Abstract
Endocytosis and mitosis are fundamental processes in a cell’s life. Nearly 50 years of research suggest that these processes are linked and that endocytosis is shut down as cells undergo the early stages of mitosis. Precisely how this occurs at the molecular level is an open question. In this review, we summarize the early work characterizing the inhibition of clathrin-mediated endocytosis and discuss recent challenges to this established concept. We also set out four proposed mechanisms for the inhibition: mitotic phosphorylation of endocytic proteins, altered membrane tension, moonlighting of endocytic proteins, and a mitotic spindle-dependent mechanism. Finally, we speculate on the functional consequences of endocytic shutdown during mitosis and where an understanding of the mechanism of inhibition will lead us in the future.
Keywords: Clathrin-mediated endocytosis, Moonlighting, Transferrin, Cell division, Prometaphase, Metaphase, Receptor trafficking
Introduction
Accurate, high-fidelity division is crucial to cell viability. It is arguably the most important time in the life of an individual cell, as the consequences of error are disastrous [1]. With such an essential job to perform, it is perhaps no surprise that this is not a time for multitasking: during mitosis, cells cease to respond to external signals, nucleic acid and protein synthesis is inhibited, and transport of vesicles between organelles is switched off [2, 3]. Clathrin-mediated endocytosis (CME) is no exception. Continually active during interphase, it is suppressed during mitosis. A process referred to as “shutdown”.
The first report of inhibition of CME during mitosis dates back to 1965 [4]. Over subsequent years, many studies confirmed and refined this theory [5–15], leading to a general consensus that endocytosis is rapidly shut down as cells enter prophase, is strongly inhibited in prometaphase and metaphase, before resuming in late anaphase/telophase and returning to normal levels by cytokinesis [15, 16]. We describe the evidence for endocytic shutdown during mitosis and discuss the possible mechanisms underlying this phenomenon.
Primer: clathrin-mediated endocytosis
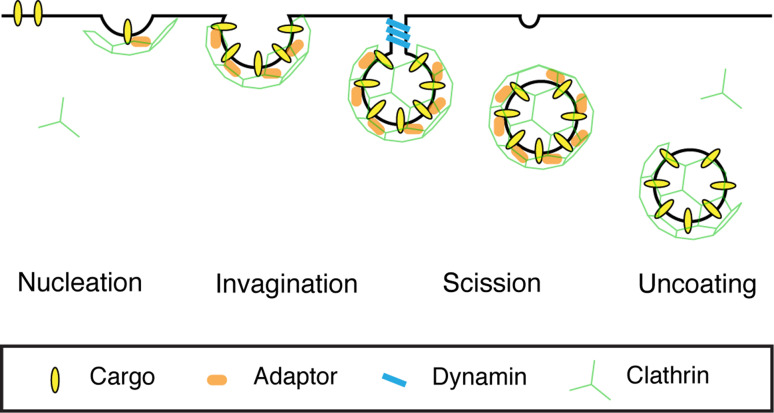
It is useful to think of CME as a process of four steps: nucleation, invagination, scission, and uncoating. These four steps, summarized in Fig. 1, comprise the central scheme of CME. Superimposed on this scheme is a basic layer of core molecules comprising: (1) cargo—ligands and transmembrane receptors that are to be internalized; (2) adaptors—recognize the cargo and membrane; (3) clathrin—forms a polyhedral cage around the vesicle and, together with adaptors, constitutes the coat; (4) dynamin—causes scission of the vesicle from the membrane, and (5) enzymes to uncoat the clathrin-coated vesicle (CCV). For an excellent historical overview, see [17]. On top of this basic layer, a second set of molecules can then be added. This layer of >40 proteins has been described over the last 15 years to modulate the lower layers, to provide control over the basic scheme of CME [18, 19]. Examples include proteins implicated in generating membrane curvature [20] or changing the lipid composition of the membrane [21].
Fig. 1.
Schematic diagram of clathrin-mediated endocytosis. The four main stages of clathrin-mediated endocytosis (CME) are shown. 1. Nucleation, where cargo is gathered into the forming pit. 2. Invagination, where the pit curves inwards. 3. Scission, where the CCV is severed from the plasma membrane. 4. Uncoating, where the clathrin coat is removed and the nascent vesicle is freed. The basic layer of molecules that drive this process is shown as indicated. The second layer of proteins that add further complexity to the pathway are not shown
The destination of CCVs derived from the plasma membrane is the early endosome. From here cargo may be sorted back to the cell surface either directly or indirectly via perinuclear recycling endosomes. Alternatively, cargo may be sorted to multivesicular bodies (MVBs), which can mature into late endosomes, which in turn can fuse to form lysosomes (sites of protein degradation) [22]. Clathrin-dependent membrane traffic analogous to CME occurs at other intracellular sites, such as endosomes or trans-Golgi network [17].
Clathrin-coated pits (CCPs) typically occupy 1–2 % of the cell surface and because the whole cycle from nucleation to uncoating occurs in ~1 min, the equivalent of the entire surface area of the cell may be internalized in approximately 1 h. A variety of receptors and transmembrane proteins are internalized constitutively, independently of ligand binding. The best-characterized example is the transferrin receptor (TfR). A large fraction of constitutively internalized receptors is recycled to the plasma membrane. Other receptors must be bound to their ligand in order for their internalization to be effected. Good examples here are receptor tyrosine kinases such as the epidermal growth factor (EGF) receptor. Finally, receptors that are internalized constitutively can have this endocytosis stimulated by ligand binding, e.g., G-protein coupled receptors.
CME is one of several routes of internalization in cells. Other routes include phagocytosis, macropinocytosis, caveolar endocytosis, and other clathrin-independent routes [23]. There is good evidence that all internalization routes are similarly shut down during mitosis (summarized in Table 1). CME is the best understood at the molecular level and is the focus of this review.
Table 1.
Summary of key studies examining endocytosis during mitosis
| Endocytic process | Observation | Phases showing inhibition | Surface receptor? | Cell line | Method | Reference |
|---|---|---|---|---|---|---|
| Formation of ferritin-containing coated vesicles (CME) | Membrane specializes and ferritin binds but invagination and separation of the vesicle from the PM is suppressed | Telophase | Yes, ferritin bound to surface but not internalized | Erythroblasts | EM | [4] |
| Phagocytosis of opsonized particles (IgG-coated erythrocytes) | Depressed during mitosis | Early prophase to early G1 | Yes, erythrocytes bound to cell surface but were not internalized | Mouse macrophage J774.1 | LM | [5] |
| Phagocytosis of non opsonized particles (latex beads) | Inhibited during mitosis (to ~13 % of interphase levels) | Early prophase to anaphase/telophase | No | Mouse macrophage J774.1 | LM | [5] |
| Fluid pinocytosis (soluble horseradish peroxidase) | Inhibited | Mid-prophase to telophase | N/A | J774, CHO | LM | [5] |
| Adsorptive pinocytosis of ConA | ConA restricted to cell surface during mitosis | Mid-prophase to telophase | Yes, ConA binds to surface throughout mitosis | J774, CHO | LM | [5] |
| Endocytosis and phagocytosis | Peroxidase, dextran, and chloroquine uptake inhibited during mitosis | N/A | No | HTC | Assay | [7] |
| Fluid pinocytosis observed by internalization of fluorescent dextran | Reduced greater than 30-fold during mitosis | Within 30 s after entry into prophase-end telophase | N/A | J774.2 | FM | [6] |
| Endocytosis of FITC-dextran | Less than 1 % of pro-metaphase, metaphase, and anaphase cells had taken up FITC-dextran | Early prophase–end telophase | N/A | A431 | FM | [9] |
| Uptake of Rhodamine-transferrin | Reduced in mitosis | Prometaphase-telophase | Yes, cells in mitosis bind RITC-transferrin on their surface | HeLa S3 | FM | [8] |
| Fluid pinocytosis measured by fluorescein-dextran and DNP-phycoerythrin uptake | Uptake inhibited | Prometaphase-telophase | Yes, DNP-Phe binds to the surface of mitotic as well as interphase cells | RBL-2H3 | FM | [10] |
| Endocytosis of DNP-BSA-gold particles via coated pits | Gold particles taken up in interphase but not mitotic cells | Metaphase and anaphase | Yes, cells in mitosis accumulate surface-bound gold particles in coated pits. | RBL-2H3 | EM | [10] |
| Coated pit invagination | All stages of coated pit invagination are present in mitotic cells | N/A | N/A | A431 | EM | [11] |
| Invagination of coated pits | Mitotic cytosol and cdc2 kinase inhibit invagination of coated pits | Prometaphase | Yes, coated pits observed in both interphase and mitotic cells | HeLa | EM | [12] |
| Endocytosis of TMA-DPH | Decreased in mitosis | N/A | Yes, cell surface doubled at the beginning of mitosis | I929 | FM | [13] |
| Endocytosis of fluorescent dextran | Number and intensity of internalized vesicles decreased to <20 % of interphase uptake in metaphase | Prometaphase-cytokinesis | Mitotic endocytosis restored by decreasing membrane tension with amphiphilic compounds | HeLa | FM | [14] |
| Endocytosis of FM1-43 | Number and intensity of internalized vesicles decreased to ~30 % of interphase uptake in metaphase | Prometaphase-anaphase | No | HeLa, NIH-3T3 | FM | [14] |
| Endocytosis of transferrin | Decreased in early mitosis | Prometaphase-anaphase/telophase | No | HeLa | FM | [15] |
The cell uses CME for a number of physiological functions. For example, controlling the number of receptors, channels, and transporters on the cell surface directly influences the excitability of the cell, the permeability of the membrane, and cell adhesion, etc. In specialized cells, such as neurons, CME is the main route for synaptic vesicles to be internalized after a burst of exocytosis [24]. Pathogens, such as viruses, toxins, and bacteria can exploit CME as an entry point to cells to cause infection. Understanding how CME is regulated will allow us to think of ways to manipulate the system using pharmacological agents so that we can, for example, prevent cellular infection.
Endocytosis is inhibited during mitosis
In 1965, Fawcett observed that both interphase and mitotic cells displayed areas of specialized plasma membrane that bind ferritin. However, in contrast to interphase cells, coated membrane areas in mitotic cells did not invaginate and separate from the plasma membrane [4]. These were the first observations which lead to the idea that endocytosis is shut down during cell division.
After Fawcett’s initial observations, a number of papers have examined mitotic shutdown of endocytosis. These studies are summarized in Table 1 and are briefly discussed here. Berlin et al. [5, 6] completed a series of elegant studies examining pinocytosis and phagocytosis of a range of ligands during defined periods of the cell cycle. During early mitosis, significant inhibition of all uptake routes was observed. It was determined that endocytic shutdown occurred within 30 s of a cell entering prophase and resumed again during telophase [6]. Subsequently, three major studies utilizing fluorescence microscopy and biochemical measurement of uptake reported an inhibition of dextran or transferrin endocytosis in cells at prometaphase through to telophase [7–9].
Further electron microscopy studies of endocytic structures in mitotic cells were also performed, which confirmed an inhibition of internalization during mitosis [10–12]. For example, Oliver et al. characterized the endocytosis of DNP-BSA-gold particles. In interphase cells, gold particles could be seen bound to CCPs at the cell surface and inside vesicles in the cell interior. Neighboring mitotic cells also accumulated surface-bound gold particles in CCPs at the cell surface but no gold labeling in the cell interior was observed [10].
There is a misconception that endocytic shutdown occurs throughout mitosis and cytokinesis, when in fact the timing of shutdown is under strict control. Many of these earlier studies observed that while endocytosis was not active during the early stages of mitosis, it resumed during later stages, typically during late anaphase or telophase (Table 1). The importance of the endocytic resumption during later stages of cell division was established by Schweitzer et al. [15], who demonstrated that membrane internalization was required during furrow ingression and abscission. This highlights the fact that if studying endocytosis during cell division, early and late stages of mitosis need to be clearly distinguished.
Questioning the dogma: evidence for continuing endocytosis in mitosis
By 2007, the shutdown of endocytosis during mitosis was enshrined in the textbooks of cell biology. However, the very existence of this inhibition was questioned by an important study [25]. Boucrot and Kirchhausen reported that endocytosis is in fact continuous throughout mitosis and that previous data showing decreased internalization had been misinterpreted due to variations in surface levels of receptors during mitosis. The authors were examining the regulation of plasma membrane surface area in mitotic cells. They observed a decrease in surface area during mitosis and they argued that this could only occur if endocytosis continues while endocytic recycling is inhibited [25].
Here, CME was examined by two principle methods. Firstly, the incorporation and subsequent loss of fluorescently labeled AP-2 patches was studied in BSC1 and HeLa cells. Although from these experiments it was concluded that CME was not affected during mitosis, it seems that the phase of mitosis examined was not carefully controlled for. For example, the quantification of AP-2 dynamics in BSC1 cells was from data pooled from cells in metaphase, anaphase, and cytokinesis. Similarly, the data presented for HeLa cells is only for cells in anaphase or later. Therefore, these data agree with the numerous studies, discussed above, that have demonstrated that endocytosis resumes from anaphase onwards and do not disagree with the notion that endocytosis is inhibited during early mitosis. In addition, the appearance and loss of AP-2 patches need not equate to productive internalization of a CCV [26]. Secondly, transferrin uptake was examined during mitosis. In agreement with other data, transferrin uptake was reduced to approximately 30 % of interphase levels in metaphase cells. However, a more substantial decrease in surface levels of bound transferrin in mitosis was observed and it was therefore concluded that the endocytic rate was actually modestly increased during prophase and metaphase compared to interphase cells [25]. In support of this observation, a decrease in surface TfR during mitosis had been previously observed in A431 cells [9], although this study noted a less marked reduction in surface TfRs on mitotic HeLa cells. This point posed a worrying question: can a decrease in surface receptors account for the observed decrease in uptake of fluorescent ligands? Obviously, controlling for cell surface receptor number is critical to conclude that endocytosis is shut down. Yet many of the classic studies in Table 1 showed evidence for abundant surface receptors [4, 5, 8–10, 12, 13]. Moreover, the other classic papers on endocytic shutdown characterized this change using morphological observations by electron microscopy [4, 12], which is independent of surface receptors or ligand uptake. Despite these contradictions, the ideas in this paper [25] became quickly established, and many key review papers that followed concluded that endocytosis continues in mitosis [27–30].
Resolving the debate: CME is inhibited during early mitosis
Fielding et al. attempted to resolve the debate over endocytic shutdown during mitosis. Initially, chimeric CD8 receptors were used to demonstrate that despite abundant surface receptor levels in mitosis, uptake of CD8 chimeras containing three major endocytic motifs (YXXΦ, [DE]XXXL[LI], and FXNPXY) was reduced to basal levels during mitosis [16]. This was examined in both large cell populations by flow cytometry and on an individual cell basis by confocal microscopy. In addition, experiments examining transferrin uptake were performed. Flow cytometry of tens of thousands of cells once again demonstrated that transferrin uptake was reduced in mitotic cells but showed that surface levels of bound transferrin were not reduced in mitotic compared to interphase cells [16]. Uptake and surface levels of endogenous TfR were also measured by flow cytometry, which clearly demonstrated a reduced rate of endocytosis during mitosis. To confirm the flow cytometry results, confocal microscopy on unsynchronized HeLa cells was also used to observe internalized transferrin while monitoring surface TfR levels. This verified that while surface levels of transferrin receptor in mitotic cells remained at least as abundant as in interphase cells, and that these receptors can be recruited to clathrin puncta at the cell surface, transferrin uptake was severely reduced. Therefore this study, which examined endogenous and exogenous receptors, in large cell populations and on an individual cell basis, in unsynchronized cells and cells synchronized by more than one method, in all cases controlling for surface receptor levels, clearly supports the many studies preceding it in concluding that endocytosis is severely inhibited during early mitosis [16].
The earlier report of continued CME during mitosis nicely explained the observed decrease in plasma membrane surface area during mitosis [25]. Since CME is shut down during early mitosis (Table 1), how can this decrease be explained? It is possible that the reported halving of surface area during mitosis was an overestimate. The plasma membrane area of a cell during mitosis is difficult to measure accurately given the extensive infoldings found on the surface [31–33]. Scanning electron micrographs show numerous microvilli on the surface of rounded mitotic cells [32]. In addition, a variable amount of plasma membrane covers the retraction fibers that hold the cell to its substrate [34]. The difficulty in measuring surface area accurately may explain why previous studies show an apparent decrease [35], increase [36], or no change [31–33]. Certainly, it is not clear how CME—if it was not shut down—could cause a 50 % decrease in surface area in such a short space of time [25]. It will be important to revisit this question with higher-resolution imaging methods in the future.
Exceptions to the rule: internalization during mitosis
Liu et al. [37] examined the endocytosis of the EGF receptor (EGFR) during mitosis. Stimulation of EGFR with EGF resulted in internalization at all stages of cell division. However, internalization occurred at a much slower rate than during interphase. In the first 10 min of stimulation there was virtually no uptake of EGF in metaphase cells. It was only after 30 min of stimulation that the total amount of internalized EGF approached that seen in interphase cells. Note that under similar conditions, uptake of transferrin was completely inhibited during early mitosis (despite abundant surface TfRs). This suggests that CME is shut down during mitosis but that stimulated EGFRs can override this inhibition to enter the cell, albeit at a reduced rate. The authors went on to demonstrate that in contrast to EGFR internalization in interphase, EGF-stimulated mitotic uptake was dependent on EGFR kinase activity and largely independent of clathrin, indicating that CME was likely not the route for EFGR internalization during mitosis [37]. In interphase cells, EGFR internalization is largely by CME but 5–15 % of internalization is via a non-CME route [38]. It remains to be determined if this balance is shifted during mitosis or if this lower capacity route can account for all of the internalization observed in mitosis [37].
The regulation of Dab2 during mitosis was studied by Chetrit et al. [39]. Dab2 is an adaptor protein that recognizes cargo with FXNXPY endocytic motifs [40]. A reduction in endocytosis of chimeric TGF-beta receptors containing the FXNPXY motif was observed during mitosis. However, it was also observed that receptors with YXXΦ endowed receptors were internalized during mitosis. This in contrast with the work described earlier using CD8 fusions containing YXXΦ, FXNPXY, or [DE]XXXL[LI] motifs, none of which were internalized during early mitosis [16]. One difference between these two studies is the way that cells were synchronized in mitosis. It is possible that cells arrested with 2-methoxyestradiol (2ME2) behave differently to cells synchronized using different compounds or indeed to mitotic cells in an unsynchronized population. Recall that the transferrin receptor, which has an intracellular YXXΦ motif, is not internalized during early mitosis in cells synchronized with different compounds or in cells growing asynchronously (Table 1; [15, 16, 24]).
Another recent study examined how planar cell polarity (PCP) proteins are distributed during cell division [41]. Celsr1 is found in puncta inside mitotic basal epidermal cells. This was not the case for any non-PCP transmembrane proteins tested. In interphase cells, no intracellular location of Celsr1 was detected. This argues for mitosis-specific internalization of Celsr1. The intracellular accumulation of Celsr1 depended on a non-canonical di-leucine motif in its cytoplasmic tail. Internalized Celsr1 co-localized with endocytic markers, particularly markers of the recycling endosome [41]. There are several explanations of the observations in regards to the apparent endocytic activity in mitosis. One is that the di-leucine motif confers the specific property of enabling endocytosis to occur during mitosis. Celsr1 could then be transported through early to recycling endosomes. However, given the large amount of data demonstrating not only a shutdown of endocytosis but also of all vesicle trafficking during mitosis, this interpretation would seem unlikely [2]. Alternatively, as the majority of Celsr1 seemed to be already vesicular by prophase, the internalization of the PCP proteins could occur at or shortly before the onset of mitosis (i.e., late G2/early prophase) with the internalized vesicles remaining in the cytosol during mitosis before being recycled back to the plasma membrane upon the well-documented restarting of membrane trafficking during telophase/cytokinesis. Note that direct evidence for mitotic internalization Celsr1 was not provided [41]. This explanation would fit well with the general view of membrane trafficking activity during mitosis but leaves the intriguing question of how this protein is internalized specifically at this stage of the cell cycle.
From these three studies it is possible that entry routes into the cell during mitosis exist, but whether they constitute an override of inhibited CME is an open question. It will be interesting to pursue whether any other cargo types can enter the cell during mitosis and to understand the route by which they are internalized. This investigation will naturally follow from understanding the mechanism of mitotic shutdown of CME.
What is the mechanism of endocytic shutdown?
Determining how CME is shut down during mitosis has proved difficult. There are four mechanisms proposed to explain how endocytosis is suppressed during mitosis, which are discussed below. It is not certain how each of these mechanisms, alone or in combination, contribute to endocytic shutdown.
The majority of the classic studies in Table 1 were carried out before many molecular details of CME were known. However, some mechanistic detail is available despite the limited molecular information. For example, there is good evidence that the shutdown may involve an inhibition of the invagination stage of CME (Fig. 1). Fawcett observed a predominance of flat, coated membrane areas on the surface of dividing erythroblasts, initially suggesting that CCV formation was inhibited at an early stage of invagination [4]. Further studies in A431 cells showed that CCPs are present at all stages of invagination in mitotic cells, but that in prometaphase there was a predominance of flatter, less invaginated CCPs [11]. Any potential mechanisms for shutdown need to account for the observed block in invagination. On the other hand, more than one mechanism may be responsible as other stages of the CCV cycle that involve different proteins are also blocked, such as uncoating [2].
Mechanism A: mitotic phosphorylation of endocytic proteins
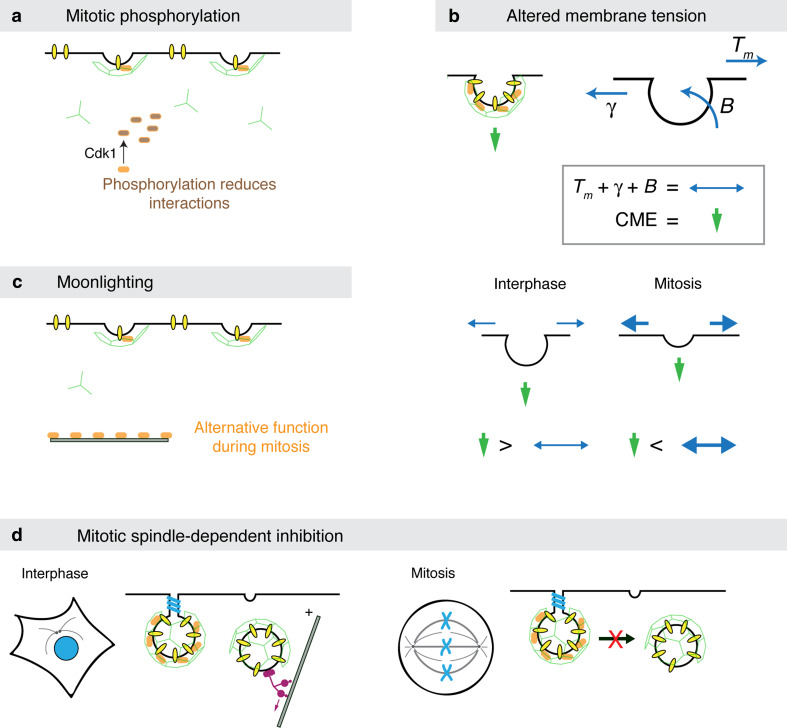
The first mechanism that was proposed to explain endocytic shutdown during mitosis was one of mitotic phosphorylation of endocytic proteins. The idea is that these phosphorylation events render the CME machinery inactive (Fig. 2a). The best evidence supporting this mechanism comes from experiments using broken cells [12]. Here, interphase or mitotic cells are broken by freeze-thawing, allowing the wash-in of various factors to test for inhibition of CCP invagination. If mitotic cytosol was washed into broken interphase cells, this inhibited invagination. This was recapitulated with purified Cdk1/cdc2, suggesting that this master mitotic kinase could be responsible for the failure of CCPs to invaginate [2, 12]. The question of what protein(s) is the target of the kinase has not been definitively answered.
Fig. 2.
Four mechanisms proposed to account for CME shutdown during mitosis. a Mitotic phosphorylation. Important molecules in the basic layer or second layer of CME proteins are phosphorylated by mitotic kinases. This phosphorylation renders them unable to interact with their partners, thus stalling CME. b Altered membrane tension. During endocytosis, the CME machinery (green arrow) must overcome three forces: the tension in the bilayer plane (T m), interactions between the membrane and cytoskeleton (γ), and the stiffness of bending a membrane (B); B depends on the radius of the invaginated membrane. The CME machinery can overcome the sum of these forces easily during interphase, but cannot do so during mitosis. c Mitotic moonlighting. This is a variation on the first mechanism where a key protein is involved in another function during mitosis and its unavailability for CME stalls the pathway. It may or may not involve mitotic phosphorylation. d Mitotic spindle-dependent inhibition. The reorganization of the microtubule network and associated motors during mitosis could inhibit late stages of CME
Several endocytic proteins are phosphorylated in mitosis. The endocytic accessory proteins Eps15 and epsin are phosphorylated in mitosis and these phosphorylation events decrease their binding to the α-adaptin subunit of AP-2 [42]. The endocytic adaptor Dab2 is phosphorylated during mitosis at multiple sites [43]. During mitosis, the localization of Dab2 is altered and it can no longer interact with clathrin [39]. It was proposed that this may regulate the internalization of receptors with the endocytic motif FXNPXY [39]. However, the evidence that these phosphorylation events would be sufficient to cause the shutdown of all endocytic events is lacking. Dynamins 1 and 2 are phosphorylated in mitosis at S778 or S764, respectively [44]. It has been proposed that the dephosphorylation of dynamins by calcineurin is important for completion of cytokinesis in a mechanism analogous to that reported at the synapse [44, 45].
Mitotic phosphoproteome studies using mass spectrometry have shown that many endocytic proteins are phosphorylated during mitosis [46]. These include those proteins in the basic layer of endocytic machinery (Fig. 1). However, whether these phosphorylation events are functionally meaningful has been difficult to determine. It is very possible that these phosphorylation events are just bystanders: a product of promiscuous mitotic kinases phosphorylating any S/TP sites that they encounter. On the other hand, the appeal of this mechanism is that its global nature could explain why other trafficking pathways are similarly inhibited during mitosis. There is good evidence that Cdk1 activity in yeast inhibits intracellular trafficking [47]. The resumption of CME in late anaphase agrees well with the decrease in kinase activity of cyclin B/Cdk1 that is induced by destruction of cyclin B by the anaphase promoting complex/cyclosome [48].
Mechanism B: altered plasma membrane tension
The second proposal to explain the shutdown of CME during mitosis is a physical mechanism. The invagination of a CCP into a CCV requires energy to deform the membrane (Fig. 2b). This is because the plasma membrane is under tension, the membrane is attached to the cytoskeleton, and bending the membrane itself requires work [49, 50]. Under normal circumstances, the forces exerted by the proteins involved in CME are far in excess of these resistance forces [51]. However, during mitosis, the cell rounds up and the membrane tension is increased significantly [14, 52]. An elegant set of experiments showed that if membrane tension in mitosis was reduced, by introducing deoxycholate, then the endocytic rate could be increased [14]. The simplest interpretation of this work is that endocytic shutdown is purely a biophysical process: the tension at the membrane during mitosis is too great to be overcome by the CME machinery. If this is correct, then other forms of regulation, e.g., mitotic phosphorylation, need not necessarily be invoked. Although it is possible that the CME machinery is “weakened” during mitosis, by a mechanism such as phosphorylation, and in the face of increased tension, it cannot complete its task.
Cells have evolved methods to overcome increased membrane tension to continue endocytosis. During interphase, if the membrane tension is increased artificially, the cell can overcome these forces, but it requires the deployment of the actin machinery [51]. This machinery, although important for endocytosis in lower species, is dispensable for CME in mammalian cells when the membrane is not under significant tension [49]. The question is whether the forces in mitosis are too great to be overcome by the actin machinery or if another layer of inhibition exists on top of the physical changes to the mitotic cell.
A potential problem with altered plasma membrane tension as a mechanism is that it does not explain why other forms of vesicle transport are also inhibited during mitosis. Transport within the Golgi, from ER to Golgi and from Golgi to plasma membrane, are all inhibited. Fusion of endocytic vesicles is also suppressed [2]. Obviously, these forms of transport could be regulated by a separate mechanism. A second problem is that the changes in tension and endocytosis are not perfectly aligned according to the literature. The increase in rounding pressure measured by Stewart et al. [52] occurred at nuclear envelope breakdown, i.e., the start of prometaphase; yet endocytosis is inhibited within 30 s of prophase entry according to an earlier study [6]. Similarly, the resumption of endocytosis in late anaphase is after the rounding pressure has decreased [15, 52].
Mechanism C: mitotic moonlighting of endocytic proteins
The final mechanism of mitotic endocytic shutdown is the “moonlighting” hypothesis (Fig. 2c). Several endocytic proteins have been shown to have alternative localizations and functions during mitosis [53–55]. There is good evidence that these mitotic functions are completely independent of the role in membrane trafficking during interphase [53, 54]. One could hypothesize that because these proteins are preoccupied by another task, they cannot participate fully in endocytosis, and thus cause the shutdown, perhaps indirectly. It is possible that moonlighting may in some cases be controlled by mitotic phosphorylation. Therefore this mechanism potentially overlaps with mechanism A.
While the moonlighting functions occur mainly when endocytosis is shut down [53], there is currently no evidence that any moonlighting behavior can cause endocytic shutdown. For example, the best-characterized moonlighting endocytic protein is clathrin itself, which is recruited to the mitotic spindle where it is required for stabilization of spindle fibers [56, 57]. Earlier reports noted the accumulation of flatter CCPs in mitosis, consistent with a reduction in free clathrin preventing further invagination [2]. The timing of the mitotic function of clathrin also agrees well with the suppression of endocytosis. However, the proportion of clathrin involved in this mitotic function is relatively small and so the localization to the spindle alone probably cannot explain a shutdown of endocytosis. Indeed CCPs and CCVs remain abundant during mitosis. Therefore, although several endocytic proteins certainly have alternative functions during mitosis, it is not clear whether these moonlighting functions can be the driving force behind endocytic shutdown.
Mechanism D: mitotic spindle-dependent mechanisms
Finally, a potential mechanism conceptually similar to “moonlighting” exists which involves the reorganization of the microtubule network and associated motors into a mitotic spindle. In interphase, an intact microtubule network exists with motor proteins, such as dynein, controlling important trafficking pathways in the endosomal network. Once the cell enters mitosis, this network is lost as all cellular forces are focused on ensuring the separation of centrosomes to form a bipolar spindle. Many sorting events at intracellular organelles rely on microtubules and motors, e.g., Golgi [58], ER [59], and endosomes/lysosomes [60]. Reorganization of the microtubule network will significantly impact these events. For CME, the later stages (but not the early steps) are sensitive to perturbation of the microtubule network [16, 60, 61], so this would not adequately explain the accumulation of shallow CCPs in mitosis [11]. The shutdown of CME aligns well with the separation of centrosomes in prophase, a dynein-dependent mechanism [62], however the resumption of CME during late anaphase occurs at a time of persistent motor activity and the continued presence of a mitotic spindle, albeit elongated. Therefore, the contribution of this mechanism to shutdown is uncertain.
Clearly more work is required to determine the precise mechanism of endocytic inhibition during mitosis. Whatever the mechanism, it must be global, since the inhibition affects all kinds of internalization (Table 1) and CME of different ligands using various intracellular sorting signals [16]. With this in mind, moonlighting of endocytic proteins (mechanism C) has the least experimental support and seems the most unlikely. Altered membrane tension (mechanism B) is the most direct explanation for endocytic shutdown and, perhaps in combination with mitotic phosphorylation of endocytic proteins (mechanism A), could explain the inhibition of endocytosis during mitosis. Many molecules involved in the CME pathway have not been investigated with respect to mitotic shutdown. For example, over the last decade, huge progress has been made in understanding membrane bending during CME [20, 63]. The mitotic block in invagination observed previously [11] could point to the class of molecules that regulate shutdown. Mechanism B does not explain why other trafficking events are also inhibited, unless the tension of intracellular organellar membranes is also increased during mitosis. Mechanisms A and D provide the best explanation for inhibition of endocytosis together with other pathways. Unraveling the mechanism behind this inhibition is the next big challenge in the field.
Resumption of clathrin-mediated endocytosis in late mitosis
CME shutdown has to end at some point, otherwise the two daughter cells cannot use CME for the many cellular processes on which it depends. Curiously, the resumption of CME does not occur in G1 (after cytokinesis), but earlier in mitosis during late anaphase [15, 16], suggesting a requirement for CME during the final stages of cell division. This makes sense because this is a time of extensive membrane remodeling: the furrow forms at the spindle midzone and invaginates before the intercellular bridge is cut in a process termed abscission [64]. In agreement with this idea, endocytosis at the plasma membrane near the spindle poles is observed in late mitosis and the traffic of vesicles toward the midzone has been reported [15, 65]. Endosomal membrane is required at the midbody for successful separation of the two daughter cells [29, 64]. It is possible that CME occurring locally could provide this membrane rather than vesicles being trafficked exclusively from distal parts of the cell [64]. The requirement for CME in successful cytokinesis has been demonstrated by inhibition of CME by dominant-negative and RNAi approaches [15, 44, 54] and from a number of studies in non-mammalian cells [66, 67]. Understanding how restarted CME fits in with other membrane-trafficking events during cytokinesis is an ongoing challenge and an important area for future work [29]. As described above, understanding the timing, the mechanisms, and the reasons for resumption of CME may also provide clues to the mechanism of endocytic shutdown by telling us how shutdown is reversed.
What is the purpose of mitotic shutdown of endocytosis and why should we care?
The question of why cells shut down endocytosis during mitosis has two possible answers. Firstly, shutdown may be a consequence of mitosis and may serve no function at all. Secondly, continued endocytosis may interfere with accurate chromosome segregation in mitosis and therefore its inhibition is required. This second explanation seems more likely because (1) many other cellular processes are also inhibited during mitosis and (2) it is not clear how CME could proceed otherwise. After a CCV is formed, it is transported via microtubules to an intracellular destination. In mitotic cells, microtubules are reorganized into the spindle apparatus and many membranous organelles are vesiculated [2]. An important experiment would be to test whether continued CME interferes with normal mitosis, but a specific method for restarting CME artificially is not currently known.
The critical reader may be wondering whether this question is worth addressing. After all, in one cell cycle lasting ~24 h the cell spends <1 h in the stages of mitosis where CME is shut down! Moreover, many cells in the body are in G0 and are not dividing readily. We would argue that there are three reasons why deciphering the molecular mechanism of endocytic shutdown during mitosis is important. First, it may inform our understanding of the basic biological mechanism of CME as shutdown is a naturally occurring inhibited state. For example, if we find that mitotic phosphorylation of a single residue in an endocytic adaptor causes shutdown, then this could be very informative. Does this phosphorylation prevent the adaptor from engaging clathrin? There is still a lot to discover about the basic mechanisms of CME. Much of what we have learned so far has been by using experimental manipulations in cells or utilizing biochemical assays in vitro. Allowing the cell to teach us how evolution generated CME inhibition could tell us more about how CME works in normal cells. Second, understanding how the cell regulates CME could lead us to mimic this event pharmacologically. Specific inhibition of CME will be useful experimentally, but more importantly, it could be used as an anti-infective as many pathogens use CME as an entry route to the cell [68]. Some progress has been made in the development of inhibitors of CME [69], but the agents we have are far from perfect with issues of specificity and selectivity. Third, overriding the inhibition pharmacologically could also be useful therapeutically. Agents that are delivered to cells via CME [70] will not be internalized in mitotic cells. In tissues that are undergoing rapid division or where cells that are dividing need to be targeted specifically, drug delivery by this route becomes a problem. For example, overriding mitotic CME shutdown could be advantageous when dividing cells need to be targeted in proliferative diseases such as cancer.
Conclusions
In summary, the inhibition of endocytosis during mitosis is a robust observation documented by various methods, in multiple cell types over the last ~50 years. There are very few documented exceptions to the rule that, from prophase to late anaphase, internalization of cargo from the cell surface is shut down. Precisely how this inhibition occurs at the molecular level remains a mystery. The leading hypothesis is that an increase in membrane tension that occurs during early mitosis is too great for the CME machinery to overcome. Whether this is because the machinery is also weakened by mitotic phosphorylation or “moonlighting” is an open question. The role of microtubules and motors in the shutdown is also unclear. Finally, whether the shutdown is necessary for normal mitosis or is simply a consequence of cell division remains to be tested.
Acknowledgments
We apologize to any authors whose work we may have omitted. We are grateful to members of the Royle lab for critically reading the manuscript. We thank one anonymous reviewer who suggested mechanism D. This work was supported by Biotechnology and Biological Sciences Research Council Project Grant BB/H015582/1. SJR is a Senior Cancer Research Fellow for Cancer Research UK.
Abbreviations
- CME
Clathrin-mediated endocytosis
- CCV
Clathrin-coated vesicle
- CCP
Clathrin-coated pit
- DNP-BSA
Dinitrophenyl-bovine serum albumin
- EGF
Epidermal growth factor
- 2ME2
Methoxyestradiol
- EM
Electron microscopy
- LM
Light microscopy
- FM
Fluorescence microscopy
References
- 1.Murray AW. A brief history of error. Nat Cell Biol. 2011;13(10):1178–1182. doi: 10.1038/ncb2348. [DOI] [PubMed] [Google Scholar]
- 2.Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- 3.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11(12):1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fawcett DW. Surface specializations of absorbing cells. J Histochem Cytochem. 1965;13(2):75–91. doi: 10.1177/13.2.75. [DOI] [PubMed] [Google Scholar]
- 5.Berlin RD, Oliver JM, Walter RJ. Surface functions during mitosis I: phagocytosis, pinocytosis and mobility of surface-bound ConA. Cell. 1978;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- 6.Berlin RD, Oliver JM. Surface functions during mitosis. II. Quantitation of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J Cell Biol. 1980;85(3):660–671. doi: 10.1083/jcb.85.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintart J, Leroy-Houyet MA, Trouet A, Baudhuin P. Endocytosis and chloroquine accumulation during the cell cycle of hepatoma cells in culture. J Cell Biol. 1979;82(3):644–653. doi: 10.1083/jcb.82.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sager PR, Brown PA, Berlin RD. Analysis of transferrin recycling in mitotic and interphase HeLa cells by quantitative fluorescence microscopy. Cell. 1984;39(2 Pt 1):275–282. doi: 10.1016/0092-8674(84)90005-9. [DOI] [PubMed] [Google Scholar]
- 9.Warren G, Davoust J, Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984;3(10):2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver JM, Seagrave JC, Pfeiffer JR, Feibig ML, Deanin GG. Surface functions during mitosis in rat basophilic leukemia cells. J Cell Biol. 1985;101(6):2156–2166. doi: 10.1083/jcb.101.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pypaert M, Lucocq JM, Warren G. Coated pits in interphase and mitotic A431 cells. Eur J Cell Biol. 1987;45(1):23–29. [PubMed] [Google Scholar]
- 12.Pypaert M, Mundy D, Souter E, Labbe JC, Warren G. Mitotic cytosol inhibits invagination of coated pits in broken mitotic cells. J Cell Biol. 1991;114(6):1159–1166. doi: 10.1083/jcb.114.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illinger D, Italiano L, Beck JP, Waltzinger C, Kuhry JG. Comparative evolution of endocytosis levels and of the cell surface area during the L929 cell cycle: a fluorescence study with TMA-DPH. Biol Cell. 1993;79(3):265–268. doi: 10.1016/0248-4900(93)90146-6. [DOI] [PubMed] [Google Scholar]
- 14.Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol. 1999;144(3):497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweitzer JK, Burke EE, Goodson HV, D’Souza-Schorey C. Endocytosis resumes during late mitosis and is required for cytokinesis. J Biol Chem. 2005;280(50):41628–41635. doi: 10.1074/jbc.M504497200. [DOI] [PubMed] [Google Scholar]
- 16.Fielding AB, Willox AK, Okeke E, Royle SJ. Clathrin-mediated endocytosis is inhibited during mitosis. Proc Natl Acad Sci USA. 2012;109(17):6572–6577. doi: 10.1073/pnas.1117401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404(1–2):173–193. doi: 10.1016/S0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traub LM. Regarding the amazing choreography of clathrin coats. PLoS Biol. 2011;9(3):e1001037. doi: 10.1371/journal.pbio.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao Y, Haucke V. Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell Mol Life Sci (CMLS) 2011;68(24):3983–3993. doi: 10.1007/s00018-011-0768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirruccello M, De Camilli P. Inositol 5-phosphatases: insights from the Lowe syndrome protein OCRL. Trends Biochem Sci. 2012;37(4):134–143. doi: 10.1016/j.tibs.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5(4):317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 23.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 24.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51(6):773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA. 2007;104(19):7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118(5):591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463(7280):464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 28.Furthauer M, Gonzalez-Gaitan M. Endocytosis and mitosis: a two-way relationship. Cell Cycle. 2009;8(20):3311–3318. doi: 10.4161/cc.8.20.9700. [DOI] [PubMed] [Google Scholar]
- 29.Neto H, Collins LL, Gould GW. Vesicle trafficking and membrane remodelling in cytokinesis. Biochem J. 2011;437(1):13–24. doi: 10.1042/BJ20110153. [DOI] [PubMed] [Google Scholar]
- 30.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habela CW, Sontheimer H. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle. 2007;6(13):1613–1620. doi: 10.4161/cc.6.13.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson CA, Trinkaus JP. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976;99(2):375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- 33.Follett EA, Goldman RD. The occurrence of microvilli during spreading and growth of BHK21-C13 fibroblasts. Exp Cell Res. 1970;59(1):124–136. doi: 10.1016/0014-4827(70)90631-2. [DOI] [PubMed] [Google Scholar]
- 34.Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009;19(4):174–179. doi: 10.1016/j.tcb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Boucrot E, Kirchhausen T. Mammalian cells change volume during mitosis. PLoS ONE. 2008;3(1):e1477. doi: 10.1371/journal.pone.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coupin GT, Muller CD, Remy-Kristensen A, Kuhry JG. Cell surface membrane homeostasis and intracellular membrane traffic balance in mouse L929 cells. J Cell Sci. 1999;112((Pt 14) 14):2431–2440. doi: 10.1242/jcs.112.14.2431. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Shi H, Chen X, Wang Z. Regulation of EGF-stimulated EGF receptor endocytosis during M phase. Traffic. 2011;12(2):201–217. doi: 10.1111/j.1600-0854.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 38.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189(5):871–883. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chetrit D, Barzilay L, Horn G, Bielik T, Smorodinsky NI, Ehrlich M. Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis. J Biol Chem. 2011;286(7):5392–5403. doi: 10.1074/jbc.M110.161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10(9):583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 41.Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011;13(8):893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Slepnev VI, Di Fiore PP, De Camilli P. The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1999;274(6):3257–3260. doi: 10.1074/jbc.274.6.3257. [DOI] [PubMed] [Google Scholar]
- 43.He J, Xu J, Xu XX, Hall RA. Cell cycle-dependent phosphorylation of disabled-2 by cdc2. Oncogene. 2003;22(29):4524–4530. doi: 10.1038/sj.onc.1206767. [DOI] [PubMed] [Google Scholar]
- 44.Chircop M, Malladi CS, Lian AT, Page SL, Zavortink M, Gordon CP, McCluskey A, Robinson PJ. Calcineurin activity is required for the completion of cytokinesis. Cell Mol Life Sci (CMLS) 2010;67(21):3725–3737. doi: 10.1007/s00018-010-0401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5(8):701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- 46.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105(31):10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCusker D, Royou A, Velours C, Kellogg D. Cdk1-dependent control of membrane-trafficking dynamics. Mol Biol Cell. 2012;23(17):3336–3347. doi: 10.1091/mbc.E11-10-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16(1):55–63. doi: 10.1016/j.tcb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier NC, Masters TA, Sheetz MP. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22(10):527–535. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Dai J, Ting-Beall HP, Sheetz MP. The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells. J Gen Physiol. 1997;110(1):1–10. doi: 10.1085/jgp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13(9):1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469(7329):226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- 53.Royle SJ. Mitotic moonlighting functions for membrane trafficking proteins. Traffic. 2011;12(7):791–798. doi: 10.1111/j.1600-0854.2011.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith CM, Chircop M. Clathrin-mediated endocytic proteins are involved in regulating mitotic progression and completion. Traffic. 2012 doi: 10.1111/tra.12001. [DOI] [PubMed] [Google Scholar]
- 55.Ma MP, Chircop M. SNX9, SNX18 and SNX33 are required for progression through and completion of mitosis. J Cell Sci. 2012 doi: 10.1242/jcs.105981. [DOI] [PubMed] [Google Scholar]
- 56.Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 2011;30(5):906–919. doi: 10.1038/emboj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Royle SJ, Bright NA, Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434(7037):1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandoval IV, Bonifacino JS, Klausner RD, Henkart M, Wehland J. Role of microtubules in the organization and localization of the Golgi apparatus. J Cell Biol. 1984;99(1 Pt 2):113s–118s. doi: 10.1083/jcb.99.1.113s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subtil A, Dautry-Varsat A. Microtubule depolymerization inhibits clathrin coated-pit internalization in non-adherent cell lines while interleukin 2 endocytosis is not affected. J Cell Sci. 1997;110(Pt 19):2441–2447. doi: 10.1242/jcs.110.19.2441. [DOI] [PubMed] [Google Scholar]
- 62.Tanenbaum ME, Medema RH. Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell. 2010;19(6):797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Kirchhausen T. Bending membranes. Nat Cell Biol. 2012;14(9):906–908. doi: 10.1038/ncb2570. [DOI] [PubMed] [Google Scholar]
- 64.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131(5):847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Warner AK, Keen JH, Wang YL. Dynamics of membrane clathrin-coated structures during cytokinesis. Traffic. 2006;7(2):205–215. doi: 10.1111/j.1600-0854.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 66.Gerald NJ, Damer CK, O’Halloran TJ, De Lozanne A. Cytokinesis failure in clathrin-minus cells is caused by cleavage furrow instability. Cell Motil Cytoskeleton. 2001;48(3):213–223. doi: 10.1002/1097-0169(200103)48:3<213::AID-CM1010>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 67.Feng B, Schwarz H, Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp Cell Res. 2002;279(1):14–20. doi: 10.1006/excr.2002.5579. [DOI] [PubMed] [Google Scholar]
- 68.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124(4):729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Kleist L, Haucke V. At the crossroads of chemistry and cell biology: inhibiting membrane traffic by small molecules. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 70.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Jin Y, Plummer MR, Pooyan S, Gunaseelan S, Sinko PJ. Endocytosis and membrane potential are required for HeLa cell uptake of R.I.-CKTat9, a retro-inverso Tat cell penetrating peptide. Mol Pharm. 2009;6(3):836–848. doi: 10.1021/mp800121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tooze J, Hollinshead M. Evidence that globular Golgi clusters in mitotic HeLa cells are clustered tubular endosomes. Eur J Cell Biol. 1992;58(2):228–242. [PubMed] [Google Scholar]