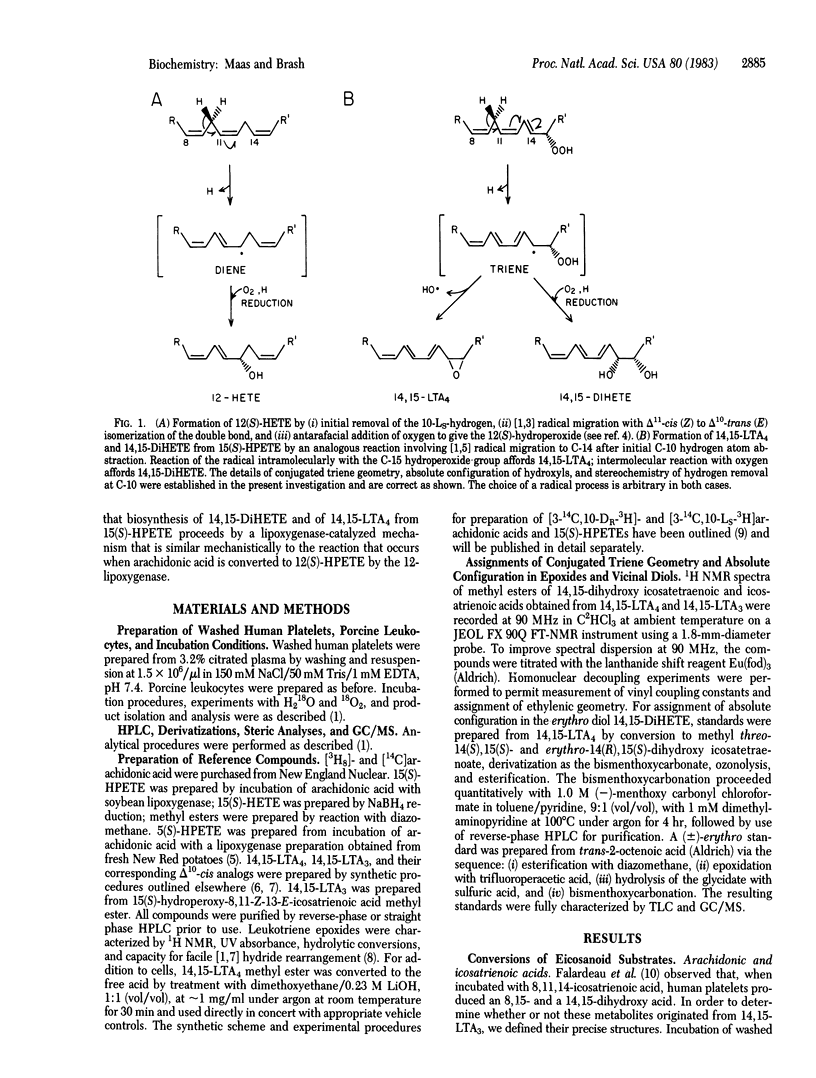
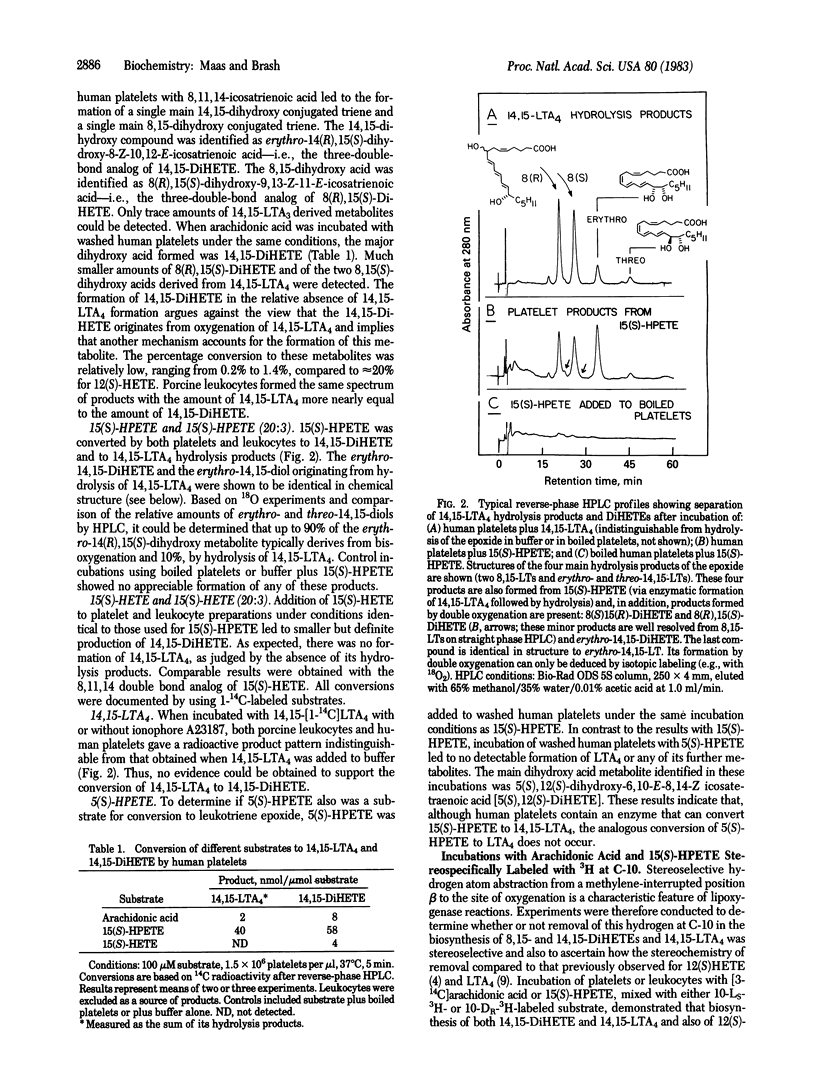
Abstract
Leukocyte preparations convert the hydroperoxy icosatetraenoic acids 5(S)-HPETE and 15(S)-HPETE to the unstable leukotriene epoxides LTA4 and 14,15-LTA4. In several ways, the conversion of 5- or 15-HPETE to leukotriene epoxide bears a formal mechanistic resemblance to the reaction catalyzed by the 12-lipoxygenase in the conversion of arachidonic acid to 12(S)-HPETE. Points of similarity include enzymatic removal of a hydrogen at carbon 10, double bond isomerization, and formation of a new carbon-to-oxygen bond. In the case of 15(S)-HPETE, two 8,15- and an erythro-14,15-dihydroxy acid (8,15- and 14,15-DiHETEs), which result from incorporation of molecular oxygen into each hydroxyl group, are coproducts in the formation of 14,15-LTA4. These facts prompted us to test the hypothesis that the biosynthesis of 14,15-LTA4 and of 8,15- and 14,15-DiHETEs from 15(S)-HPETE occurs by a mechanism similar to that observed in lipoxygenase reactions. Based on the results presented here, we conclude that the biosynthesis of 14,15-LTA4 and of 8,15- and 14,15-DiHETEs from 15(S)-HPETE occurs via a common intermediate and that, moreover, the formation of these metabolites from 15(S)-HPETE is catalyzed by an enzyme with many mechanistic features in common with the 12-lipoxygenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Falardeau P., Hamberg M., Samuelsson B. Metabolism of 8,11,14-eicosatrienoic acid in human platelets. Biochim Biophys Acta. 1976 Aug 23;441(2):193–200. doi: 10.1016/0005-2760(76)90162-4. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Hamberg G. On the mechanism of the oxygenation of arachidonic acid by human platelet lipoxygenase. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1090–1097. doi: 10.1016/0006-291x(80)91584-3. [DOI] [PubMed] [Google Scholar]
- Maas R. L., Brash A. R., Oates J. A. A second pathway of leukotriene biosynthesis in porcine leukocytes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5523–5527. doi: 10.1073/pnas.78.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. L., Brash A. R., Oates J. A. Novel leukotrienes and lipoxygenase products from arachidonic acid. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:29–44. [PubMed] [Google Scholar]
- Maas R. L., Ingram C. D., Taber D. F., Oates J. A., Brash A. R. Stereospecific removal of the DR hydrogen atom at the 10-carbon of arachidonic acid in the biosynthesis of leukotriene A4 by human leukocytes. J Biol Chem. 1982 Nov 25;257(22):13515–13519. [PubMed] [Google Scholar]
- Rådmark O., Lundberg U., Jubiz W., Malmsten C., Samuelsson B. New group of leukotrienes formed by initial oxygenation at C-15. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:61–70. [PubMed] [Google Scholar]
- Sok D. E., Chung T., Sih C. J. Mechanisms of leukotriene formation: hemoglobin-catalyzed transformation of 15-HPETE into 8,15-DiHETE and 14,15-DiHETE isomers. Biochem Biophys Res Commun. 1983 Jan 14;110(1):273–279. doi: 10.1016/0006-291x(83)91291-3. [DOI] [PubMed] [Google Scholar]


