Abstract
Chemical potentials or standard chemical potentials of bound ligands cannot be used to follow the step-by-step transfer of free energy from one ligand to another in a free energy transducing cycle. The basic difficulty is that, in most states of the cycle, separate ligand free energies are not even defined because, when ligands are bound on the enzyme, the interaction free energy of the complex cannot simply be assigned to ligands nor in general even be divided between two ligands if both are bound. This is a mutual, indivisible free energy among enzyme and ligands. Separate ligand free energies are well defined only at the complete cycle level, where the enzyme drops out of consideration (returns to its original state). Other types of free energy are also considered in order to discuss recent work of Tanford. In principle, the kinetics and mechanism can be followed in molecular or atomic detail through the steps of a transduction cycle, but the transfer of free energy from one ligand to another cannot be so followed.
Full text
PDF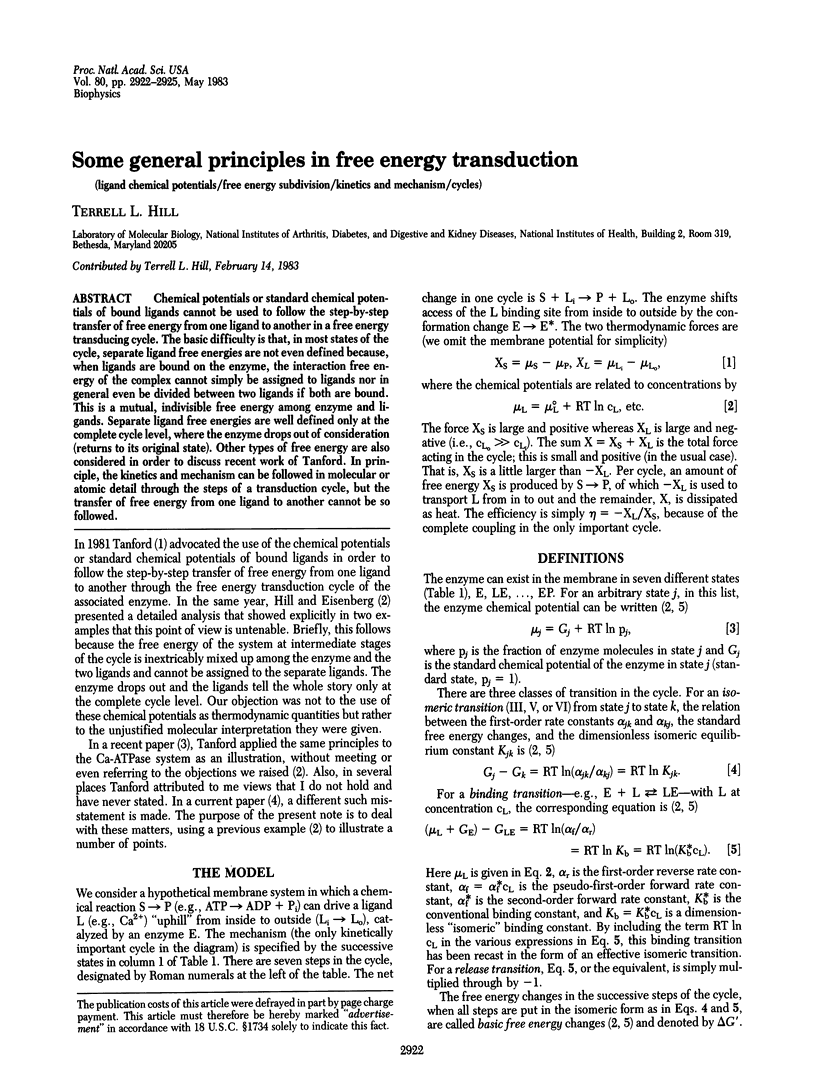



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill T. L. Derivation of the relation between the linear Onsager coefficients and the equilibrium one-way cycle fluxes of a biochemical kinetic diagram. Proc Natl Acad Sci U S A. 1983 May;80(9):2589–2590. doi: 10.1073/pnas.80.9.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E. Can free energy transduction be localized at some crucial part of the enzymatic cycle? Q Rev Biophys. 1981 Nov;14(4):463–511. doi: 10.1017/s0033583500002468. [DOI] [PubMed] [Google Scholar]
- Tanford C. Chemical potential of bound ligand, an important parameter for free energy transduction. Proc Natl Acad Sci U S A. 1981 Jan;78(1):270–273. doi: 10.1073/pnas.78.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Mechanism of active transport: free energy dissipation and free energy transduction. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6527–6531. doi: 10.1073/pnas.79.21.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]


