Abstract
Background
Recurrent affective problems are predictive of cognitive impairment, but the timing and directionality, and the nature of the cognitive impairment, are unclear.
Aims
To test prospective associations between life-course affective symptoms and cognitive function in late middle age.
Method
A total of 1668 men and women were drawn from the Medical Research Council National Survey of Health and Development (the British 1946 birth cohort). Longitudinal affective symptoms spanning age 13-53 years served as predictors; outcomes consisted of self-reported memory problems at 60-64 years and decline in memory and information processing from age 53 to 60-64 years.
Results
Regression analyses revealed no clear pattern of association between longitudinal affective symptoms and decline in cognitive test scores, after adjusting for gender, childhood cognitive ability, education and midlife socioeconomic status. In contrast, affective symptoms were strongly, diffusely and independently associated with self-reported memory problems.
Conclusions
Affective symptoms are more clearly associated with self-reported memory problems in late midlife than with objectively measured cognitive performance.
Studies suggest that affective problems are associated with cognitive decline in later life. In a recent systematic review of 51 studies, depression in particular was associated with increased risk of dementia (where specified, mostly Alzheimer’s disease but also vascular dementia); with evidence of a dose-response effect in regard to frequency and severity, and a smaller number of studies also implicating bipolar disorder.1 In addition, some studies suggest that depression predicts mild cognitive impairment, although design inconsistencies (such as the nature of the sample and length of follow-up) have resulted in conflicting findings for this outcome.2
There are at least three possible scenarios underlying these associations:1-3 anxiety and depression directly cause cognitive impairment, or at least lower the threshold for its manifestation (such as through glucocorticoid neurotoxicity or through social pathways to lower cognitive reserve); anxiety and depression are emotional responses to emerging cognitive impairment; anxiety and depression are risk indicators, being a manifestation of a shared neuropathological substrate that underlies both cognitive decline and mood disturbance. Long-term prospective studies lend weight to the first scenario, since depressive symptoms in midlife, particularly recurrent symptoms, are shown to precede dementia onset by decades.4,5 However, there are important uncertainties that require investigation: (a) timing: depression shows continuity over the life course,6,7 so it is likely that this risk begins early and is cumulative, yet this has not been studied in this context; furthermore, since most studies in this area have been conducted on old age samples it is unclear whether associations with cognition are already evident before this stage of the life course (a reasonable possibility if depression predicts mild cognitive impairment); (b) causal direction: lower childhood cognition is associated with higher frequency of adult affective symptoms,8-15 so the association between recurrent depressive symptoms and cognitive decline may reflect an even longer-standing effect of poor cognitive development; (c) type of cognition affected: emotional status may be a better predictor of self-reported memory problems than of tested cognitive performance.16-18
Addressing these uncertainties, the Medical Research Council (MRC) National Survey of Health and Development (NSHD), the oldest of the UK birth cohorts, provides a unique opportunity to test associations between lifetime affective problems in terms of poor emotional adjustment and cognitive function in a cohort reaching retirement age, while controlling for childhood cognition as well as other potential confounders. We hypothesised that a lifelong pattern of affective symptoms is associated with a faster rate of cognitive decline in late middle age, independently of level of childhood cognition and other key confounders.
Method
Participants
The MRC NSHD originally consisted of a socially stratified sample of 5362 children born within marriage in 1 week in March 1946 in England, Scotland and Wales.19 Of these, the study team was still in contact with 3163 prior to the most recent assessment, conducted between 2006 and 2011 when survey members were aged 60-64 years (henceforth 60+ years). Contact was not attempted for the remaining 2199 (718 deaths, 567 living abroad, 594 prior refusals and 320 permanently lost).
The most recent data collection began with a postal questionnaire sent to the target 3163 sample.20 This was followed by an invitation to an assessment by trained nurses at one of six clinical research facilities (based in Cardiff, Birmingham, Edinburgh, London (at University College London and St Thomas’ hospitals) and Manchester) or, if preferred, at a home visit. This was supplemented by a further questionnaire sent ahead to those who agreed to a clinic or home visit. These questionnaires, along with additional questions asked by the nurses, updated information on general health, household composition, family structure, socioeconomic status, daily function, life events and lifestyle. Of the target sample of 3163, 2661 (84%) provided some information, of whom 2229 (70%) completed either a clinic visit (n = 1690) or a home visit (n = 539).21
The study protocol received ethical approval from the Greater Manchester Local Research Ethics Committee for the four English sites; the Scotland A Research Ethics Committee approved the data collection taking place in Edinburgh. Written informed consent was obtained from the participants at each stage of data collection.
Cognitive outcomes
At either the clinic or home visit participants undertook tests of memory and information processing previously given at ages 53 years,22 and based on widely used task paradigms. The memory task consisted of a 15-item word learning task devised by the NSHD. Each word was shown for 2 s. When all 15 words were shown the study member was asked to write down as many of these as possible, in any order. The total number of words correctly recalled over three identical trials was summed to provide an overall score for short-term verbal memory (maximum 45). After a 1 min processing speed task (see below), an uncued delayed free recall trial was administered. Processing speed was assessed by a visual search task, where study members were required to cross out the letters P and W, randomly embedded within a page of other letters, as quickly and accurately as possible within 1 min. Search speed was represented by the position reached at the end of this interval (maximum 600) and search accuracy was represented by the number of target letters correctly crossed out within this interval (maximum 84). Cognitive impairment was defined as performance at least 1 standard deviation below the mean for each of these test scores. In addition to cognitive test performance, self-reported memory impairment was defined as answering ‘often’ or ‘very often’ to experiencing problems over the past 12 months with at least one of: finding the right word; remembering things; remembering where something was put.
Lifetime affective measures
A life-course mental health phenotype based on longitudinal latent class analysis of measures at ages 13, 15, 36, 43 and 53 years is available in NSHD. This characterises propensity for anxiety and depression throughout the period over these years as a parsimonious number of profiles,6 the validity of which has been demonstrated through associations with early developmental indicators.7 At ages 13 and 15 years teacher ratings of behaviour and emotionality were obtained using a forerunner of the Rutter A scale;23 relevant items referred to anxiety, timidity, fearfulness, diffidence and avoidance of attention. Frequency and severity of common symptoms of depression and anxiety were also assessed in adulthood, with the Present State Examination (PSE) at 36 years,24 the Psychiatric Symptom Frequency scale (PSF) at 43 years,25 and the 28-item General Health Questionnaire (GHQ-28) at age 53 and 60+.26 All of these measures except the GHQ-28 at 60+ were used to characterise the longitudinal profiles of affective symptoms (Fig. 1). Briefly, a single factor score representing symptoms of depression and anxiety with each of the above measurements was estimated after confirmatory factor analysis. Due to the skewed distributions of these scores, a categorical variable with four groups (absence of symptoms, occasional symptoms, moderate symptoms and severe symptoms) was defined at each age.
Fig. 1.
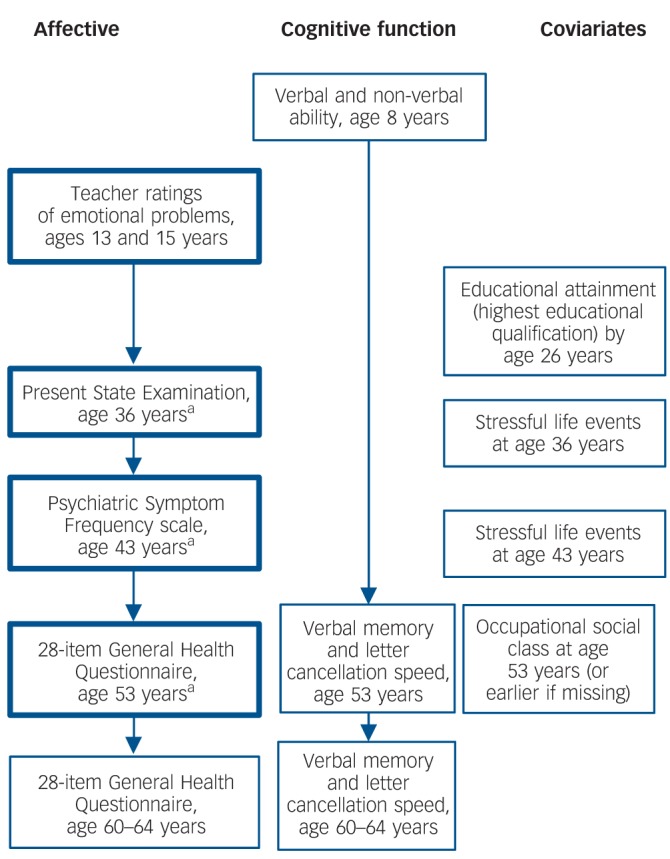
Flow diagram for National Survey of Health and Development data used in this study.
a. Anxiolytic and antidepressant medication use was recorded at these ages. Boxes in bold are affective symptom variables used for defining longitudinal profiles.7
Longitudinal latent class analysis of these categorical variables identified six distinct profiles:7 lifetime absence of symptoms (44.8% of sample), repeated moderate symptoms (33.6%), adult-onset moderate symptoms (11.3%), adolescent symptoms with good adult outcome (5.8%), adult-onset severe symptoms (2.9%) and repeated severe symptoms over the life course (1.7%). Because selection for non-missing covariate data (see below) resulted in a very small n for the repeated severe symptoms profile (n = 15), these survey members were treated as missing data.
As an additional marker of affective problems, study members were asked about prescription use at ages 36, 43 and 53 years.27 For the present study, use of anxiolytic (British National Formulary section 4.1.2) or antidepressant (British National Formulary section 4.3) medication at any of these ages (yes/no) was used as the indicator variable.28
Covariates
The following variables were treated as confounders: childhood cognitive ability, since this is associated with affective symptoms in this cohort,13,14 with implications for reverse causality; educational attainment and midlife occupational social class, since these are well known to be associated with affective problems and cognitive function; and affective symptoms concurrent with the cognitive outcomes, measured by the GHQ-28, since this may part-account for any associations between the mental health profiles and memory at this age. Age at which the cognitive outcomes were tested (ranging from 60 to 64 years), and corresponding cognitive scores at 53 years (baseline for change; see Statistical analysis) were also adjusted.
Childhood cognitive ability at age 8 years was represented as the sum of four tests of verbal and non-verbal ability devised by the National Foundation for Educational Research.29 These tests were (a) reading comprehension (selecting appropriate words to complete 35 sentences); (b) word reading (ability to read and pronounce 50 words); (c) vocabulary (ability to explain the meaning of 50 words); and (d) picture intelligence, consisting of a 60-item non-verbal reasoning test. Test-retest correlation coefficients indicated high reliability for each of these.29 Educational attainment by age 26 years was dichotomised into those with advanced (‘A level’, taken during the final year of secondary/high school) or higher (university or equivalent) qualifications v. those below this level. Midlife occupational social class was used at age 53 years, or earlier than this if missing. This was coded according to the UK Registrar General, and dichotomised into manual v. non-manual.
To explore any associations between mental health and the cognitive outcomes, the possible role of stressful life events was investigated. Classification of these was based on previous work using NSHD,30 which distinguished family, interpersonal and economic stressors in NSHD at ages 36 and 43 years that led to a life change from those that did not, since the former had stronger associations with mental health. For the present study these were summed over both ages and recoded into a single variable with three levels: (a) no event either reported or that led to a life change; (b) at least one event that led to a life change; (c) two or more events leading to a life change.
Preliminary analysis revealed that the gender mental health profile interaction was non-significant at the 5% level for all fully adjusted models (see below); gender was therefore used as a covariate rather than stratifying variable.
Statistical analysis
Linear regression was used to test associations between the mental health profiles and the memory outcomes as continuous variables, adjusting for the potential confounders (gender, childhood cognition, educational attainment, midlife occupational attainment, affective symptoms (GHQ-28) at the time of the outcomes, and age at testing of the latter). Logistic regression was used to test associations between the mental health profiles and cognitive impairment (1 standard deviation below the mean for test scores, and presence of self-reported memory impairment), also adjusting for the above potential confounders. For all multivariable analyses of cognitive test scores, conditional models of change were used to test associations between the mental health profiles and degree of cognitive decline by additionally adjusted for the corresponding continuous cognitive score at age 53 years.22 For consistency with this, the analysis with self-reported memory impairment as the outcome was adjusted for the delayed memory score at 53 years.
For all multivariable analyses, the mental health latent classes were entered as categorical variables, with lifetime absence of symptoms as the reference group. All other variables were entered as either continuous (childhood cognition and the GHQ-28 at 60+ years) or binary.
Results
A total of 1668 study members (53% of the target sample at 60+ years) had non-missing data for all variables incorporated in these analyses. Those without memory scores at age 60+ for any reason were more likely to be male, have less than advanced educational attainment, to be in a manual occupation in midlife, to have lower cognitive test scores at age 8 years and to have repeated severe affective symptoms (all P<0.001). However, there was no difference between those with and without cognitive scores at age 60+ and the total GHQ-28 score at the same age (P = 0.26).
Table 1 shows mean cognitive test scores at 60+ by the mental health profiles. There was a tendency for those with adult-onset moderate symptoms to have higher scores, otherwise scores appeared to be broadly similar between the profiles.
Table 1.
Means and standard deviations for the cognitive test scores at 60+ years by the mental health profiles
| Mean (s.d.) |
|||||
|---|---|---|---|---|---|
| n | Short-term memory | Delayed memory | Letter search speed | Letter search accuracy | |
| No signs/symptoms |
741 |
24.22 (6.04) |
8.45 (2.57) |
263.69 (70.9) |
24.59 (4.84) |
| Repeated moderate symptoms |
466 |
24.17 (6.08) |
8.43 (2.53) |
263.93 (70.52) |
24.45 (4.82) |
| Adult-onset moderate symptoms |
262 |
24.84 (6.06) |
8.69 (2.61) |
275.62 (69.84) |
25.33 (5.02) |
| Adolescent-onset symptoms with good adult outcome |
142 |
24.27 (5.58) |
8.5 (2.33) |
263.48 (75.05) |
24.08 (4.79) |
| Adult-onset severe symptoms | 57 | 24.14 (6.02) | 8.42 (2.82) | 270.14 (66.35) | 24.3 (4.9) |
Tables 2 and 3 show results of the multivariable regression analyses for cognitive test scores, as continuous measures (linear regression) and as impaired/unimpaired scores (logistic regression). No clear impression emerged from these analyses. If anything there was a trend towards those with affective symptoms showing lower odds of memory and search speed impairment, although these associations were not significant at the 5% level. The single exception was significantly better short-term memory in those with adolescent-onset problems with good adult outcome, compared with those without evidence of affective problems. This was observed with short-term memory as a continuous and a threshold score, but was not significant at the 5% level for delayed memory. These results were not substantially different when outcomes at 60-64 years only were used, i.e. without conditioning these on their corresponding age 53 scores (data not shown).
Table 2.
Regression coefficients representing associations between mental health latent classes and memory at 60+ yearsa
| Short-term memory | P | Delayed memory | P | |
|---|---|---|---|---|
| Continuous score,b regression coefficient | ||||
| No signs/symptoms | Reference | Reference | ||
| Repeated moderate symptoms | 0.06 (-0.46 to 0.57) | 0.83 | –0.11 (–0.38 to 0.16) | 0.44 |
| Adult-onset moderate symptoms | –0.17 (–0.79 to 0.45) | 0.59 | –0.05 (–0.38 to 0.27) | 0.75 |
| Adolescent-onset symptoms with good adult outcome | 0.93 (0.16 to 1.71) | 0.02 | 0.36 (–0.04 to 0.77) | 0.08 |
| Adult-onset severe symptoms |
0.53 (–0.69 to 1.74) |
0.40 |
–0.06 (–0.7 to 0.57) |
0.85 |
| Odds of impairment,c odds ratio (95% CI) | ||||
| No signs/symptoms | Reference | Reference | ||
| Repeated moderate symptoms | 0.93 (0.64 to 1.35) | 0.70 | 1.04 (0.71 to 1.53) | 0.84 |
| Adult-onset moderate symptoms | 0.82 (0.5 to 1.32) | 0.41 | 0.81 (0.49 to 1.34) | 0.42 |
| Adolescent-onset symptoms with good adult outcome | 0.55 (0.31 to 1.00) | 0.05 | 0.58 (0.32 to 1.06) | 0.08 |
| Adult-onset severe symptoms | 0.64 (0.27 to 1.54) | 0.32 | 1.16 (0.48 to 2.80) | 0.73 |
All coefficients are adjusted for gender, childhood cognition, educational attainment, midlife occupational social class, corresponding memory score at 53 years, total 28-item General Health Questionnaire (GHQ-28) score at 60+ years, and age memory was tested (60-64 years).
Coefficients represent rate of decline in memory from 53 to 60+ years for each mental health latent class compared with those with no signs/symptoms (positive coefficients represent slower decline).
Odds ratios for scoring 1 standard deviation below the mean for each mental health latent class compared with those with no symptoms.
Table 3.
Regression coefficients representing associations between mental health latent classes and visual search performance at 60+ yearsa
| Search speed | P | Search accuracy | P | |
|---|---|---|---|---|
| Continuous score,b regression coefficient | ||||
| No signs/symptoms | Reference | Reference | ||
| Repeated moderate symptoms | 0.05 (–6.96 to 7.05) | 0.99 | –0.05 (–0.56 to 0.45) | 0.84 |
| Adult-onset moderate symptoms | 0.05 (–8.40 to 8.51) | 0.99 | 0.04 (–0.56 to 0.65) | 0.88 |
| Adolescent-onset symptoms with good adult outcome | 3.72 (–6.81 to 14.24) | 0.49 | 0.03 (–0.73 to 0.79) | 0.94 |
| Adult-onset severe symptoms |
9.54 (–6.96 to 26.04) |
0.26 |
–0.16 (–1.35 to 1.02) |
0.79 |
| Odds of impairment,c odds ratio (95% CI) | ||||
| No signs/symptoms | Reference | Reference | ||
| Repeated moderate symptoms | 0.80 (0.57 to 1.14) | 0.22 | 0.85 (0.58 to 1.26) | 0.42 |
| Adult-onset moderate symptoms | 0.67 (0.42 to 1.07) | 0.09 | 0.7 (0.41 to 1.19) | 0.19 |
| Adolescent-onset symptoms with good adult outcome | 0.79 (0.47 to 1.34) | 0.39 | 0.71 (0.39 to 1.29) | 0.26 |
| Adult-onset severe symptoms | 0.58 (0.24 to 1.38) | 0.22 | 0.85 (0.36 to 2.02) | 0.71 |
All coefficients are adjusted for gender, childhood cognition, educational attainment, midlife occupational social class, corresponding visual search score at 53 years, and total 28-item General Health Questionnaire (GHQ-28) score at 60+ years, and letter search was tested (60-64 years).
Coefficients represent rate of decline in search performance from 53 to 60+ years for each mental health latent class compared with those with no symptoms (positive coefficients represent slower decline).
Odds ratios for scoring 1 standard deviations below the mean for each mental health latent class compared with those with no symptoms.
Table 4 shows the proportion of those with self-reported memory impairment within each mental health profile. Those with moderate (repeated and adult-onset) symptoms, and those with adult-onset severe symptoms, were more likely to report memory problems than those without symptoms, and those with adolescent-onset problems with good adult outcome. This was particularly pronounced for those with adult-onset severe symptoms. This table also shows odds of self-reported memory impairment by these profiles based in multivariable logistic regression. All mental health profiles were associated with this outcome at the 5% level or less, with the exception of those with adolescent-onset problems with good adult outcome. Those with adult-onset severe symptoms had nearly three times the odds of self-reported memory impairment compared with those without affective problems. These results were essentially unchanged when the analysis was re-run with the variable representing life-changing stressors included.
Table 4.
Regression coefficients (odds ratios) representing associations between mental health latent classes and self-reported memory problems at 60+ years (n = 1668)a
| Self-reported memory impairment, n (%) |
Self-reported memory problems, OR (95% CI) |
P | |
|---|---|---|---|
| No signs/symptoms | 103 (13.9) | Reference | |
| Repeated moderate symptoms |
109 (23.4) |
1.4 (1.02-1.92) |
0.04 |
| Adult-onset moderate symptoms |
68 (26.0) |
1.68 (1.16-2.42) |
0.006 |
| Adolescent-onset symptoms with good adult outcome |
18 (12.7) |
0.82 (0.48-1.42) |
0.48 |
| Adult-onset severe symptoms | 28 (49.1) | 2.98 (1.6-5.51) | 0.001 |
Coefficients are adjusted for gender, childhood cognition, educational attainment, midlife occupational social class, delayed memory score at 53 years, and total 28-item General Health Questionnaire (GHQ-28) score at 60+ years.
These patterns of associations were essentially unchanged when analyses were repeated with anxiolytic or antidepressant medication (see Method) added as a covariate, and when study members who had taken either of these medications were removed.
Associations between self-reported memory impairment and tested cognitive performance, although mostly significant at the 5% level, were of modest magnitude: 23.8% of those with self-reported memory problems also had impaired short-term memory (P = 0.03); figures for impaired delayed memory, search speed and search accuracy were 26.1% (P = 0.01), 21.2% (P = 0.24) and 27.3% (P = 0.004) respectively. Consistent with this, the association between delayed memory at 53 years and odds of self-reported memory impairment in the multivariable analysis was negligible (odds ratio (OR) = 1.0, 95% CI 0.99-1.00, P = 0.99).
Discussion
Main findings
In this population-based prospective longitudinal cohort study, with particiants now in late midlife, there was no clear pattern of association between affective problems over the life course and rate of decline in tested verbal memory and information processing (letter search) from 43 to 60+ years. This was the case when these variables were analysed as continuous scores, or as binary outcomes representing potential impairment (1 standard deviation below the mean); and when the analyses were controlled for reverse causality, i.e. from possible selection into affective problems by childhood cognition and from affective symptoms concurrent with the cognitive outcomes, as well as for confounding from educational and occupational attainment. The only significant finding for measured cognition was of slower memory decline in those with adolescent-onset affective problems that showed remission in adulthood. However, affective symptoms were more strongly and more diffusely associated with self-reported memory impairment, with approximately 50% raised odds of this impairment in those with moderate symptoms (lifetime and adult-onset), and nearly treble the odds in those with severe (adult-onset) symptoms in similarly controlled analyses.
Strengths and limitations
The main limitation of this study was a disproportionate loss to follow-up of those who were socioeconomically disadvantaged, had lower cognitive function in childhood and persistent severe affective symptoms. This probably led to an underestimation of the strength of association between affective symptoms and these outcomes. However, we have no reason to believe that this would have altered the pattern of associations observed, including the differential pattern of association with measured cognition and self-reported cognitive impairment. Nevertheless, the number of participants with persistent severe mental symptoms was too small for inclusion in these analyses after selection for non-missing data. Thus, we cannot rule out the possibility that the lack of an association for lifetime symptoms was a type II error arising from low power to demonstrate a dose-response effect with respect to symptom severity.
Against these limitations, important strengths should be emphasised. First, the NSHD is a prospective life-course study drawn from the general UK population. Second, a lifetime mental health phenotype was derived from measures in adolescence and adulthood, which is more robust and less prone to measurement error than information at a single time point, and which more accurately captures the population heterogeneity of symptom profiles over the life course.7 Third, as noted, a range of potential confounders were available, including childhood cognitive function, as well as educational and occupational attainment.
Implications
With these strengths and weaknesses in mind, how might these findings be understood? Evidence suggests that depression is associated with dementia, particularly Alzheimer’s disease and vascular dementia.1 On this basis it might be argued that an inverse association between affective symptoms and cognitive test scores was not observed in NSHD because study members were still relatively young, and that associations will be more likely in this cohort in older age, when cognitive decline is more marked and clinical outcomes are more frequent. Yet it is clear that many neurodegenerative diseases have a long preclinical phase, during which molecular pathology progressively accumulates; we might therefore have expected evidence of associations between lifetime affective symptoms and subtle cognitive impairment or decline in a cohort that is now old enough to be at risk of neurodegeneration.31 This might be consistent with a large systematic review that suggests lower volume of the hippocampus, an important neural substrate for memory and a primary sight for neurodegeneration in Alzheimer’s disease, in people who are depressed in midlife and earlier,32 with additional evidence of reversal of this reduction with antidepressant treatment.33 However, the implications of these subtle changes for cognitive function are unclear. Indeed, if anything there was evidence of a non-significant trend for lower odds of impaired cognitive function in those with affective symptoms in the present study. This ‘protective’ effect was most clearly observed in those with adolescent-onset affective problems with good adult outcome, who had significantly better short-term memory than those without symptoms. However, this needs to be replicated and further investigated before the interesting possibility that this mental health profile reflects a process of resilience can be taken seriously.
In striking contrast, affective symptoms were clearly associated with self-reported memory impairment. This is consistent with previous studies showing that emotional status is a better predictor of subjective memory than objective cognitive performance, whether measured by symptom checklist16 or by personality traits.17,18 This suggests that self-reported memory complaints are more a part of the symptomatology of common mental disorder than an essential part of an amnestic syndrome; and that presence of these complaints as a requirement for ascertaining mild cognitive impairment34 may lead to some degree of misclassification of this syndrome. Notably, we did not find that significant adverse life events accounted for associations between affective symptoms and self-reported memory problems, possibly suggesting that this association reflects a long-standing common trait. A further clinical implication of this study is that people with self-reported memory problems should be carefully reviewed for anxiety and depression. Continued follow-up of this cohort will determine whether or not an association between persistent affective problems and measured memory impairment emerges in old age.
Acknowledgments
The authors are grateful to NSHD study members who took part in this latest data collection for their continuing support. We thank members of the NSHD scientific and data collection team at the following centers: MRC Unit for Lifelong Health and Ageing, University College London; Wellcome Trust (WT) Clinical Research Facility (CRF) Manchester; WTCRF at the Western General Hospital in Edinburgh; WTCRF at University Hospital Birmingham; WTCRF at University College London Hospital; CRF at the University Hospital of Wales; CRF and Twin Research Unit at St Thomas’ Hospital London; NatCen.
Footnotes
Declaration of interest
P.B.J. has received research grant support from GlaxoSmithKline, a speaker’s honorarium from Eli Lilly and is a co-inventor of patent PCT/GB2005/003279 (methods for assessing psychotic disorders). J.H.B. is an employee of Cambridge Cognition Ltd, and is a co-inventor of patent PCT/GB2005/003279 (methods for assessing psychotic disorders).
Funding
This study was sponsored by Wellcome Trust grant 088869/B/09/Z, the UK MRC and the UK Department of Health (National Institute for Health Research).
References
- 1. da Silva J, Gonçalves-Pereira M, Xavier M, Mukaetova-Ladinska EB. Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry 2013; 202: 177–86 [DOI] [PubMed] [Google Scholar]
- 2. Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco A, Imbimbo B, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 2010; 18: 98–116 [DOI] [PubMed] [Google Scholar]
- 3. Geda YE. Blowing hot and cold over depression and cognitive impairment. Neurology 2010; 75: 12–4 [DOI] [PubMed] [Google Scholar]
- 4. Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010; 75: 27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia. Neurology 2010; 75: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutter M, Kim-Cohen J, Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. J Child Psychol Psychiatry 2006; 47: 276–95 [DOI] [PubMed] [Google Scholar]
- 7. Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Biol Psychiatry 2007; 62: 1265–71 [DOI] [PubMed] [Google Scholar]
- 8. Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, et al. A 35-year longitudinal assessment of cognition and midlife depression symptoms: the Vietnam era twin study of aging. Am J Geriatr Psychiatry 2011; 19: 559–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gale CR, Batty GD, Tynelius P, Deary IJ, Rasmussen F. Intelligence in early adulthood and subsequent hospitalisation and admission rates for the whole range of mental disorders: longitudinal study of 1,049,663 men. Epidemiology 2010; 21: 70–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gale CR, Hatch SL, Batty G, Deary IJ. Intelligence in childhood and risk of psychological distress in adulthood: the 1958 National Child Development Survey and the 1970 British Cohort Study. Intelligence 2009; 37: 592–9 [Google Scholar]
- 11. Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry 2009; 166: 50–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of five specific psychiatric disorders in mid life: the Vietnam Experience Study. Arch Gen Psychiatry 2008; 65: 1410–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatch SL, Jones PB, Kuh D, Hardy R, Wadsworth MEJ, Richards M. Childhood cognitive ability and adult mental health in the British 1946 birth cohort. Soc Sci Med 2007; 64: 2285–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richards M, Maughan B, Hardy R, Hall I, Strydom A, Wadsworth M. Long-term affective disorder in people with mild learning disability. Br J Psychiatry 2001; 179: 523–7 [DOI] [PubMed] [Google Scholar]
- 15. Martin LT, Kubzansky LD, Lewinn KZ, Lipsitt LP, Satz P, Buka SL. Childhood cognitive performance and risk of generalized anxiety disorder. Int J Epidemiol 2007; 36: 769–75 [DOI] [PubMed] [Google Scholar]
- 16. Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging 1996; 11: 272–9 [DOI] [PubMed] [Google Scholar]
- 17. Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Troller JN, Draper B, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010; 18: 701–10 [DOI] [PubMed] [Google Scholar]
- 18. Pearman A, Storandt M. Predictors of subjective memory in older adults. J Gerontol B Psychol Sci Soc Sci 2004; 59: P4–6 [DOI] [PubMed] [Google Scholar]
- 19. Wadsworth MEJ, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 national birth cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006; 35: 49–54 [DOI] [PubMed] [Google Scholar]
- 20. Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, et al. Updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol 2011; 40: e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stafford M, Black S, Shah I, Hardy R, Pierce M, Richards M, et al. Using a birth cohort to study ageing: representativeness and response rates in the National Survey of Health and Development. Eur J Ageing 2013; 10: 145–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richards M, Shipley B, Fuhrer R, Wadsworth MEJ. Cognitive ability in childhood and cognitive decline in mid-life: longitudinal birth cohort study. BMJ 2004; 328: 552–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richards M, Abbott R. Childhood Mental Health and Adult Life Chances in Post-War Britain: Insights from Three National Birth Cohort Studies. Centre for Mental Health, 2009. [Google Scholar]
- 24. Wing JK, Cooper JE, Sartorius N. Present State Examination. Institute of Psychiatry, 1974. [Google Scholar]
- 25. Lindelow M, Hardy R, Rodgers B. Development of a scale to measure symptoms of anxiety and depression in the general UK population: the psychiatric symptom frequency scale. J Epidemiol Community Health 1997; 51: 549–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979; 9: 139–45 [DOI] [PubMed] [Google Scholar]
- 27. Colman I, Croudace TJ, Wadsworth MEJ, Kuh D, Jones PB. Psychiatric outcomes 10 years after treatment with antidepressives or anxiolytics. Br J Psychiatry 2008; 193: 327–31 [DOI] [PubMed] [Google Scholar]
- 28. British Medical Association & Royal Pharmaceutical Society of Great Britain British National Formulary (March issue). BMJ Books & Pharmaceutical Press, 2011. [Google Scholar]
- 29. Pigeon DA. Tests used in the 1954 and 1957 surveys. In The Home and the School (Appendix 1) (ed. Douglas JWB.). Macgibbon & Kee, 1964. [Google Scholar]
- 30. Hatch SL, Mishra G, Hotopf M, Jones PB, Kuh D. Appraisals of stressors and common mental disorder from early to mid-adulthood in the 1946 British birth cohort. J Affect Disord 2009; 119: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rowe CC, Ellis KA, Rimajovae M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010; 31: 1275–83 [DOI] [PubMed] [Google Scholar]
- 32. McKinnon MC, Yucel K, Nazarov A, MacGueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 2009; 34: 41–54 [PMC free article] [PubMed] [Google Scholar]
- 33. Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry 2012; November 6 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 34. Petersen RC. Mild cognitive impairment. N Engl J Med 2011; 364: 2227–34 [DOI] [PubMed] [Google Scholar]


