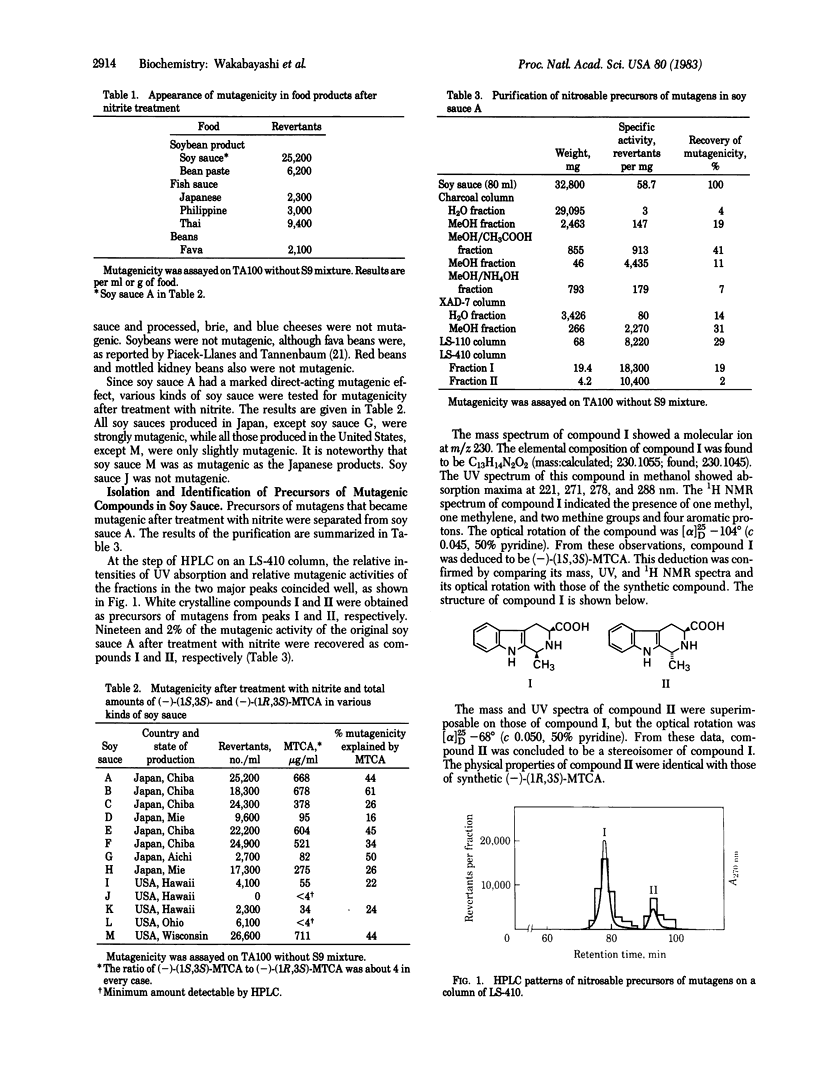
Abstract
After treatment with nitrite, Japanese soy sauce was strongly mutagenic to Salmonella typhimurium TA100 without S9 mixture. Two precursors of the mutagen were isolated from Japanese soy sauce, and these were identified as (-)-(1S,3S)-1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid [(-)-(1S,3S)-MTCA] and its stereoisomer (-)-(1R,3S)-MTCA. After treatment with nitrite, 1-mg samples of these compounds induced 17,400 and 13,000 revertants of TA100, respectively, without S9 mixture. Quantitative analysis of various kinds of soy sauces produced in Japan showed the presence of 82-678 micrograms of MTCA per ml. The mutagenicities of these compounds with nitrite accounted for 16-61% of the total mutagenicity of soy sauce with nitrite. Most soy sauces produced in the United States were less mutagenic than those produced in Japan and little, if any, of these two precursors of the mutagen was found in them. A major reaction product of (-)-(1S,3S)-MTCA and nitrite was a compound having a nitroso substitution at position N-2, but this compound was not mutagenic. Thus, the mutagen(s) formed from (-)-(1S,3S)-MTCA and nitrite was a minor product(s), and its specific mutagenic activity must be very high.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braestrup C., Nielsen M., Olsen C. E. Urinary and brain beta-carboline-3-carboxylates as potent inhibitors of brain benzodiazepine receptors. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2288–2292. doi: 10.1073/pnas.77.4.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossi A., Focella A., Teitel S. Alkaloids in mammalian tissues. 3. Condensations of L-tryptophan and L-5-hydroxytryptophan with formaldehyde and acetaldehyde. J Med Chem. 1973 Apr;16(4):418–420. doi: 10.1021/jm00262a027. [DOI] [PubMed] [Google Scholar]
- Cuello C., Correa P., Haenszel W., Gordillo G., Brown C., Archer M., Tannenbaum S. Gastric cancer in Colombia. I. Cancer risk and suspect environmental agents. J Natl Cancer Inst. 1976 Nov;57(5):1015–1020. doi: 10.1093/jnci/57.5.1015. [DOI] [PubMed] [Google Scholar]
- Doll R. Strategy for detection of cancer hazards to man. Nature. 1977 Feb 17;265(5595):589–596. doi: 10.1038/265589a0. [DOI] [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Fajen J. M., Carson G. A., Rounbehler D. P., Fan T. Y., Vita R., Goff U. E., Wolf M. H., Edwards G. S., Fine D. H., Reinhold V. N-nitrosamines in the rubber and tire industry. Science. 1979 Sep 21;205(4412):1262–1264. doi: 10.1126/science.472741. [DOI] [PubMed] [Google Scholar]
- HAENSZEL W. Variation in incidence of and mortality from stomach cancer, with particular reference to the United States. J Natl Cancer Inst. 1958 Aug;21(2):213–262. [PubMed] [Google Scholar]
- Hirono I., Mori H., Yamada K., Hirata Y., Haga M. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. J Natl Cancer Inst. 1977 Apr;58(4):1155–1157. doi: 10.1093/jnci/58.4.1155. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Adams J. D., Brunnemann K. D., Hecht S. S. Assessment of tobacco-specific N-nitrosamines in tobacco products. Cancer Res. 1979 Jul;39(7 Pt 1):2505–2509. [PubMed] [Google Scholar]
- LIJINSKY W., SHUBIK P. BENZO(A)PYRENE AND OTHER POLYNUCLEAR HYDROCARBONS IN CHARCOAL-BROILED MEAT. Science. 1964 Jul 3;145(3627):53–55. doi: 10.1126/science.145.3627.53. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Wang H. I., Yeh Y. C. The mutagenicity of soy bean sauce. Food Cosmet Toxicol. 1979 Aug;17(4):329–331. doi: 10.1016/0015-6264(79)90324-9. [DOI] [PubMed] [Google Scholar]
- MacDonald W. C., Dueck J. W. Long-term effect of shoyu (Japanese soy sauce) on the gastric mucosa of the rat. J Natl Cancer Inst. 1976 Jun;56(6):1143–1147. doi: 10.1093/jnci/56.6.1143. [DOI] [PubMed] [Google Scholar]
- Maekawa A., Ogiu T., Onodera H., Furuta K., Matsuoka C., Ohno Y., Odashima S. Carcinogenicity studies of sodium nitrite and sodium nitrate in F-344 rats. Food Chem Toxicol. 1982 Feb;20(1):25–33. doi: 10.1016/s0278-6915(82)80005-7. [DOI] [PubMed] [Google Scholar]
- Ohta T., Isa M., Suzuki Y., Yamahata N., Suzuki S., Kurechi T. Formation of mutagens from tryptophan by the reaction with nitrite. Biochem Biophys Res Commun. 1981 May 15;100(1):52–57. doi: 10.1016/s0006-291x(81)80061-7. [DOI] [PubMed] [Google Scholar]
- Piacek-Llanes B. G., Tannenbaum S. R. Formation of an activated N-nitroso compound in nitrite-treated fava beans (Vicia faba). Carcinogenesis. 1982;3(12):1379–1384. doi: 10.1093/carcin/3.12.1379. [DOI] [PubMed] [Google Scholar]
- Sander J., Bürkle G. Induktion maligner Tumoren bei Ratten durch gleichzeitige Verfütterung von Nitrit und sekundären Aminen. Z Krebsforsch. 1969 Sep 25;73(1):54–66. [PubMed] [Google Scholar]
- Sugimura T., Fujimura S. Tumour production in glandular stomach of rat by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1967 Dec 2;216(5118):943–944. doi: 10.1038/216943a0. [DOI] [PubMed] [Google Scholar]
- Sugimura T. Mutagens, carcinogens, and tumor promoters in our daily food. Cancer. 1982 May 15;49(10):1970–1984. doi: 10.1002/1097-0142(19820515)49:10<1970::aid-cncr2820491005>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Fett D., Young V. R., Land P. D., Bruce W. R. Nitrite and nitrate are formed by endogenous synthesis in the human intestine. Science. 1978 Jun 30;200(4349):1487–1489. doi: 10.1126/science.663630. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976 Dec;14(6):549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H., Marquardt H., Hirota N., Mori H., Williams G. M. Induction of cancer of the glandular stomach in rats by an extract of nitrite-treated fish. J Natl Cancer Inst. 1980 Jan;64(1):163–167. [PubMed] [Google Scholar]
- Witter J. P., Gatley S. J., Balish E. Evaluation of nitrate synthesis by intestinal microorganisms in vivo. Science. 1981 Jul 24;213(4506):449–450. doi: 10.1126/science.7244641. [DOI] [PubMed] [Google Scholar]
- Witter J. P., Gatley S. J., Balish E. Nitrate and nitrite: origin in humans. Science. 1979 Sep 28;205(4413):1335–1337. doi: 10.1126/science.205.4413.1335. [DOI] [PubMed] [Google Scholar]
- Yang C. S. Research on esophageal cancer in China: a review. Cancer Res. 1980 Aug;40(8 Pt 1):2633–2644. [PubMed] [Google Scholar]



