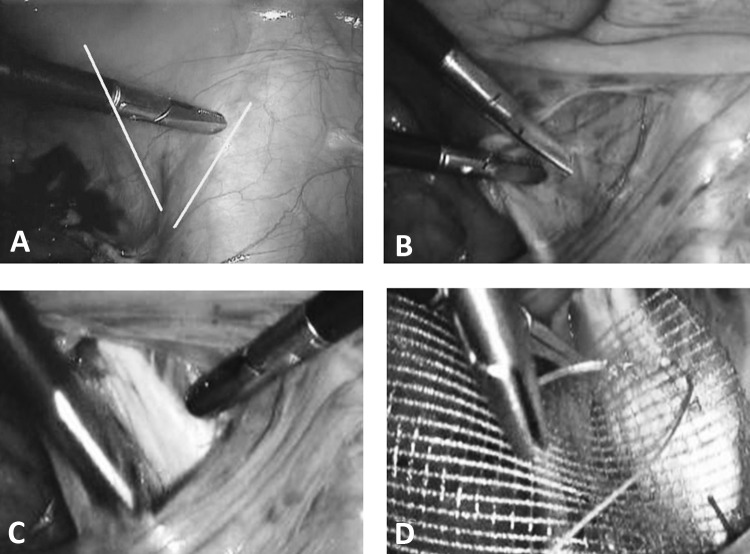
The next step is to coagulate and separate the round ligament near the pelvic side wall (Figure 2A). Afterward, the peritoneum is further incised (Figure 2B). The anterior leaf of the broad ligament is opened up to the bladder fold, and the bladder is pushed downward. The posterior leaf of the broad ligament is displayed, and the ureters are lateralized (Figure 2C). Subsequently, the retroperitoneal space is exposed, the course of the ureters is demonstrated, and the exit of the uterine artery from the iliac artery is visualized (Figure 2D). The crossing point of the uterine artery and the ureter is exposed, and the uterine artery is coagulated (Figure 3). The bladder pillar is identified, coagulated, and separated (Figure 3). This is followed by separation of the ovary and the tube from the uterus. If the adnexa, after sufficient distancing of the ureters, are to be dissected with the uterus, the infundibulopelvic ligament is coagulated and the mesosalpinx and the mesovarium are dissected in the direction of the fenestration. The fenestration is a useful point of orientation to safeguard the ureter. The removal of the fallopian tubes has become a new surgical trend based on evidence that ovarian cancer can be prevented to at least some extent. This possibility should be discussed between the patient and her surgeon.18,19 A second point of consideration concerning the removal of the fallopian tubes and ovaries is the uncommon but reported complication of fallopian tube prolapse after laparoscopic hysterectomy.20
Figure 2.
A, Dissection of round ligament. B, Opening of anterior broad ligament. C, Fenestration of broad ligament. D, Crossing point of uterine artery and ureter.
Figure 3.

Coagulation of visualized uterine artery under exclusion of ureter.
Step 2: technique 2—classic intrafascial hysterectomy.
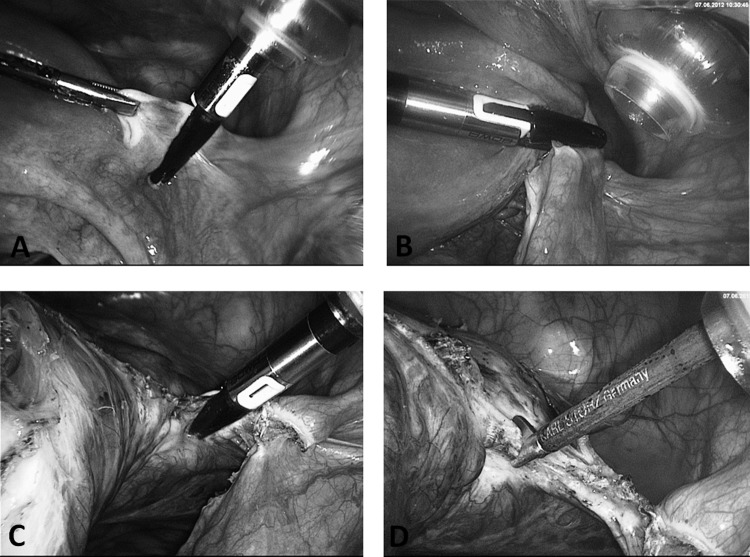
Alternatively, the main parametrium is dissected in small steps down to the vaginal vault, with the ureters a safe distance away in the pelvic wall (Figure 4). After exposure and preparation of the uterine vessels, they are coagulated and dissected (Figures 4C and 4D). The same procedure is performed contralaterally.
Figure 4.
A, Separation of round ligament with NightKNIFE (BOWA, Gomaringen, Germany). B, Division of ovarian ligament. C, Preparation of uterine wall/broad ligament parallel to ascending branch of uterine artery under safe distance to ureter (lying in lateral pelvic wall retroperitoneally). D, Cutting after coagulation of skeletonized uterine artery.
The key elements, pushing the bladder down from the anterior vaginal fornix before incision, as well as distancing the ureters from the uterine vessels at the cervical/vaginal level, are only safely facilitated by stretching the manipulator firmly cranially and to the contralateral side of the preparation (Figures 5A and 5B).
Figure 5.
A, Demonstration of prominent vessel stump. Opening of bladder peritoneum (B) and preparation of bladder peritoneum (C) with aid of CO2. Intra-abdominal pressure is opening the right preparation layer between the vaginal wall and the bladder pillar. D, Colpotomy with monopolar hook on Hohl manipulator.
Step 3.
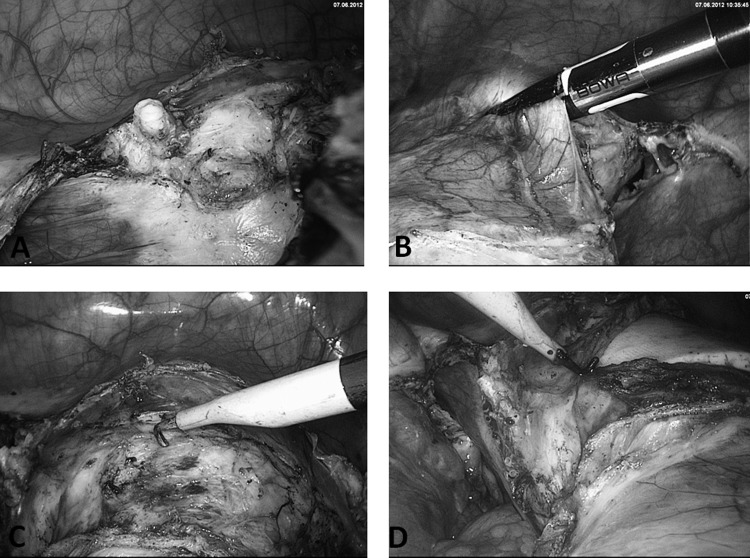
The uterus is separated from the vagina using a monopolar hook electrode, which is guided along the ceramic rim of the manipulator (Figures 5C and 5D). The uterus is then extracted through the vagina or positioned in the vagina to prevent loss of intra-abdominal pressure (Figure 6A). In cases of large benign uteri, distinguishable myomas can be enucleated or the large uterus cut into several smaller pieces so that the fragmented uterus can be extracted through the vagina. As a result, the incision in the lower abdomen can remain at 5 mm and postoperative pain or the risk of hernia is minimized. Alternatively, a 10- to 12-mm electromorcellator is used to dissect the material, which is then extracted through the abdominal wall.
Figure 6.
A, Withdrawal of uterus and deposition in vagina. B–D, First corner stitch on right and second corner stitch on right side through medial part of cardinal ligament, using suture modified by van Herendael.
Step 4: classic closure.
After the uterus has been removed, the vaginal cuff and the peritoneum are closed—for example, with 2 Z-stitches by use of No. 0 Vicryl or with a running suture with or without separate knotting of the lateral edges.
An alternative closure technique emphasizing prolapse prevention will be described in the next section.
2: TECHNIQUE FOR STABLE FIXATION OF VAGINAL OR CERVICAL STUMP
Vaginal Closure With Te Linde Suture Modified by van Herendael
It is well known that hysterectomy is associated with an increased risk of pelvic organ prolapse, with multiparous women at particular risk. This can lead to subsequent pelvic organ prolapse surgery. Particularly with the higher life expectancy of women today, organ prolapse may be a problem later in life and present difficulties during surgical repair (thrombosis, embolism, infection).21
The suture technique of Te Linde, known from abdominal hysterectomy, for the closure of the vagina has been modified by Bruno van Herendael to be used laparoscopically.22,23 After cautious coagulation of the vaginal edge,24 suturing of the vagina can begin. To avoid postoperative necrotic areas of the vaginal stump, coagulation is carried out very cautiously. Remaining slight bleedings are rectified by the following sutures incorporating the complete vaginal wall. Either the uterus is still in the vagina or a glove filled with swabs is placed in the vagina to avoid a loss of pneumoperitoneum. Usually, a curved needle and No. 2–0 PDS are used for single-knot suturing with extracorporeal knots and intracorporeal safety knots. Alternatively, sledge needles or even straight needles can be used for easy handling in and out of the 5-mm trocars. No. 2–0 PDS extracorporeal knots are used for the following reasons:
The monofilament thread slides easily through the tissues and does not cause additional damage.
The monofilament PDS material minimizes the risk of vaginal stump infection.
The long half-life of the suture material minimizes the risk of a vaginal stump dehiscence.24
Extracorporeal knots provide extra strength.
Optionally, both sacrouterine ligaments may be attached to the posterior vaginal wall to prevent vaginal prolapse (McCall culdoplasty).
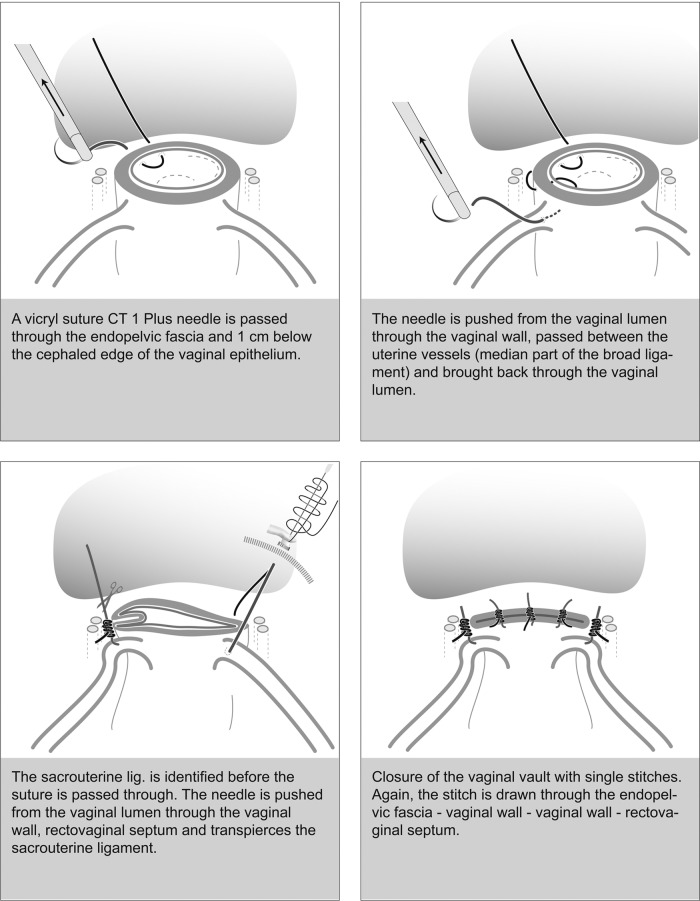
Corner Sutures
We start with the right vaginal corner suture piercing the pericervical ring, followed by the corresponding vaginal epithelium. We pierce away from the urinary bladder to minimize the risk of bladder laceration during suturing (Figures 6B and 7). In the second step the needle passes through the medial aspect of the cardinal ligament in front of the uterine vessels (Figures 6B and 7). The second step involves the structures supporting vaginal wall suspension (Figures 6C and 7). Subsequent back stitching of the vagina is followed by passing the needle through the vaginal epithelium and then, in the third step, through the sacrouterine ligament (Figures 6D and 7). The last step can be omitted when the ligament is stitched once or twice again to shorten it. In cases of an existing descensus, this is of extreme necessity.
Figure 7.
Schema for vaginal closure.
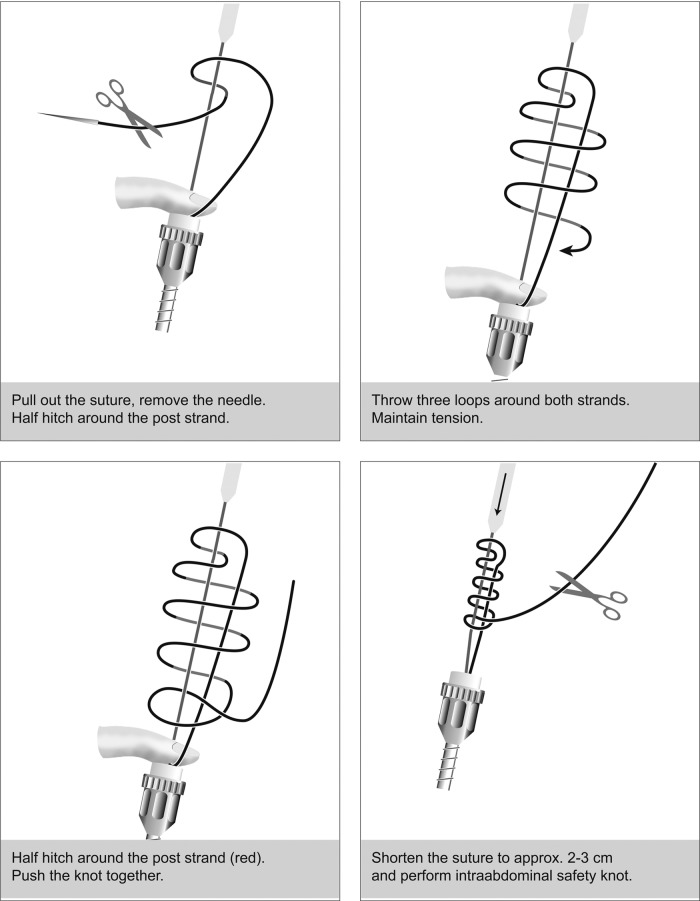
The needle can now be withdrawn, and the suture is completed (eg, with an extracorporeal Roeder knot, secured by 2 or 3 intracorporeal knots) (Figure 8). This procedure is repeated on the contralateral side and ensures that all parts of the endopelvic fascia (vesicouterine ligament, cardinal ligament, and sacrouterine ligament) are connected (Figures 8 and 9A).
Figure 8.
Extracorporeal knotting technique with Roeder knot.
Figure 9.
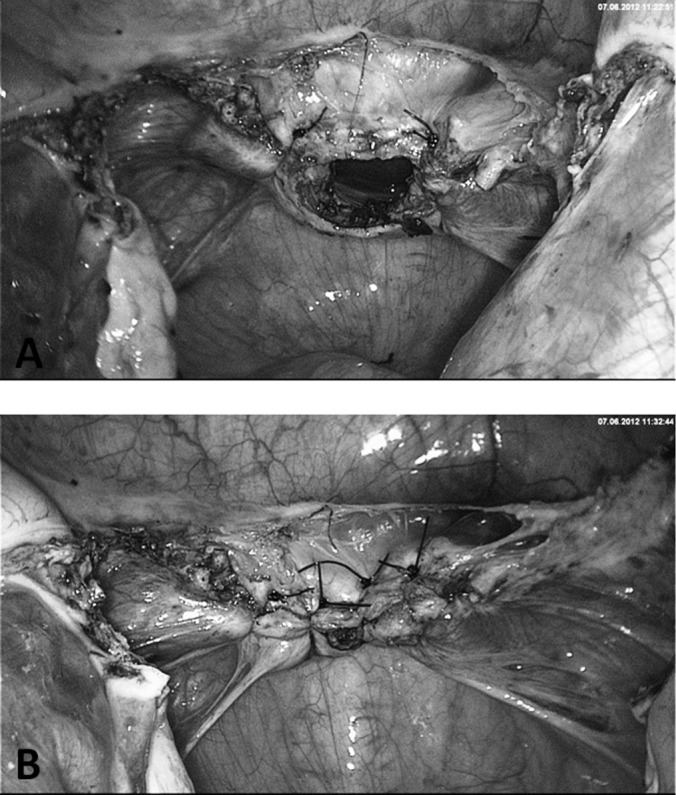
A, Closure of both vaginal edges under involvement of sacrouterine ligaments for vault prevention. B, Closure of vaginal gap with 2 to 3 Z- or U-stitches.
Vaginal Closure
The remaining vaginal opening can now be closed with 2 U- or Z-stitches. These guarantee both vertical and horizontal compression of the tissue and minimize the risk of a vaginal stump hematoma. Neither peritonealization nor drainage is necessary (Figures 7 and 9B). Physiological re-peritonealization occurs the first 2 weeks after the operation. Any additional peritoneal suturing might cause encapsulation of seroma or hematoma and increases postoperative infections and pain. If still inside, the uterus or the swab-filled glove is now taken out of the vagina.
At the end of the procedure, the abdominal cavity is irrigated with normal saline solution and drained. Normally, no drains are left in situ.
We established this suture technique because it provides a good fixation of the vaginal end and has other safety advantages:
The suture is applied parallel to the urethra, so a kinking of the ureters is avoided because the thread is led through the medial part of the cardinal ligament along the anterior-posterior access.
Another safety aspect is the compression of small vessels between the vaginal wall and uterine artery within the cardinal ligament, which minimizes the bleeding risk.
Despite good suspension, there is no relevant displacement of the vaginal access dorsally, which could possibly later increase the risk of a cystocele.
An alternative preparation emphasizing correction of pre-existing descensus is discussed in the next section.
3. NEW METHOD OF PECTOPEXY TO CORRECT PRE-EXISTING DESCENSUS SITUATION
Although sacral colpopexy still remains a gold standard in prolapse surgery, the laparoscopic approach has not really been accepted in broad clinical use.25–29 Laparoscopic methods are underestimated because of the successful marketing of medical devices and the placement of vaginal meshes. On the basis of the recommendation of the Food and Drug Administration not to use vaginal meshes for first-line therapy, it seems to be important to promote laparoscopic procedures. The advantages of postoperative well-being, a short recovery time, fewer scars, less postoperative pain, and shorter hospitalization are well known.30 Applying meshes facilitates a controlled and tension-free surgery.29,30
Major difficulties of sacral colpopexy are ileus and defecation difficulties caused by less space in the lower pelvis. Obesity is one of the major risks for vault prolapse and is always a challenge for the surgeon. The sigmoid colon in these patients is often enlarged by fatty tissue and fills the minor pelvis. In this case there is less space for a sacropexy and constriction phenomena can occur.
The number of obese women is rising.31 Especially obese patients benefit from less wound infection after laparoscopy compared with open surgery. The NPP was developed in 2007, particularly for those cases in which it is difficult to reach the os sacrum.32
During the period of application, it became evident that all patients can be operated by the NPP and the anatomic and functional results are comparable with those after sacropexy. The iliopectineal ligament has long been used as an anchoring structure in incontinence surgery, as described by Burch-Cohen,33 Marshall-Marchetti,34 and other researchers. We use the ligament more laterally as a double anchoring point for fixation of the mesh.32
The NPP is an alternative technique to sacropexy, indicated particularly for obese patients or if the sacrum cannot be reached easily (in cases of diverticulitis, previous surgeries, and multiple adhesions). In comparison with the sacropexy, the technique is easy to learn and has a shorter operative time with the same positive results.35 No preoperative bowel preparation is necessary, and the technique can be combined with all other laparoscopic procedures. Antibiotic prophylaxis with 1.5 g of cefuroxime and 0.5 g of metronidazole intravenously is accepted as the standard technique (Figure 10).
Figure 10.
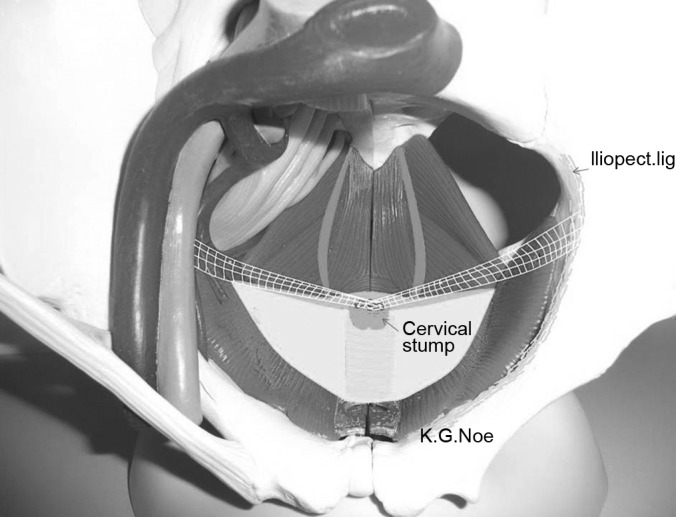
Orientation for placement of mesh for pectopexy.
Operative Technique
1. Presentation of iliopectineal ligament bilaterally.
Major orientation points are the round ligament and the ileo-pectineal ligament, which normally form a V (Figure 11A). The peritoneum is opened along the round ligament up to the middle point. The fat tissue can be pushed downward. Sometimes, lymph structures have to be separated (Figure 11B).
Figure 11.
A, Ligamentum rotundum and ligamentum ileo-pectineum providing major orientation points. B, Lymph node structures identified above ligamentum ileo-pectineum. C, Pecten ossis pubis after removal of overlying structures. D, Fixation of mesh on right iliopectineal ligament.
The tissue has to be bluntly dissected caudally. The pecten ossis pubis is found under the ligament. Some lymph nodes may be identified—in this case they should be pushed off. The end part of the iliopectineal ligament is the fixation point (Figure 11C).
This step is repeated contralaterally. Sometimes, a connecting vein between the external iliac vein and the obturator vein is found; however, this is a variability of position. In most cases this vessel can be spared.
2. Stump preparation.
If possible, we prefer fixation of the cervix after a laparoscopic subtotal hysterectomy (or LASH). Mesh fixation is also possible after TLH in the same manner, fixing the mesh on the vaginal stump. The peritoneum is opened down to the cervix. A larger preparation is not necessary.
Under sufficient pressure, the bladder can be well identified. An area of 4 × 4 cm at the iliopectineal ligament is exposed for fixation of the mesh.
3. Mesh.
We use a 3 × 16–cm mesh. It is bilaterally fixed with 2 stitches on the right iliopectineal ligament with nonabsorbable sutures (No. 0 with attached needle) (Figure 11D).
Intracorporeal monofilament sutures are preferable to the application of staples. The mesh is very stable but thin. It is not possible to fix it with a stapler without going into the bony substance. This increases the risk of osteomyelitis.
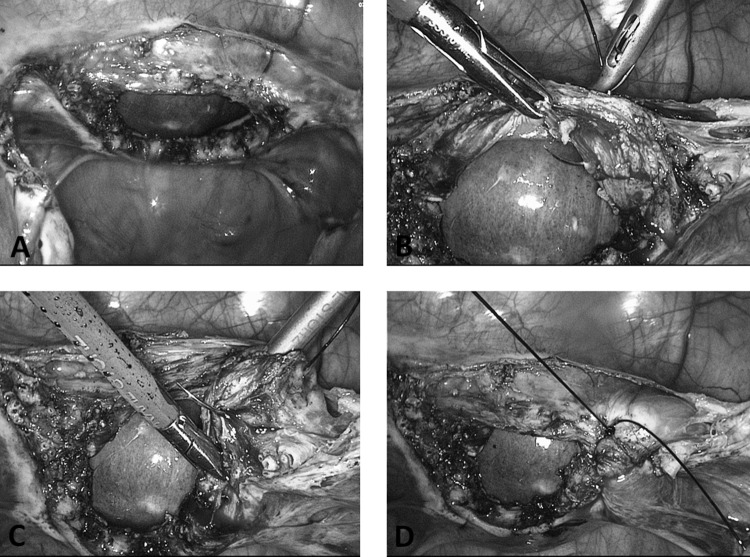
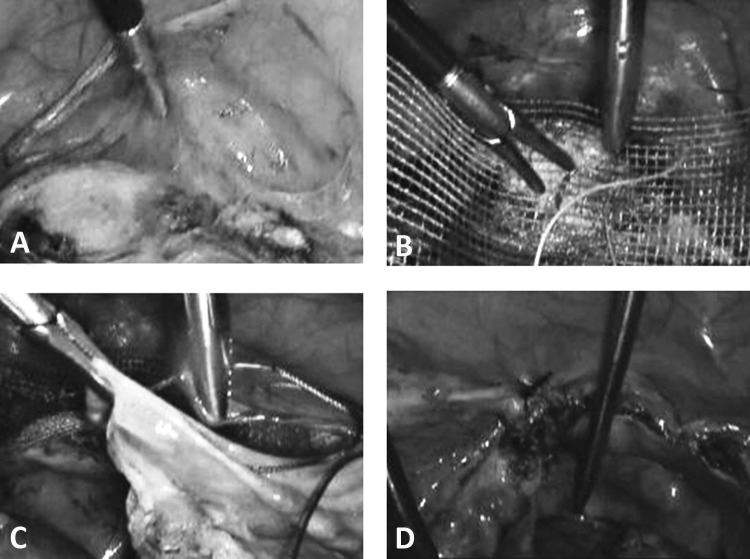
The center of the mesh is fixed with the same thread on the cervix or with No. 0 PDS on the prepared vaginal stump for vaginal fixation of the mesh (Figures 12A and 12B). In the latter case, a polyfilamental suture should not be used because of the wicking. It is difficult to avoid penetration of the vaginal tissue because it measures only 1 to 2 cm after hysterectomy. PDS suturing material is well suited to guarantee the complete grafting of the mesh onto the vagina. If the mesh is too long, it can easily be shortened by “darts” on both sides. In rare cases there is too much tension. In such a case it is preferable to split the net in the center and suture it with a patch of another mesh. After fixation of the mesh, a complete closure of the peritoneum should be performed to avoid incarcerations, hernias, or adhesions (Figures 12C and 12D).
Figure 12.
A. Preparation of cervical stump after supracervical hysterectomy has been performed. Alternatively, the mesh can be placed on the vaginal stump after TLH. B. Fixation of mesh on cervical stump. C. Closure of peritoneum with running suture (eg, Vicryl). D. Result after closing of peritoneum with mesh behind the peritoneum.
CONCLUSIONS
The transformation of the classic intrafascial hysterectomy to the laparoscopic hysterectomy, in this case the total atraumatic intrafascial laparoscopic hysterectomy, represents the first component in the prevention of descensus. The application of the uterine manipulator and the structured procedure have made laparoscopic hysterectomy much safer.16
In a comparison of different methods to avoid prolapse at vaginal hysterectomy, the McCall culdoplasty proved to be the most effective technique both short term and after 3 years.36 A similar procedure is used for abdominal hysterectomy.37,38 This method was modified by Harry Reich and adapted for laparoscopic procedures. A disadvantage is the unification of the 2 lateral sutures in the midline, which results in an unphysiological narrowing of the apical pole of the vagina. The transposition of the ureters carries the risk of kinking and therefore the suture modified by van Herendael,23 which circumvents this risk, should be applied.
The technique of pectopexy is relatively new and represents an alternative to the established methods of vaginal prolapse correction. Experienced surgeons can learn this technique very quickly. The pectopexy widens the portfolio of surgical possibilities, particularly in difficult surgical conditions. The technique guarantees a stable fixation of the pelvic floor and allows the surgeon good control of tension. In >300 procedures, no relevant intraoperative or postoperative complications occurred. Even the well-known ileus risk at sacropexy was not observed. In combination with laparoscopic colposuspension techniques, treatment of most cases of pelvic floor prolapse can be performed without introducing foreign material into the vagina.
Contributor Information
Ibrahim Alkatout, Department of Gynecology and Obstetrics, University Hospitals Schleswig-Holstein, Kiel, Germany..
Liselotte Mettler, Department of Gynecology and Obstetrics, University Hospitals Schleswig-Holstein, Kiel, Germany..
Goentje Peters, Department of Gynecology and Obstetrics, University Hospitals Schleswig-Holstein, Kiel, Germany..
Günter Noé, Department of Gynecology and Obstetrics, Dormagen Hospital, Dormagen, Germany..
Bernd Holthaus, Department of Gynecology and Obstetrics, St Elisabeth Hospital, Damme, Germany..
Walter Jonat, Department of Gynecology and Obstetrics, University Hospitals Schleswig-Holstein, Kiel, Germany..
Thoralf Schollmeyer, Department of Gynecology and Obstetrics, University Hospitals Schleswig-Holstein, Kiel, Germany..
References:
- 1. Lefebvre G, Allaire C, Jeffrey J, et al. SOGC clinical guidelines. Hysterectomy. J Obstet Gynaecol Can. 2002;24(1):37–61, quiz 74–36 [PubMed] [Google Scholar]
- 2. Stang A, Merrill RM, Kuss O. Hysterectomy in Germany: a DRG-based nationwide analysis, 2005–2006. Dtsch Arztebl Int. 2011;108(30):508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brummer TH, Seppala TT, Harkki PS. National learning curve for laparoscopic hysterectomy and trends in hysterectomy in Finland 2000–2005. Hum Reprod. 2008;23(4):840–845 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention Women's Reproductive Health: Hysterectomy Fact Sheet. Atlanta: Centers for Disease Control and Prevention; 2009 [Google Scholar]
- 5. Hanstede MM, Burger MJ, Timmermans A, Burger MP. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–225 [DOI] [PubMed] [Google Scholar]
- 6. Bundesministerium des Innern Demography Report: Report of the German Federal Government on the Demographic Situation and Future Development of the Country. Berlin: German Federal Ministry of the Interior; 2011 [Google Scholar]
- 7. Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104(3):489–497 [DOI] [PubMed] [Google Scholar]
- 8. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506 [DOI] [PubMed] [Google Scholar]
- 9. Symmonds RE, Pratt JH. Vaginal prolapse following hysterectomy. Am J Obstet Gynecol. 1960;79:899–909 [DOI] [PubMed] [Google Scholar]
- 10. Toozs-Hobson P, Boos K, Cardozo L. Management of vaginal vault prolapse. Br J Obstet Gynaecol. 1998;105(1):13–17 [DOI] [PubMed] [Google Scholar]
- 11. Marchionni M, Bracco GL, Checcucci V, et al. True incidence of vaginal vault prolapse. Thirteen years of experience. J Reprod Med. 1999;44(8):679–684 [PubMed] [Google Scholar]
- 12. Swift SE, Pound T, Dias JK. Case-control study of etiologic factors in the development of severe pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(3):187–192 [DOI] [PubMed] [Google Scholar]
- 13. Reich H. Laparoscopic oophorectomy and salpingo-oophorectomy in the treatment of benign tubo-ovarian disease. Int J Fertil. 1987;32(3):233–236 [PubMed] [Google Scholar]
- 14. Mettler L, Semm K, Lehmann-Willenbrock L, Shah A, Shah P, Sharma R. Comparative evaluation of classical intrafascial-supracervical hysterectomy (CISH) with transuterine mucosal resection as performed by pelviscopy and laparotomy—our first 200 cases. Surg Endosc. 1995;9(4):418–423 [DOI] [PubMed] [Google Scholar]
- 15. Semm K. Hysterectomy via laparotomy or pelviscopy. A new CASH method without colpotomy [in German]. Geburtshilfe Frauenheilkd. 1991;51(12):996–1003 [DOI] [PubMed] [Google Scholar]
- 16. Hohl MK, Hauser N. Safe total intrafascial laparoscopic (TAIL) hysterectomy: a prospective cohort study. Gynecol Surg. 2010;7(3):231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schüssler B, Scheidel P, Hohl MK. Hysterektomie update. Frauenheilkunde Aktuell. 2008;3:4–12 [Google Scholar]
- 18. Kramer L. Mixed reviews on removing fallopian tubes to prevent ovarian cancer. CMAJ. 2013;185(9):E391–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurman RJ, Shih I-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol. 2011;42(7):918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caceres A, McCarus SD. Fallopian tube prolapse after total laparoscopic hysterectomy. Obstet Gynecol. 2008;112(2 Pt 2):494–495 [DOI] [PubMed] [Google Scholar]
- 21. Altman D, Falconer C, Cnattingius S, Granath F. Pelvic organ prolapse surgery following hysterectomy on benign indications. Am J Obstet Gynecol. 2008;198(5):572.e1–572.e6 [DOI] [PubMed] [Google Scholar]
- 22. Thompson JD, Warshaw J. Hysterectomy. In: Rock JA, Thompson JD, eds. Te Linde's Operative Gynecology. 9th ed Philadelphia: Lippincott Raven; 1996:771–854 [Google Scholar]
- 23. van Herendael B. Strategies to prevent vaginal vault descent during hysterectomy. In: Mettler L, ed. Manual of New Hysterectomy Techniques. New Delhi: Jaypee Brothers Medical Publishers; 2007:82–85 [Google Scholar]
- 24. Hur HC, Guido RS, Mansuria SM, Hacker MR, Sanfilippo JS, Lee TT. Incidence and patient characteristics of vaginal cuff dehiscence after different modes of hysterectomies. J Minim Invasive Gynecol. 2007;14(3):311–317 [DOI] [PubMed] [Google Scholar]
- 25. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2004(4):CD004014. [DOI] [PubMed] [Google Scholar]
- 26. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805–823 [DOI] [PubMed] [Google Scholar]
- 27. Beer M, Kuhn A. Surgical techniques for vault prolapse: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2005;119(2):144–155 [DOI] [PubMed] [Google Scholar]
- 28. David-Montefiore E, Barranger E, Dubernard G, Nizard V, Antoine JM, Darai E. Functional results and quality-of-life after bilateral sacrospinous ligament fixation for genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2007;132(2):209–213 [DOI] [PubMed] [Google Scholar]
- 29. Rivoire C, Botchorishvili R, Canis M, et al. Complete laparoscopic treatment of genital prolapse with meshes including vaginal promontofixation and anterior repair: a series of 138 patients. J Minim Invasive Gynecol. 2007;14(6):712–718 [DOI] [PubMed] [Google Scholar]
- 30. Gadonneix P, Ercoli A, Salet-Lizee D, et al. Laparoscopic sacrocolpopexy with two separate meshes along the anterior and posterior vaginal walls for multicompartment pelvic organ prolapse. J Am Assoc Gynecol Laparosc. 2004;11(1):29–35 [DOI] [PubMed] [Google Scholar]
- 31. Irvine L, Shaw R. The effects of patient obesity in gynaecological practice. Curr Opin Obstet Gynecol. 2003;13:179–184 [Google Scholar]
- 32. Banerjee C, Noe KG. Laparoscopic pectopexy: a new technique of prolapse surgery for obese patients. Arch Gynecol Obstet. 2011;284(3):631–635 [DOI] [PubMed] [Google Scholar]
- 33. Burch JC. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele and prolapse. Am J Obstet Gynecol. 1961;81:281–285 [DOI] [PubMed] [Google Scholar]
- 34. Williams G, Richardson A. Transplantation of external oblique aponeurosis: an operation for prolapse of the vagina following hysterectomy. Am J Obstet Gynecol. 64(3):552–558 [DOI] [PubMed] [Google Scholar]
- 35. Noe KG, Spuntrup C, Anapolski M. Laparoscopic pectopexy: a randomised comparative clinical trial of standard laparoscopic sacral colpo-cervicopexy to the new laparoscopic pectopexy. Short-term postoperative results. Arch Gynecol Obstet. 2012;287(2):275–280 [DOI] [PubMed] [Google Scholar]
- 36. Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180(4):859–865 [DOI] [PubMed] [Google Scholar]
- 37. Wall LL. A technique for modified McCall culdeplasty at the time of abdominal hysterectomy. J Am Coll Surg. 1994;178(5):507–509 [PubMed] [Google Scholar]
- 38. Ostrzenski A. A new, simplified posterior culdoplasty and vaginal vault suspension during abdominal hysterectomy. Int J Gynaecol Obstet. 1995;49(1):25–34 [DOI] [PubMed] [Google Scholar]