Abstract
Objective
The inability to successfully treat women with ovarian cancer is due to the presence of metastatic disease at diagnosis and the development of platinum resistance. Ovarian cancer metastasizes throughout the peritoneal cavity by attaching to and invading through the mesothelium lining the peritoneum using a mechanism that involves α4β1 integrin and its ligand (vascular cell adhesion molecule) VCAM-1. Integrin α4β1 expression on tumor cells is known to confer protection from therapy in other cancers, notably multiple myeloma. We evaluated the role of α4β1 integrin in response to platinum-based therapy in a mouse model of peritoneal ovarian cancer metastasis by treatment with a humanized anti-α4β1 integrin function-blocking antibody.
Methods
Integrin α4β1 expression on primary human ovarian cancer cells, fallopian tube and ovarian surface epithelium and fresh tumor was assessed by flow-cytometry. The therapeutic impact of anti- α4β1 treatment was assessed in murine models of platinum-resistant peritoneal disease and in vitro using the platinum resistant ovarian cancer cell lines.
Results
Treatment of tumor-bearing mice with human-specific α4β1 integrin function-blocking antibodies, anti-VCAM-1 antibody or carboplatin alone had no effect on tumor burden compared to the IgG control group. However, the combined treatment of anti-α4β1 integrin or anti-VCAM-1 with carboplatin significantly reduced tumor burden. In vitro, the combination of carboplatin and anti-α4β1 integrin antibodies resulted in increased cell death and doubling time.
Conclusions
Our findings support a role for α4β1 integrin in regulating treatment response to carboplatin, implicating α4β1 integrin as a potential therapeutic target to influence platinum responsiveness in otherwise resistant disease.
INTRODUCTION
Ovarian cancer is the most lethal gynecologic malignancy. The inability to successfully treat women with ovarian cancer is due primarily to the advanced stage at diagnosis and the development of platinum resistance. Up to 75% of ovarian cancer patients are diagnosed with metastatic disease and experience a 5-year survival of less than 30%. While most patients respond favorably to initial treatment (cytoreductive surgery with platinum-based chemotherapy), greater than 80% will recur often with disease that is platinum resistant. The ability to overcome resistance would provide a significant advance in the treatment of women with ovarian cancer.
Ovarian cancer metastasizes throughout the peritoneal cavity where it invades the mesothelium, a single cell layer of mesothelial cells that lines the peritoneal cavity and surrounds structures within the cavity. Mesothelial invasion, an unfavorable prognostic indicator [1], is mediated in part by the interaction of α4β1 integrin and its ligand, vascular cell adhesion molecule-1 (VCAM-1), which is expressed preferentially on the mesothelium of ovarian cancer patients [2–4]. Integrin α4β1 is more commonly known for its expression on leukocytes, where it regulates leukocyte trafficking during infection and in autoimmune diseases including multiple sclerosis and Crohn’s disease. Natalizumab (or Tysabri™) is a humanized anti-α4β1 integrin function-blocking antibody approved for the treatment of these autoimmune conditions [5]. While abnormal integrin expression is frequently observed in cancer leading to numerous clinical trials with inhibitors targeting various integrin receptors [5], the expression and function of α4β1 integrin in cancer remains unclear with few exceptions. In particular, α4β1 integrin expression on tumor cells in multiple myeloma promotes bone marrow homing and protects the cancer cells from chemotherapy [6–9]. Given this known role for α4β1 to confer treatment resistance in some disease sites, we investigated whether its inhibition influences progression and treatment response in ovarian cancer using a mouse model of peritoneal metastasis and platinum-resistant cancer cells.
MATERIALS and METHODS
Antibodies
Rat anti-mouse VCAM-1 hybridoma clone M/K-2.7 and rat anti-mouse α4β1 integrin clone PS/2 [10], mouse anti-human α4β1 integrin clone HP1/2 (US Patent # 6,602,503) [11] and humanized anti-human α4β1 integrin (natalizumab or Tysabri™, US Patent # 5,840,299, provided by Biogen Idec, Inc.) [12] were used for animal studies.
Cell Lines
SKOV3LucD3 cells (Caliper Life Sciences; grown in McCoy’s 5A media supplemented with 10% FBS) were passed once through the peritoneal cavity of athymic nude mice (SKOV3ip1Luc cells) to improve tumor take and kinetics of tumor progression. A2780Cis cells (Sigma-Aldrich, Inc.) were transduced with lentivirus-expressing luciferase and propagated in RPMI1640 supplemented with 10% FBS and 2 mM glutamine with the addition of 1 μM cisplatin (Sigma-Aldrich, Inc.). Primary human ovarian cancer (n=8) and fallopian tube epithelial cell lines (n=2) were generated as described previously [13].
Human Tissue Samples
Following IRB approval, de-identified samples of ovarian tumors (n=3), normal ovaries (n=10) and normal fallopian tubes (n=7) were obtained surgery following appropriate pathological evaluation. The tissue was minced and digested with 180 units/ml collagenase at 37° C, for 16 hours. Remaining large tissue pieces were discarded, and the cells pelleted, washed in FACS buffer (PBS with 2% FBS), and resuspendend for flow cytometry staining and analysis.
Mouse Studies
Mouse experiments were performed in accordance with the policies and procedures established by the UVA Animal Care and Use Committee. NCR nude mice (6–8 weeks, Taconic Laboratories) were injected intraperitoneally (IP) with 106 SKOV3ip1Luc (n=10 per treatment group) or A2780CisLuc (n=5 per treatment group) (both platinum resistant) cells as previously described [2]. Treatment consisting of 25 mg/kg carboplatin once weekly and/or 200 μg of the indicated antibodies twice weekly was initiated 1 week after tumor initiation and delivered IP for 3 or 4 weeks to mice with A2780CisLuc or SKOV3ip1Luc, respectively. Tumor burden was monitored weekly by measuring light emission as an indication of luciferase activity using an IVIS imaging system (Molecular Imaging Core, University of Virginia). Total flux (photons/second) was determined for the entire abdominal cavity. Upon experimental termination, mice were euthanized and tumor burden evaluated upon necropsy by counting the number of tumor nodules, and weighing the omentum (primary site of tumor implantation) and any additional tumor nodules. Formalin-fixed, paraffin-embedded tissues were, sectioned and H&E stained to evaluate microscopic tumor burden, the extent of peritoneal invasion, and mitotic index. Additionally, sections were subjected to immunohistochemistry for Ki67, cleaved caspase 3, cleaved PARP, F4/80 (macrophage) or CD31 (endothelial cell). Peritoneal lavage was performed prior to necropsy to isolate cells for flow cytometry.
Flow Cytometry
Primary human ovarian cancer and fallopian tube epithelial cells (106 cells/ml) were stained with anti-human antibodies directed against CD49d (α4 integrin), CD45 (pan leukocyte marker) and EpCAM (epithelial cell marker); isotype antibodies were used as negative controls (all from Millipore). Cells from mouse lavage were stained with anti-mouse CD45. The stained samples were examined using a FACSCalibur cytometer (BD Biosciences) and data analyzed using FlowJo software version 9.4.10 for Macintosh (Tree Star).
Cell Proliferation and Viability
Cell growth was determined using CyQuant (Invitrogen) according to manufacturer’s instructions or by counting trypan blue stained cells. Briefly, 1,500 SKOV3ip1Luc or A2780Cis cells were plated, treated 24 hours later with 10 μg/ml of the indicated antibodies and/or the indicated concentrations of carboplatin and cultured for up to 7 days. For trypan experiments, SKOV3ip1 cells were plated in complete media. After 24 hours, cells were treated with the indicated concentrations of carboplatin ± 10 μg/ml of the indicated antibodies in serum-free media. Cells were harvested, stained with trypan blue and counted for viable and dead cells 24 and 48 hours after treatment. The number of dead cells compared to the total cell count was used to determine the percent dead. The 24 and 48-hour viable cell counts were used to calculate the doubling time with the following formula:
where Nf = 48-hour cell counts and Ni = 24-hour cell counts.
Statistical Analysis
Human tissue, flow cytometry, and animal experiments were analyzed using ANOVA followed by Bonferroni Correction for all combinations. Cell culture experiments were analyzed using 2-way ANOVA followed by Bonferroni Correction for all combinations.
RESULTS
Expression of α4β1 integrin in ovarian cancer patients
Previous reports demonstrate α4β1 integrin expression on ovarian cancer cell lines where it correlates with the extent of mesothelial invasion in cell culture models [2]; however, its expression on patient tumor cells has not been evaluated. Cell surface expression was measured by flow cytometry on single cell suspensions of, ovarian surface epithelium, tumor, or fallopian tube epithelium. The percent of α4β1 integrin positive epithelial cells was determined by gating for α4 integrin, EpCAM positive cells and against CD45 positive leukocytes (Table 1). While α4 integrin pairs with either β1 or β7 integrins, β7 integrin is restricted to hematopoietic cells; therefore, α4 integrin expression likely reflects the relative level of α4β1 integrin expression. Epithelial cells from all three tissues expressed α4β1 integrin demonstrating that α4β1 integrin expression is not induced during transformation. Examination of A2780 (sensitive) and A2780Cis (resistant) cells demonstrated similar levels of α4 integrin expression (Supplemental Table). However, since its ligand, VCAM-1 is induced on the mesothelium of women with metastatic ovarian cancer [2], α4β1 integrin has the potential of being a viable therapeutic target.
Table 1.
Percent of α4 integrin positive cells from each human tissue.
Mean ± std. dev.
Significantly different from “Tumor” determined by 1-way ANOVA followed by Bonferroni’s Multiple Comparison Test
Inhibiting α4β1 integrin sensitizes ovarian cancer to carboplatin in vivo
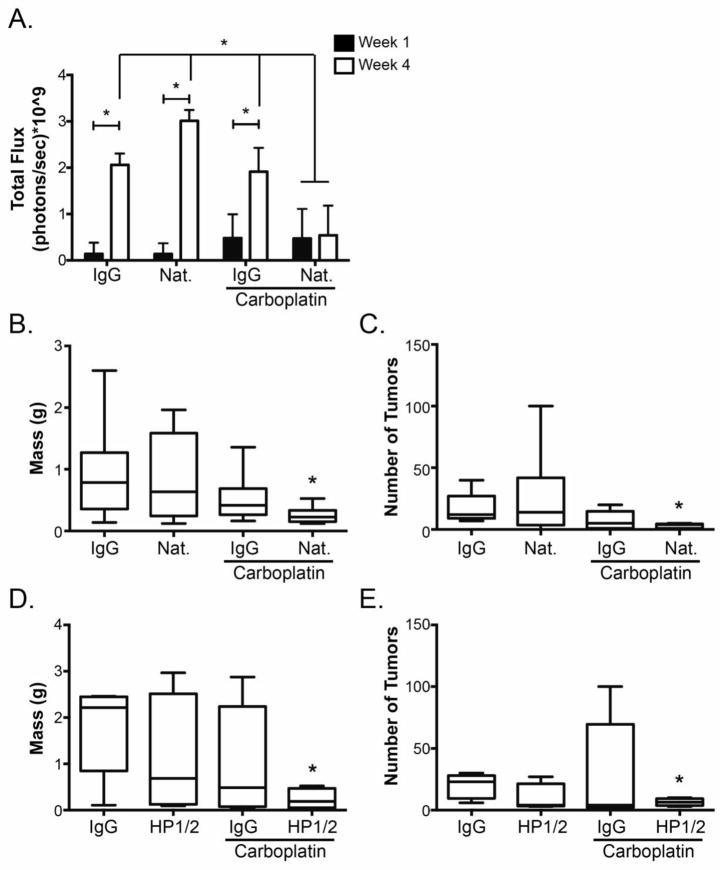
Intraperitoneal (IP) injection of human ovarian cancer cells into immune compromised mice mimics many aspects of advanced disease. One week after IP injection of luciferase-expressing SKOV3ip1 cells into athymic nude mice, baseline images were obtained, total photon flux was determined to confirm that mice harbored equivalent tumor burden (Fig. 1A), and treatment was initiated. After 4 weeks of treatment, in vivo imaging demonstrated increased total photon flux in IgG, natalizumab or carboplatin + IgG treatment groups; however, there was no change in photon flux in the group that received carboplatin with natalizumab (Fig. 1A). Mice were euthanized and tumor burden determined. Consistent with the in vivo imaging data, treatment with natalizumab and carboplatin resulted in a significant decrease in omentum/tumor weight (Fig. 1B) and tumor nodule count (Fig. 1C) compared to control mice or those treated with either agent alone. Similarly, growth of cisplatin-resistant A2780 cells (A2780Cis) was diminished significantly following combination treatment with carboplatin and a second α4β1 integrin antibody, HP1/2, which recognizes a distinct but overlapping epitope targeted by natalizumab; neither carboplatin nor HP1/2 alone had any impact on tumor burden in mice harboring A2780Cis tumors (Figs. 1D and 1E) indicating that functional blockade of α4β1 integrin renders tumors derived from platinum-resistant ovarian cancer cells responsive to carboplatin treatment.
Fig. 1. Inhibition of α4β1 integrin sensitizes platinum resistant ovarian tumors to carboplatin chemotherapy.
Mice were injected with SKOV3ip1Luc (n=10 per treatment group) (a-c) or A2780Cis (n=5 per treatment group) (d, e) cells. One week later treatment consisting of control IgG, natalizumab (Nat.), or HP1/2 in the presence or absence of carboplatin as indicated was initiated. (A) Baseline images of light emission from luciferase-expressing cells were taken just prior to the start of treatment (week 1, dark bars) and after 4 weeks of treatment (open bars). Data represent the mean total flux in photons per second ± std. dev. measured from the entire abdominal cavity of each mouse. *p < 0.05, week 4 relative to week 1, determined by 2-way ANOVA followed by Bonferroni multiple comparisons test. (B) Five weeks after SKOV3ip1Luc tumor initiation (4 weeks of treatment), mice were euthanized; omentum and visible tumors were isolated from surrounding tissues and weighed. Data represent mean ± std. dev. of 10 mice per group. *p < 0.05 relative to IgG control determined by 1-way ANOVA followed by Bonferroni multiple comparisons test for all combinations. (C) Individual visible tumor nodules within the abdominal cavity were enumerated following euthanization as described in (B). In some cases, the mice had too many nodules to count in which case they were assigned a value of 100. Data represent mean ± std. dev. of 10 mice per group. * p < 0.05 relative to IgG control determined by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. (D) Four weeks after A2780Cis tumor initiation (3 weeks of treatment), mice were euthanized; omentum and visible tumors were isolated from surrounding tissues and weighed. Data represent mean ± std. dev. of 5 mice per group. * p < 0.05 relative to IgG control determined by 1-way ANOVA followed by Bonferroni multiple comparisons test for all combinations. (E) Individual visible tumor nodules within the abdominal cavity were enumerated following euthanization as described in (D). Mice with too many nodules to count were assigned a value of 100. Data represent mean ± std. dev. of 5 mice per group. * p < 0.05 relative to IgG control determined by Kruskal-Wallis test followed by Dunn’s multiple comparisons test.
Previous work demonstrated a role for VCAM-1, a ligand of α4β1 integrin, in ovarian cancer metastatic progression [2]. Therefore, we tested the ability of VCAM-1 blockade to improve the response to carboplatin therapy. One week following IP injection of SKOV3ip1Luc cells, mice were treated with control IgG, a function-blocking antibody directed against mouse VCAM-1 (M/K 2.7), carboplatin and IgG, or carboplatin and M/K 2.7. As with natalizumab, the combination of carboplatin and M/K 2.7 treatment significantly decreased omentum/tumor weight compared to control or either treatment alone (Fig. 2A); however, there was no difference in the number of macroscopic tumor nodules (Fig. 2B). While this result is slightly less effective than treatment anti-α4β1 integrin antibodies and carboplatin, it supports the notion that targeting α4β1 integrin by inhibiting one ligand enhances the efficacy of carboplatin in peritoneal ovarian cancer growth.
Fig. 2. Inhibition of VCAM-1 cooperates with carboplatin to decrease ovarian cancer growth in vivo.
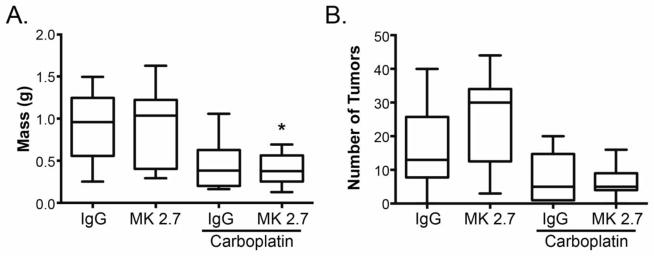
Mice were injected with SKOV3ip1Luc cells, and one week later treatment consisting of control IgG, anti-mouse VCAM-1 blocking antibody (MK 2.7) with or without carboplatin was initiated. (A) Five weeks after SKOV3ip1Luc tumor initiation (4 weeks of treatment), mice were euthanized; omentum and visible tumors were isolated from surrounding tissues and weighed. Data represent mean ± std. dev. of 10 mice per group. *p < 0.05 relative to IgG control determined by 1-way ANOVA followed by Bonferroni multiple comparisons test for all combinations. (B) Individual visible tumor nodules within the abdominal cavity were enumerated following euthanization as described in (A). In some cases, the mice had too many nodules to count in which case they were assigned a value of 100. Data represent mean ± std. dev. of 10 mice per group.
Inhibiting leukocyte infiltration has no effect on tumor growth
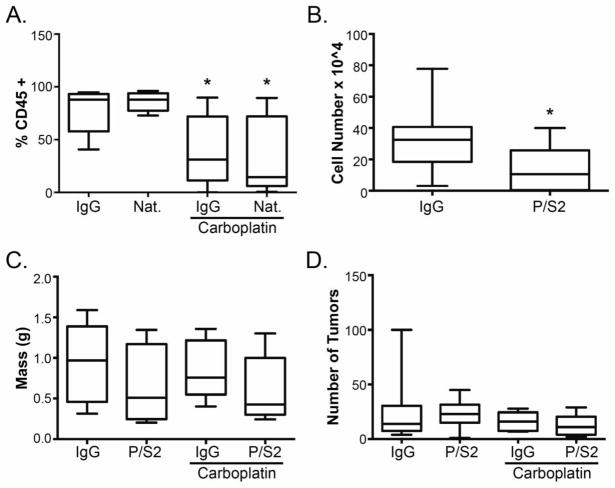
Leukocytes express α4β1 integrin, which facilitates extravasation and entry into the peritoneal cavity following interaction with VCAM-1 [14, 15], and monocytic cells, specifically, have been implicated in ovarian cancer progression [16, 17]. While natalizumab and HP1/2 are specific for human α4β1 integrin, it is possible that sufficient cross-reactivity with mouse α4β1 integrin could inhibit leukocyte infiltration to reduce tumor growth. Furthermore, M/K 2.7 recognizes mouse VCAM-1 and is expected to block leukocyte infiltration [10]. To address this possibility, peritoneal lavage samples from the mice were stained for the pan-leukocyte marker, CD45 and examined by flow cytometry. Treatment with natalizumab had no effect on the percentage of leukocytes relative to control IgG treated mice (Fig. 3A). Additionally, immunohistochemical staining of tumor sections revealed no difference in the number of macrophages identified by F4/80 positivity (data not shown). Targeting leukocytes directly using the anti-mouse α4β1 integrin function-blocking antibody, PS/2, in tumor-bearing mice resulted in a significant decrease in the number of CD45 positive cells in peritoneal lavage samples (Fig. 3B) but had no effect on tumor progression as a single agent or in combination with carboplatin as determined by omentum/tumor weight and nodule count (Figs. 3C and 3D). These observations demonstrate that leukocyte infiltration is not a critical component of α4β1 integrin-dependent tumor response to carboplatin. Rather, they imply that inhibition of α4β1 integrin enhances the cytotoxic effects of carboplatin.
Fig. 3. Leukocyte trafficking and ovarian cancer growth in vivo.
(A) Cells obtained from peritoneal lavage of mice treated as in Figure 1 were stained for the pan-leukocyte marker, CD45 and analyzed by flow cytometry. Data represent mean percentage of CD45+ cells ± std. dev. (n=10 per group). * p < 0.05 relative to IgG control determined by 1-way ANOVA followed by Bonferroni multiple comparisons test for all combinations. (B) Athymic nude mice harboring SKOV3ip1 cells were treated with the anti-mouse monoclonal antibody, P/S2. Five weeks after treatment, cells obtained from peritoneal lavage were stained for CD45 and analyzed by flow cytometry. Data represent the mean number of CD45+ cells ± std. dev. (n=10 per group). * p < 0.05 relative to IgG control, Student’s t test. (C, D) One week following IP injection of SKOV3ip1 cells, mice were treated with control IgG or P/S2 with or without carboplatin. Four weeks after treatment initiation, mice were euthanized; omentum and tumor weight (C) and macroscopic tumor nodule counts (D) were determined as described in Figures 1 and 2.
Functional blockade of α4β1 integrin increases ovarian cancer cell death and doubling time in response to carboplatin in vitro
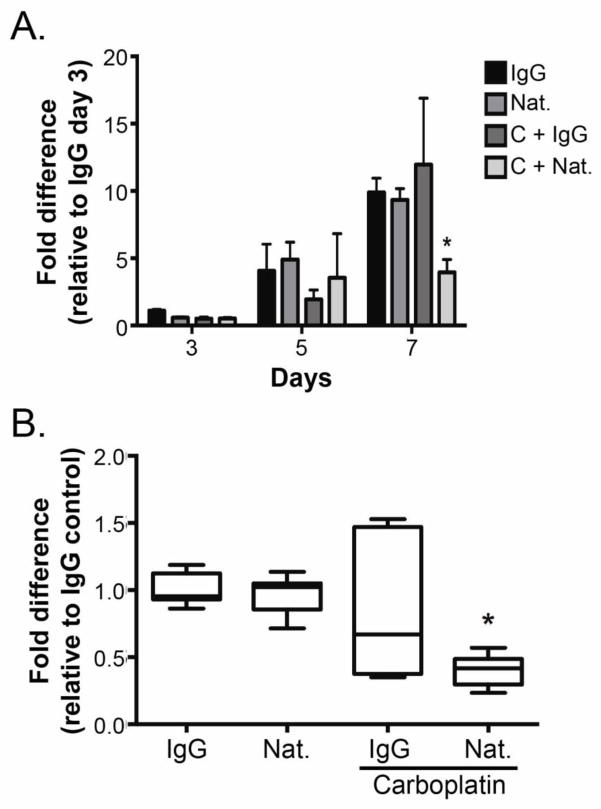
To investigate the contribution of α4β1 integrin to ovarian cancer cell growth and response to carboplatin, SKOV3ip1 cells were treated with natalizumab or control IgG in the presence or absence of 0.1 μg/ml carboplatin. No change in cell number was observed after 3 or 5 days of any treatment (Fig. 4A). However, treatment with the combination of α4β1 integrin blockade and carboplatin for 7 days resulted in a significant decrease in cell number compared to either treatment alone or control (Fig. 4A). Indeed, the combination treatment resulted in a 60% decrease in cell number compared to IgG treated cells (Fig. 4B).
Fig. 4. Effects of carboplatin and natalizumab on ovarian cancer cell growth in vitro.
(A) Growth of SKOV3ip1cells cultured in the presence of the indicated treatments (Nat. = natalizumab; C = carboplatin) was determined 3, 5, and 7 days after treatment using CyQuant. Fluorescence intensity from a single experiment performed in triplicate was normalized to the day 3 IgG control. Data represent mean ± std. dev. * p < 0.05 relative to all day 7 treatments determined by 2-way ANOVA followed by Bonferroni multiple comparisons test. (B) SKOV3ip1Luc cells were cultured in the presence of the indicated treatments for 7 days. Growth was determined as described in (A). Data represent mean ± std. dev. of 3 independent experiments performed in triplicate normalized to the IgG control. * p < 0.05 relative to all day 7 treatments determined by 2-way ANOVA followed by Bonferroni multiple comparisons test.
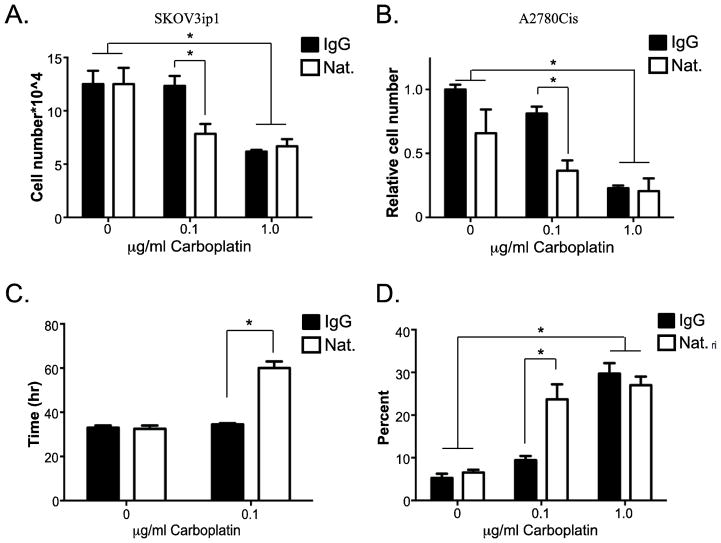
Since anti-α4β1 integrin antibody and carboplatin inhibited cell growth in 7 days, we reasoned that it occurred in conjunction with depletion of nutrients and/or serum components. As a result of the heterogeneous blood flow within tumors, micro-domains within the tumor are deprived of oxygen, nutrients and other serum factors [18] perhaps resembling the conditions achieved in the 7-day growth assay. Therefore, the change in SKOV3ip1 and A2780Cis cell number was measured in the absence of serum with the indicated treatments (Fig. 5). Serum-starved SKOV3ip1 cells increased more than 10-fold in 48 hours indicating that serum is not necessary to promote growth for this period of time. Treatment with natalizumab alone had no effect on cell adhesion (data not shown) or cell growth. Additionally, a dose-response assessment revealed that 0.1 μg/ml carboplatin had no effect on growth; however, 1.0 μg/ml carboplatin reduced cell growth by 50% (Figs. 5A and 5B). Intriguingly, inhibition of α4β1 integrin shifted the dose response of carboplatin such that 0.1 μg/ml reduced cell growth by 50% demonstrating that natalizumab rendered cells sensitive to an order of magnitude less carboplatin. SKOV3ip1 cells double every 33 hours, which remains unaltered upon treatment with natalizumab or 0.1 μg/ml carboplatin alone (Fig. 5C); natalizumab plus 0.1 μg/ml carboplatin increased the doubling time to 64 hours (Fig. 5C). The change in doubling time was not due to cell cycle arrest as we observed no difference in cell cycle progression determined by flow cytometry of propidium iodide stained cells with or without BrdU incorporation (data not shown).
Fig. 5. Effects of carboplatin and natalizumab on serum-free ovarian cancer cell growth in vitro.
SKOV3ip1 or A2780Cis cells were cultured in serum-free media in the presence of control IgG or natalizumab (Nat.) with increasing concentrations of carboplatin as indicated. After 2 days, cells were counted following trypan blue staining. (A and B) The number of viable cells was determined. (B) Cell counts were normalized to IgG, no carboplatin control. (C) Doubling time was calculated using viable cell counts obtained 24 and 48 hours after treatment as indicated. (D) The percentage of dead cells was determined 48 hours after treatment following trypan blue staining. Data represent mean ± std. dev. of 3 independent experiments. * p < 0.05 determined by 2-way ANOVA followed by Bonferroni multiple comparisons test.
To determine whether the combination treatment induced cell death, cells were stained with trypan blue and counted. Enumeration of the dead cells demonstrated that 1 μg/ml carboplatin resulted in approximately 27% cell death regardless of the presence of α4β1 integrin function-blocking antibodies, while 0.1 μg/ml carboplatin alone had no effect (Fig. 5D). Natalizumab alone also had no effect on cell death; however, when combined with 0.1 μg/ml carboplatin, it promoted death in 25% of the population (Fig. 5D). Additional studies demonstrated no change in caspase cleavage, PARP cleavage or nucleosome release following natalizumab and 0.1 μg/ml carboplatin treatment (data not shown) indicating that apoptosis might not be the mechanism of cell death. Together, these observations indicate that functional blockade of α4β1 integrin directly increases ovarian cancer cell death in response to carboplatin treatment and provide pre-clinical evidence to support targeting α4β1 integrin in the treatment of platinum-resistant ovarian cancer.
DISCUSSION
The data presented here support a role for α4β1 integrin in the regulation of ovarian cancer cell response to platinum-based chemotherapy. Function-blocking antibodies directed against α4β1 integrin or its ligand, VCAM-1 sensitized advanced peritoneal disease to carboplatin in two different platinum-resistant mouse models. This effect was due to directly targeting the tumor cell expression of α4β1 integrin because inhibiting leukocyte infiltration had no effect on tumor growth and treatment of cultured ovarian cancer cells with α4β1 integrin blocking antibodies increased sensitivity to carboplatin by an order of magnitude. These observations provide evidence supporting the evaluation of α4β1 integrin as a therapeutic target to enhance treatment response to platinum agents in ovarian cancer.
Integrins regulate many aspects of tumorigenesis and progression including cell proliferation, survival, migration and invasion, and their differential expression in cancer has been exploited to generate targeted inhibitors [5]. Unlike many tumors, we found that α4 integrin expression is not altered upon transformation; it is expressed on tumor cells as well as the ovarian surface epithelium and fallopian tube epithelium. Furthermore, the level of expression does not change with increasing platinum resistance. However, expression of its counter receptor, VCAM-1 is induced on the mesothelium of ovarian cancer patients with peritoneal metastasis [2–4], thus providing support to target this tumor-specific interaction for the treatment of metastatic ovarian cancer.
Inhibition of α4β1 integrin rendered peritoneal ovarian tumors sensitive to carboplatin. Interestingly, the effect of inhibiting VCAM-1 was not as dramatic resulting in decreased tumor weight but no change in the number of tumor nodules. One possibility is that natalizumab has a higher affinity for its target than the anti-VCAM-1 blocking antibody employed in this study. Alternatively, α4β1 integrin has additional ligands, including fibronectin, which is expressed in the tumor microenvironment [19]. Natalizumab would inhibit the interaction of α4β1 integrin with all ligands while functional blockade of VCAM-1 would only target one.
The mechanism(s) by which inhibition of α4β1 integrin cooperates with carboplatin to inhibit ovarian cancer growth are unclear. Our results reveal an increased doubling time of the population and increased cell death. While integrins have been implicated in the regulation of cell proliferation and cell cycle progression, we were unable to detect alterations in cell cycle progression following treatment (data not shown). Inhibition of α4β1 integrin did not affect cell adhesion, which promotes cell survival. Ovarian cancer cells express many different integrin heterodimers that could mediate adhesion in vitro (data not shown, [20–22]). Therefore, it is not unexpected that inhibiting one would impact adhesion. Additionally, the contribution of adhesion to ovarian cancer cell survival is questionable as tumor cells often grow in suspension in ascites [23]. Comprehensive analysis of apoptosis failed to identify it as the mechanism of cell death raising the possibility that alternative mechanisms, including necrosis are involved.
The ability of α4β1 integrin blockade to enhance the treatment response to carboplatin in resistant ovarian cancer cells raises the intriguing possibility of using it to treat this subset of patients. Since natalizumab is approved for the treatment of multiple sclerosis, Crohn’s disease and inflammatory bowel disease, it is possible that it could transition more readily for use in ovarian cancer. Unfortunately, natalizumab is associated with the development of a rare, life-threatening virus-induced progressive multifocal leukoencephalopathy (PML) that requires strict monitoring of patients receiving treatment [24], which has resulted in caution with regard to its use in oncology particularly with immune compromising chemotherapies [25]. However, PML is reversible upon withdrawl of natalizumab raising the possibility that ovarian cancer patients could be treated with natalizumab and platinum agents for limited periods of time under careful supervision. Moreover, small molecule, non-peptide inhibitors of α4β1 integrin have been tested for the treatment of asthma, ulcerative colitis and multiple sclerosis [26]. In particular, firategrast, an oral, short-acting α4β1 integrin inhibitor, showed efficacy in a randomized, double-blind, placebo-controlled Phase II trial for multiple sclerosis with no indication of PML or reactivation of its causative agent (JC virus) [27]. Together with the findings reported here, this provides optimism for the use of α4β1 integrin targeted agents in the treatment of advanced ovarian cancer and warrants additional investigation.
Supplementary Material
Highlights.
α4β1 integrin is expressed on patient-derived ovarian cancer cells as well as fallopian tube epithelium and ovarian surface epithelium.
Inhibition of α4β1 integrin increased the response of platinum-resistant ovarian tumors to carboplatin in a mouse model of peritoneal disease.
Carboplatin together with α4β1 integrin blockade increased cell death and doubling time of ovarian cancer cells in vitro.
Acknowledgments
This work was supported by grants from the University of Virginia Women’s Oncology Research Fund and Cancer Center and NCI R01 CA142783 to J.K.S-D. J.M.S. was supported by Gynecologic Oncology Fellowship program. We thank the members of the University of Virginia Women’s Oncology Group for their insight and comments during the course of this work, and Dr. Rodney Rocconi for critical review of the manuscript.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell DA, Longacre TA, Prat J, Kohn EC, Soslow RA, Ellenson LH, Malpica A, Stoler MH, Kurman RJ. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Human pathology. 2004;35:934–948. doi: 10.1016/j.humpath.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer research. 2009;69:1469–1476. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Zhang J, Li H, Lu Z, Shan W, Mercado-Uribe I, Liu J. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. American journal of translational research. 2013;5:336–346. [PMC free article] [PubMed] [Google Scholar]
- 4.Scalici JM, Thomas S, Harrer C, Raines TA, Curran J, Atkins KA, Conaway MR, Duska L, Kelly KA, Slack-Davis JK. Imaging vcam-1 as an indicator of treatment efficacy in metastatic ovarian cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1883–1889. doi: 10.2967/jnumed.112.117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman SL, Picard M. Integrins as therapeutic targets. Trends in pharmacological sciences. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Damiano JS, Dalton WS. Integrin-mediated drug resistance in multiple myeloma. Leukemia & lymphoma. 2000;38:71–81. doi: 10.3109/10428190009060320. [DOI] [PubMed] [Google Scholar]
- 7.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, Mundy GR, Yoneda T. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 8.Olson DL, Burkly LC, Leone DR, Dolinski BM, Lobb RR. Anti-alpha4 integrin monoclonal antibody inhibits multiple myeloma growth in a murine model. Molecular cancer therapeutics. 2005;4:91–99. [PubMed] [Google Scholar]
- 9.Schmidmaier R, Morsdorf K, Baumann P, Emmerich B, Meinhardt G. Evidence for cell adhesion-mediated drug resistance of multiple myeloma cells in vivo. The International journal of biological markers. 2006;21:218–222. doi: 10.1177/172460080602100404. [DOI] [PubMed] [Google Scholar]
- 10.Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. The Journal of cell biology. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffer SG, Hemler ME, Lobb RR, Tizard R, Osborn L. Molecular mapping of functional antibody binding sites of alpha 4 integrin. The Journal of biological chemistry. 1995;270:14270–14273. doi: 10.1074/jbc.270.24.14270. [DOI] [PubMed] [Google Scholar]
- 12.Leger OJ, Yednock TA, Tanner L, Horner HC, Hines DK, Keen S, Saldanha J, Jones ST, Fritz LC, Bendig MM. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Human antibodies. 1997;8:3–16. [PubMed] [Google Scholar]
- 13.Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH, Ferriss JS, Dutta A. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13:899–911. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannistra SA, Ottensmeier C, Tidy J, DeFranzo B. Vascular cell adhesion molecule-1 expressed by peritoneal mesothelium partly mediates the binding of activated human T lymphocytes. Experimental hematology. 1994;22:996–1002. [PubMed] [Google Scholar]
- 15.Jonjic N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. The Journal of experimental medicine. 1992;176:1165–1174. doi: 10.1084/jem.176.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Frontiers in immunology. 2011;2:29. doi: 10.3389/fimmu.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer research. 2007;67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 18.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer research. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 19.Witz CA, Montoya-Rodriguez IA, Cho S, Centonze VE, Bonewald LF, Schenken RS. Composition of the extracellular matrix of the peritoneum. Journal of the Society for Gynecologic Investigation. 2001;8:299–304. doi: 10.1016/s1071-5576(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 20.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecologic oncology. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 21.Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. The American journal of pathology. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecologic oncology. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 23.Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecologic oncology. 2009;113:143–148. doi: 10.1016/j.ygyno.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Warnke C, Menge T, Hartung HP, Racke MK, Cravens PD, Bennett JL, Frohman EM, Greenberg BM, Zamvil SS, Gold R, et al. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors and can it be avoided? Archives of neurology. 2010;67:923–930. doi: 10.1001/archneurol.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Massague J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5520–5525. doi: 10.1158/1078-0432.CCR-11-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nature reviews Drug discovery. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 27.Miller DH, Weber T, Grove R, Wardell C, Horrigan J, Graff O, Atkinson G, Dua P, Yousry T, Macmanus D, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet neurology. 2012;11:131–139. doi: 10.1016/S1474-4422(11)70299-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.