Abstract
The NR4A is a subfamily of the orphan nuclear receptors (NR) superfamily constituted by three well characterized members: Nur77 (NR4A1), Nurr1 (NR4A2) and Nor 1 (NR4A3). They are implicated in numerous biological processes as DNA repair, arteriosclerosis, cell apoptosis, carcinogenesis and metabolism. Several studies have demonstrated the role of this subfamily on glucose metabolism, insulin sensitivity and energy balance. These studies have focused mainly in liver and skeletal muscle. However, its potential role in white adipose tissue (WAT), one of the most important tissues involved in the regulation of energy homeostasis, is not well-studied. The aim of this work was to elucidate the regulation of NR4A in WAT under different physiological and pathophysiological settings involved in energy balance such as fasting, postnatal development, gender, hormonal deficiency and pregnancy. We compared NR4A mRNA expression of Nur77, Nurr1 and Nor 1 and found a clear regulation by nutritional status, since the expression of the 3 isoforms is increased after fasting in a leptin-independent manner and sex steroid hormones also modulate NR4A expression in males and females. Our findings indicate that NR4A are regulated by different physiological and pathophysiological settings known to be associated with marked alterations in glucose metabolism and energy status.
In addition to its role as an energy reservoir tissue1, the WAT exerts an impact on whole-body metabolism as an active endocrine organ capable of synthesizing a number of biologically active compounds (adipokines) regulating metabolic homeostasis. WAT is implicated in the development of several diseases as type II diabetes and cardiovascular failure2. Furthermore WAT is also essential for the postnatal development, puberty and fertility3.
The NR4A is a subfamily of orphan nuclear receptors (NR) superfamily that in mammals consists in three homologous isotypes: Nur77 (NR4A1), Nurr1 (NR4A2) and Nor 1 (NR4A3). NR4A receptors are transcription factors (contrary to classical ligand active nuclear receptor) whose activity is controlled primarily at the level of protein expression and/or post-translational modification. The three proteins bind to a specific site called NGFI-B response element (NRBE: AAAGGTCA) as monomers4,5. These NRs have aminoacid residue homology of 97% in the DNA-binding domains, 20–30% in the N-terminal transactivation domains and 60–65% in the C-terminal ligand-binding domain6
NR4A receptors are expressed as early response genes in response to physiological and pathological stimuli including fatty acids, stress, prostaglandins, growth factors, calcium and inflammatory cytokines, peptide hormones and neurotransmitters7, and are implicated in important biological processes including carcinogenesis/apoptosis control, inflammation, vascular disease, dopaminergic neuron development and metabolism8.
NR4A are widely expressed in different key metabolic demanding tissues, including skeletal muscle, liver, WAT and heart9. Most of the studies have focused mainly in the action of NR4As in glucose metabolism in liver and muscle. The activity of NR4A receptors as transcriptional regulators of glucose metabolism in liver activates gluconeogenic genes10, enhancing hepatic glucose production and elevating plasma blood glucose. Otherwise Tontonoźs laboratory found that NR4A expression is induced by cAMP axis10 in response to glucagon and fasting. In skeletal muscle NR4A is a transcriptional regulator of glycolysis, regulate the expression of genes related with glucose utilization11, and are implicated in oxidative metabolism and mitochondrial activity12,13.
According with these findings obtained in rodents several human studies have linked obesity and diabetes with NR4A receptors14,15,16 increasing the interest for this nuclear receptors as an attractive novel therapeutic potential target.
Although some reports have shown that NR4A is expressed in WAT and regulates glucose metabolism, almost all of them were performed in vitro. In 3T3-L1 cells, NR4As are induced by insulin14 and their relation with adipogenesis is controversial, since some groups did not find any implication of NR4As in adipogenesis17 whereas others demonstrated an inhibition of adipogenesis by NR4As18. In vivo data of NR4As on WAT are limited to only one report indicating that Nur77 modulates some of the actions of an α-MSH (α-melanocyte-stimulating hormone) analog19, suggesting an important endogenous role for NR4As in WAT metabolism. In this regard, previous reports have shown that mice lacking Nur77 are more sensitive to diet-induced obesity, as these mice gain more fat than the control mice11,20
In spite of these evidences suggesting an important role of NR4As on adipocytes, the physiological of these receptors in WAT remains largely unknown.
Therefore, the aim of the present study was to investigate the regulation of NR4A expression in rat WAT in different physiological conditions in relation with nutritional status, age and gender, pregnancy and hormone deficiency.
Results
Influence of food deprivation, leptin and food composition on Nur4r expression
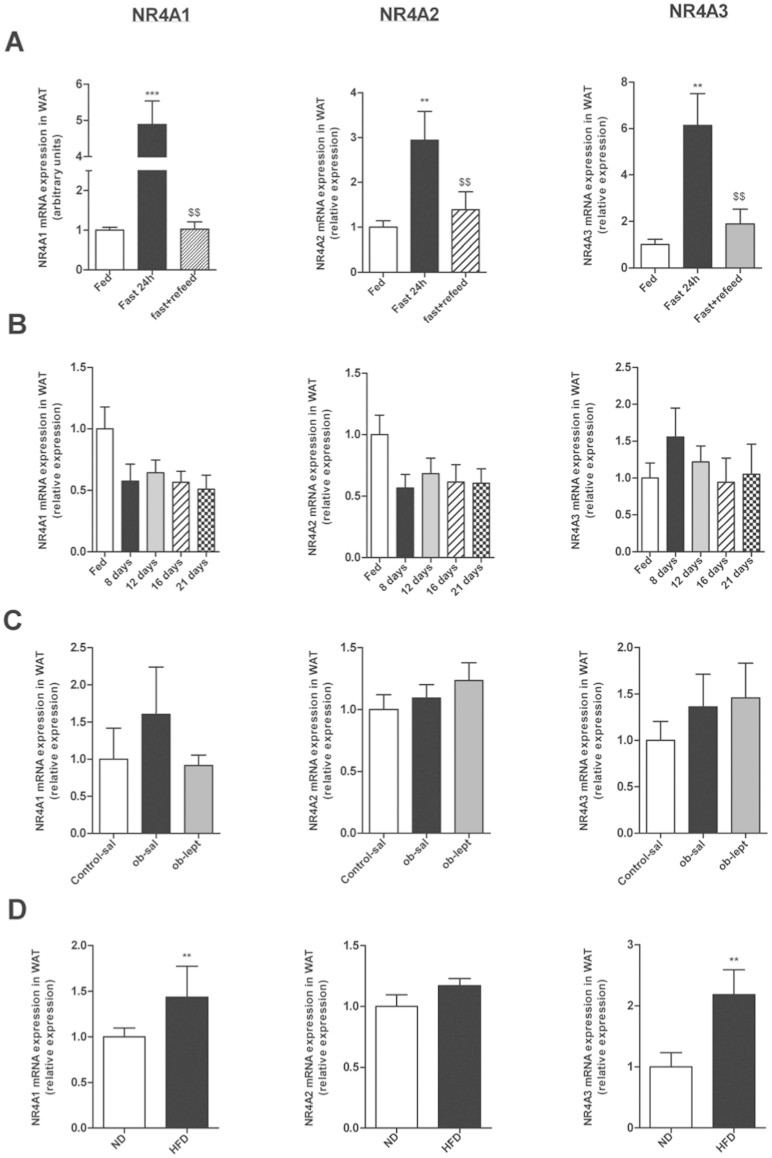
First we sought to investigate the effect of food status on NR4As (Nur77, Nurr1 and Nor-1) mRNA expression in WAT. We started analyzing the effect of fasting and we found that after 24 h of fasting there was an increase on mRNA expression for the 3 studied isotypes of NR4A (Fig 1A). This effect was reverted when the rats were refed, producing a decrease of mRNA levels until basal levels similar to those observed in fed ad libitum rats. The leptin level in this group is represented in Figure 2C.
Figure 1. Nutritional status and leptin exert an effect on NR4A1, NR4A2 and NR4A3 mRNA expression in gWAT.
(A). Effect of fed ad libitum, 24h-fasting, and 24 h-refeding on gWAT mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (Fed). (B). Effect of chronic food restriction (70%) on gWAT mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (Fed). (C). Effect of leptin in ob/ob mice on gWAT mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (saline). (D). Effect of high fat diet (HFD) on mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (Standard diet). Expression of indicated mRNAs was normalized to that of HPRT and the resultant value for each group was normalized to expression of the target gene in control. Data are expressed as mean ± SEM (n = 7–8 animals per group). *p < 0.05; **p < 0.01; ***p < 0,001 vs controls. $$ p < 0,01 vs 24 h-fasting group.
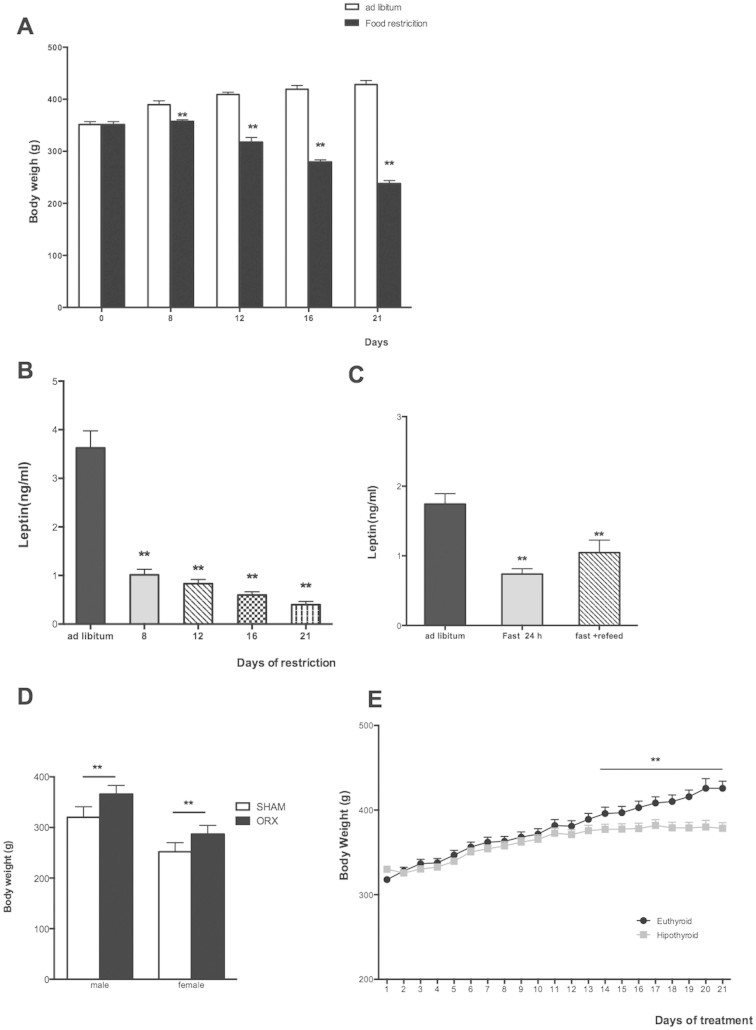
Figure 2. Effects of caloric restriction, fasting, ORX and thyroid status in body weight and leptin levels.
(A) Body weight (g) along all the experiment of rats feed ad libitum or with 8, 12,16 or 21 days of chronic food restriction (70%). (B) Plasma leptin levels (ng/ml) along all the experiment of rats feed ad libitum or with 8, 12,16 or 21 days of chronic food restriction (70%). (C) Plasma leptin levels (ng/ml) in rats feed ad libitum, 24 h-fasting, and 24 h-refeding. (D) Body weight (g) in control (Sham) and two weeks after orchidectomy (ORX) in males and females rats. (E) Body weight (g) along 21 days of AMT treatment (hypothyroid) and control rats (euthyroid).
The next step was to investigate the effect of chronic food restriction (70% of normal intake) along 8, 12, 16 and 21 days. However, we could not find any significant difference in the NR4As mRNA expression (Fig. 1B).
After this, and with the reduction of body weight and leptin levels in the caloric restriction group (Fig. 2A, B) we questioned if the effect of fasting could be mediated by leptin. For this purpose we used a mouse model deficient for leptin (Ob). We compared control mice, ob/ob mice (leptin deficiency) and ob/ob mice treated with leptin and we failed to observe any difference in the mRNA expression of any of the isophorms (Fig. 1C).
Finally we analyzed if the diet composition could affect NR4As expression. We fed rats with standard diet (ND) and with high fat diet (HFD) during 12 weeks. The HFD produced an increase of mRNA expression for Nur77 and Nor-1 in WAT, whereas we did not find any effect on Nurr1.
Influence of age and gender on NR4A mRNA expression
The WAT has very important implications in postnatal development and puberty, thereby we analyzed WAT NR4A expression at different stages of development in males and females.
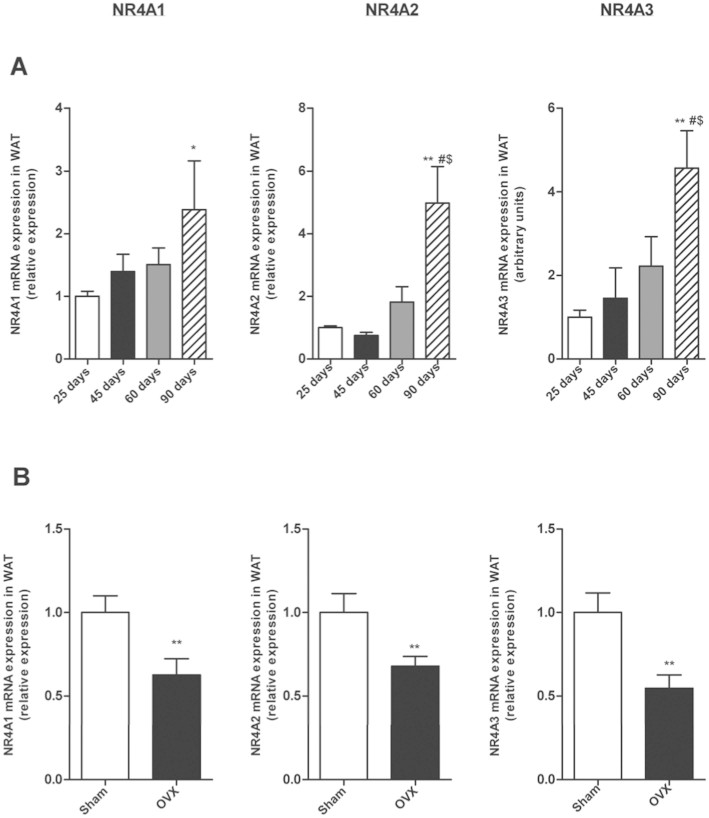
First we measured mRNA expression at 25, 45, 60 and 90 days postnatal development in female rats. The expression of mRNA for Nur77, Nurr1 and Nor-1 increased along all the development with the highest levels for the 3 isophorms at 90 days postnatal development (Fig. 3A). Since at 90 day-old rats are adult and all the gonadal axis is completely developed, we investigated if sexual hormones could be implicated in the increase of NR4As mRNA levels. For this purpose, we used ovariectomized female rats (Fig. 2D) and found decreased WAT NR4As mRNA expression compared with control females (sham-operated controls). This data suggests that estrogens are implicated in the up-regulation of NR4As in the WAT during the postnatal development in female rats (Fig. 3B).
Figure 3. Postnatal development, age and sex steroids affect NR4A1, NR4A2 and NR4A3 mRNA expression in gWAT of female rats.
(A). mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (25 days-old rats) during postnatal development in female rats. (B) mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (Sham) two weeks after ovariectomy (OVX). Expression of indicated mRNAs was normalized to that of HPRT and the resultant value for each group was normalized to expression of the target gene in control. Data are expressed as mean ± SEM (n = 7–8 animals per group). *p < 0.05; **p < 0.01 vs controls. # p< 0,05 vs 45 days-old rats. $ p < 0,05 vs 60 days-old rats.
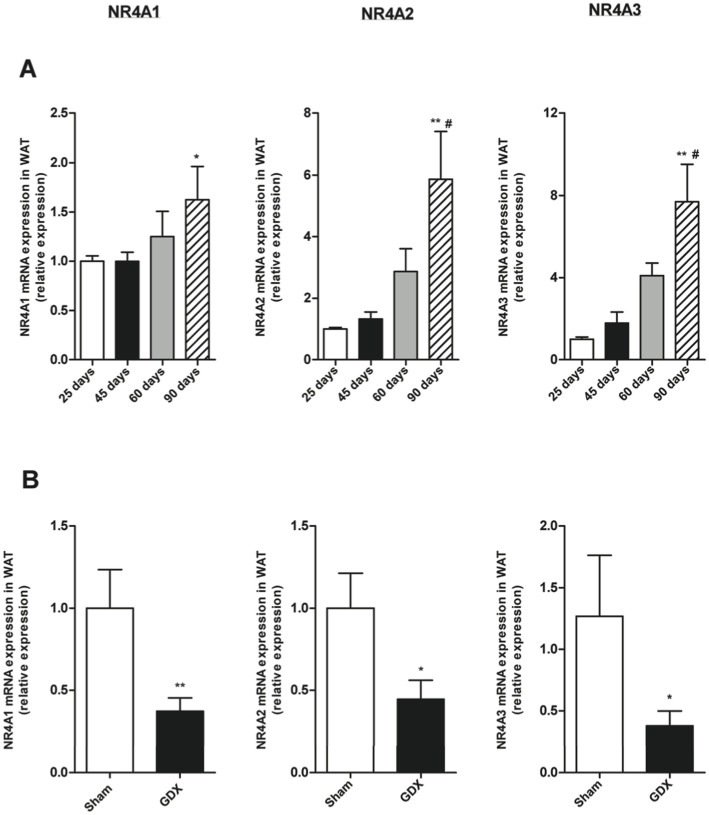
We next analyzed NR4As mRNA expression at 25, 45, 60 and 90 days of age in male rats and found that similarly to females, the mRNA expression for the 3 receptors in WAT increased throughout the development in males with highest levels at 90 days (Fig. 4A). In order to study the influence of sexual hormones, in this case testosterone, on NR4As expression, we used gonadectomized males (Fig. 2D) and found that the lack of gonads decreased NR4As mRNA expression in WAT compared with control males (sham-operated controls). This data suggests that testosterone modulates NR4As levels in WAT (Fig. 4B).
Figure 4. Effects of age and sex steroids on NR4A1, NR4A2 and NR4A3 mRNA expression in gWAT of male rats.
(A) mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (25 days-old rats) during postnatal development in male rats. (B) mRNA expression of NR4A1, NR4A2 and NR4A3 relative to control (Sham) two weeks after gonadectomy (GDX). Expression of indicated mRNAs was normalized to that of HPRT and the resultant value for each group was normalized to expression of the target gene in control. Data are expressed as mean ± SEM (n = 7–8 animals per group). *p < 0.05; **p < 0.01 versus controls. # p < 0,05 versus 45 days-old rats.
Influence of anterior pituitary hormones
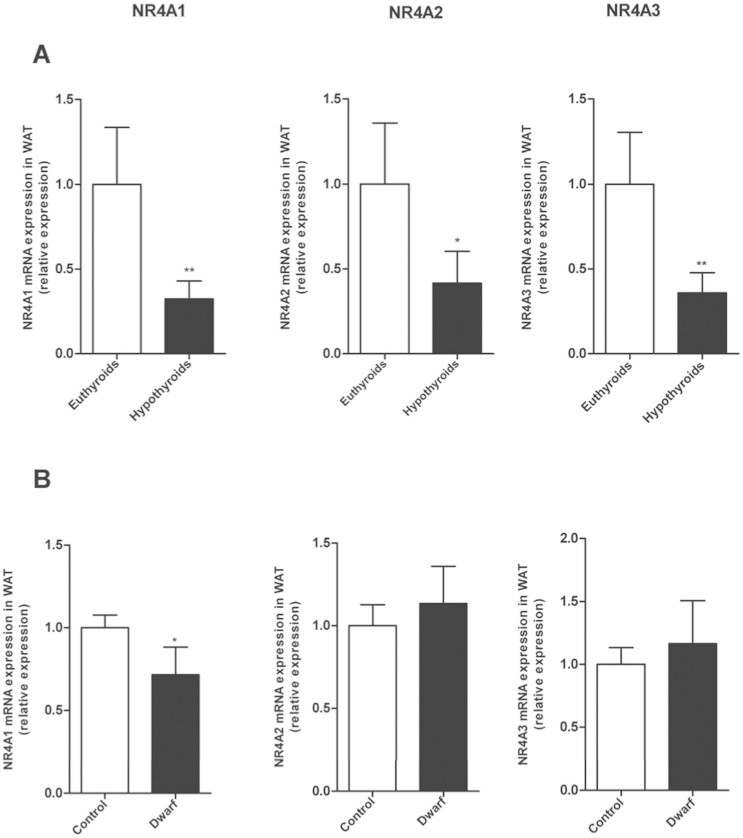
In addition to the assessment of the impact of alterations in the hypothalamus-pituitary-gonadal axis, we determined whether NR4As mRNA expression was dependent of alterations in the pituitary function, as these hormone and/or their targets glands are strongly implicated in the regulation of energy balance. As thyroid hormones play a crucial role in the regulation of metabolism, and hypothyroidism produced an impaired in weigh gain. (Fig. 2E) we analyzed if thyroid hormone deficiency could affect NR4A levels. Hypothyroidism decreased mRNA expression of Nur77, Norr1 and Nor-1 in WAT compared with euthyroids rats (Fig. 5A).
Figure 5. Analysis of pituitary factors on gWAT NR4A1, NR4A2 and NR4A3 mRNA expression.
(A) Effects of thyroid status on gWAT mRNA levels of NR4A1, NR4A2 and NR4A3 relative to control (Euthyroid). (B) Effects of GH deficiency on gWAT mRNA levels of NR4A1, NR4A2 and NR4A3 relative to control (Lewis Expression of indicated mRNAs was normalized to that of HPRT and the resultant value for each group was normalized to expression of the target gene in control. Data are expressed as mean ± SEM (n = 7–8 animals per group). *p < 0.05; **p < 0.01 versus controls.
GH (Growth hormone) plays an important role in the control of metabolic homeostasis such as body composition and glucose and lipid metabolism21. Thus, we studied the effect of GH deficiency (dwarf rats) on WAT NR4As levels and found a down-regulation of Nur77 mRNA expression in GH-deficient rats compared with controls. The other isotypes, Norr1 and Nor-1, did not present any difference in the expression in comparison to their controls (Fig. 5B).
Effects of the pregnancy
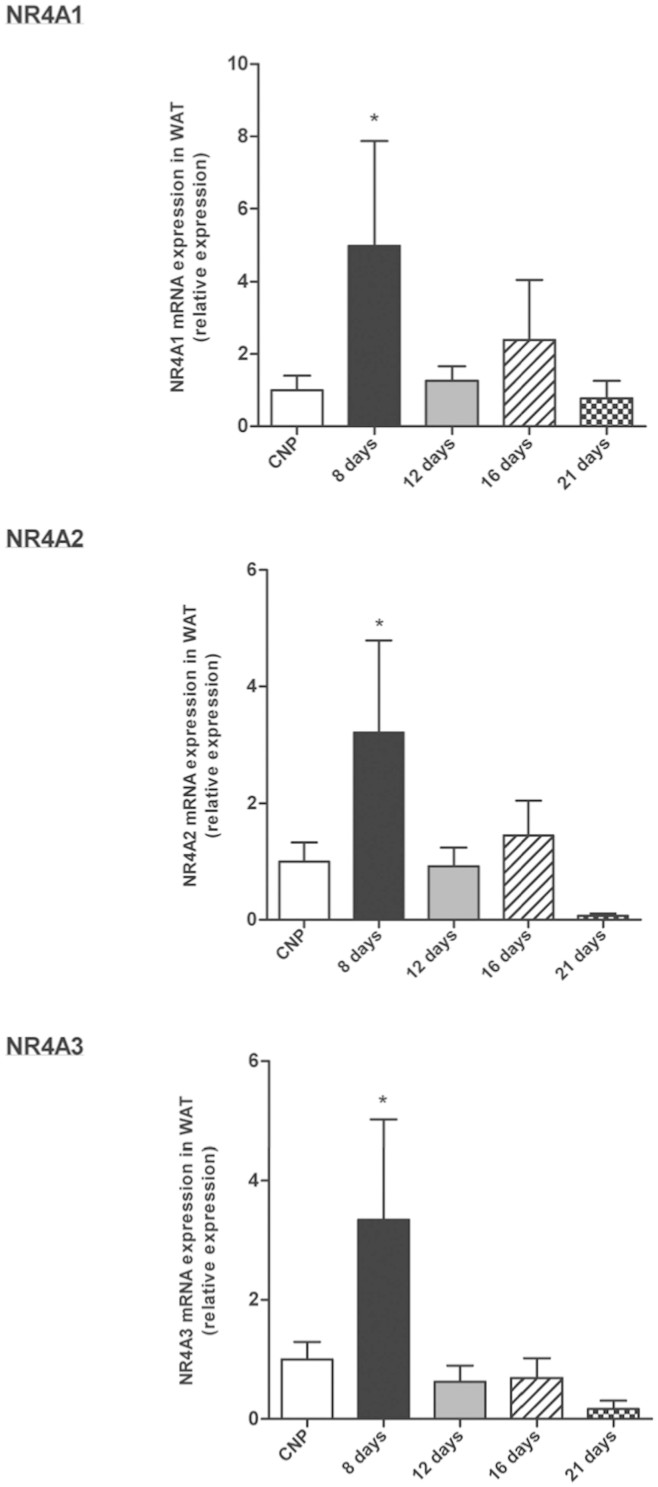
Finally we decided to analyze NR4A mRNA expression levels in a special physiological model associated to important metabolic and hormonal alterations, which promote adipose tissue accretion, as is the pregnancy. We analyzed Nur77, Nurr1 and Nor-1 during different days of gestation (8, 12,16 and 21 days) and we found an increased of Nur77, Nurr1 and Nor-1 mRNA expression for the 3 isophorms in day 8 of gestation, with a further decrease again from day 12 to 21 until control (non-pregnant) (Fig. 6).
Figure 6. Effects of gestation on NR4A1, NR4A2 and NR4A3 mRNA expression in gWAT.
mRNA expression of NR4A1 (A), NR4A2 (B) and NR4A3 (C) relative to control (control non-pregnant) throughout gestation. Expression of indicated mRNAs was normalized to that of HPRT and the resultant value for each group was normalized to expression of the target gene in control. Data are expressed as mean ± SEM (n = 7–8 animals per group). *P < 0.05 versus controls. Data are expressed as mean ± SEM (n = 7–8 animals per group). *p < 0.05; **p < 0.01 versus controls.
Discussion
In the present study, we demonstrated that the members of the NR4A subfamily are regulated in the WAT by different conditions associated with metabolic changes such as nutritional status, sex steroids, pituitary hormones and pregnancy.
Previous reports indicated that NR4As are associated with changes in fat mass in both rodents and humans. For instance, mice lacking Nur77 gain more fat when fed a HFD11,20 and Nur77 is a mediator of fasting-induced Pparγ2 regulation in WAT22. The siRNA injection of Nor-1 in hypothalamus of mice produced a suppression of food intake and body weight23. In morbid obese patients the expression of NR4As in the WAT was upregulated when compared to lean subjects whereas their expression was normalized after fat loss15. Thereby, we first analyzed the influence of nutritional status (fasting and chronic food restriction) on NR4As expression. In agreement with a previous report22 and with data obtained in muscle and liver24, we found that fasting produced an up-regulation of Nur77 in WAT, and this effect was reversed when rats returned to the fed status. The present works demonstrates that these changes were not specific to Nur77, because Nurr1 and Nor-1 showed an identical profile in the WAT of fasted and refed rats. Contrary to the findings obtained after fasting, we failed to detect any significant change in the expression of NR4As in the WAT of rats challenged to a chronic food restriction. Thus, our data indicate that NR4As might play an important role in the metabolic adaptations of the WAT to nutritional status at short- but not long-term. Furthermore, our results indicate that chronic food restriction modulates NR4A receptors in a tissue-specific manner since it was previously demonstrated that NR4As expression was increased in rat skeletal muscle and liver following food restriction24.
As one of the most important changes in the fasting state is the decrease of leptin levels, we evaluated whether leptin was mediating fasting-induced NR4As expression. To test this hypothesis, we analyzed NR4As expression in leptin deficient (ob/ob) mice treated with saline or leptin. Although leptin decreased food intake and body weight (data not shown) we did not find any difference in the expression of NR4A receptors between control mice and ob/ob mice with or without leptin. Overall, our results indicate that the effect of fasting on NR4As expression is independent of leptin. Indeed, fasting is associated to changes of multiple hormones and factors other than leptin, so further studies will be necessary the precise mechanism controlling the expression of NR4S in the WAT after fasting.
Diet-induced obesity (DIO) in rodents represents a robust experimental model of human obesity. Since previous studies demonstrated that the lack of Nur77 made animals more susceptible to gain fat when fed a HFD11,20, we next measured NR4As expression in the WAT of DIO rats. Obese mice exhibit a reduced β-adrenergic response25 and it was demonstrated that β-adrenergic signaling induces the expression of mRNAs of NR4As in WAT26. Unexpectedly, we found that WAT Nur77 and Nor-1 levels were increased in DIO rats in comparison to lean rats. Although the explanation for these results is still not clear, it might be possible that the increased WAT NR4As levels after three months on HFD represents a compensatory response. Although the regulation of these receptors in the WAT of obese rodents seems to be quite complex because WAT NR4As were up-regulated in DIO rats while NR4As expression remained unchanged in the WAT of obese ob/ob mice, it is worthy to note that ob/ob mice exhibit severe endocrine alterations, including hypogonadism, GH deficiency and altered thyroid hormone levels27, which could differentially modulate NR4As expression in WAT.
During postnatal development there is a significant accretion of fat mass in both males and females, which is accompanied by important changes in the levels of different hormones regulating energy balance and also insulin resistance28,29. When we measured the expression of NR4As in WAT of males and females throughout their lifespan, we found that the mRNA levels of Nur77, Nurr1 and Nor-1 were increased along development, with the highest levels at 90 days postnatal in both males and females. These results indicate that the expression of these receptors in WAT is not affected by gender, but the age has an important influence on WAT NR4A levels. In this sense, it is well known that estrogen deficiency in female animals is associated with hyperphagia, increased body weight and adiposity30 and serum testosterone levels are inversely associated with whole body and fat mass31. To test the hypothesis that increased Nur77, Nurr1 and Nor-1 levels were related to sex steroids, we carried out additional experiments in ovariectomized and orchidectomized rats. In both cases the absence of endogenous production of sex steroids (estrogens or testosterone) produced a decrease of WAT Nur77, Nurr1 and Nor-1 expression, indicating that sex steroids are regulating NR4A expression.
Thyroid hormones and GH are pituitary hormones that play a key role in the regulation of metabolic homeostasis and adipose tissue metabolism32. Thyroids hormones (T3 and T4) have been recognized as critically important for development, tissue differentiation, and maintenance of metabolic balance in mammals33. We found decreased mRNA levels for Nur77, Nurr1 and Nor-1 in the WAT of hypothyroid rats, suggesting that thyroid hormones are important modulators of NR4As. Our results also indicate that this regulation is tissue-specific, because a previous report showed that that Nr4a1 was increased in both male and female hypothyroid mice34. GH also influences many metabolic processes, such as body composition and glucose and lipid metabolism21. We only found a downregulation in WAT Nur77 expression in dwarf rats when compared to control rats, whereas the other 2 isoforms remained unchanged. These findings suggest that GH levels differentially modulate these receptors.
Pregnancy is a physiological model characterized by increased food intake and fat mass35 and during this period there are clear changes in several mechanisms related to glucose homeostasis36. Here we found that Nur77, Nurr1 and Nor-1 mRNA levels in WAT were increased in the first week of gestation but decreased thereafter returning to levels observed in non-pregnant rats. Nur77, Nur77 and Nor-1 modulate insulin action and glucose transport in both rodents and humans11,14 and the changes in their WAT expression occur in the second week of gestation, when the sensitivity of the maternal tissues for insulin decreases37. Thus, it is possible that the high NR4A expression in the beginning of the pregnancy and decreased after second week is reflecting the impaired insulin sensitivity during pregnancy and could be mediated, at least partially by NR4A.
In summary, our data demonstrate that: a) NR4A subfamily (Nur77, Nurr1 and Nor-1) are similarly regulated in WAT; b) WAT Nur77, Nurr1 and Nor-1 expression are regulated by nutritional status and food composition in a leptin-independent manner; c) WAT Nur77, Nurr1 and Nor-1 expression is increased throughout postnatal development and this up-regulation is mediated by testosterone and oestrogens; d) WAT NR4A mRNA levels are regulated by changes in the pituitary function, specifically thyroid hormone deficiency decreases all NR4A receptors and GH deficiency decreases only Nur77; e) WAT NR4A mRNA levels are increased in the first week of gestation and decrease after the second week. The results obtained in this report show the regulation of mRNA levels in white adipose tissue in vivo. We were unsuccessful in measuring protein levels for Nr4a gene products, since antibodies failed to detect endogenous levels of the different NR4A isoforms. Because protein levels not always mimic its mRNA levels, further studies analyzing protein expression will be necessary for a better understanding of the molecular mechanisms regulating.
Methods
Animals
Male and female Sprague–Dawley rats, male C57BL6J mice and male ob/ob mice were housed in air-conditioned rooms (22–24°C) under a 12:12 h light/dark cycle and fed standard rat chow and water ad libitum.
After the experiment, rats and mice were sacrificed and all tissues were rapidly explanted and snap frozen on dry ice. Tissues were collected and frozen at −80°C until they were used. The animal procedures were conducted according to the principles approved by the Animal Care Research Bioethics Committee form University of Santiago de Compostela.
Effects of food deprivation on NUR77, NURR1 and NOR-1 expression in gWAT
This study examined the effects of fasting in 10-weeks-old male Sprague-Dawley rats in NR4A family mRNA expression. The control group was fed ad libitum, two groups were deprived of food for 24 h, while another group was fasted for 24 h and refed for 24 h (n = 8/group)38,39. All animals had free access to tap water (n = 8/group).
Effects of ICV leptin administration on NUR77, NURR1 and NOR-1 expression in gWAT
For this study, surgery was performed in wt mice and ob/ob mice. Brain cannula was placed stereotaxically into the lateral ventricle as previously described39,40. A catheter tube was placed, and after 5 days of recovery, mice were injected with 3 μg of leptin and sacrificed at 6 hours.
Effect of chronic food restriction on NUR77, NURR1 and NOR-1 expression in gWAT
This study examined the effects of chronic food restriction on gWAT NR4A family mRNA expression in 10-week-old male rats. Rats were randomly assigned to one of the two dietary groups on day 1 as previously describe41,42: rats were either fed ad libitum or food restricted to 30% of the amount of food consumed by ad libitum fed rats the day before. Rats were sacrificed after 8, 12 and 21 days on the restricted diet (n = 8/group).
Effect of high fat diet on NUR77, NURR1 and NOR-1 expression in gWAT
Eight-week-old male Sprague- Dawley rats were fed standard chow (standard diet) or high fat diet (Research Diets 12451; 45% fat, 4,73 kcal/g, Research Diets, New Brunswick, NJ) during 8 weeks.
Effect of gestation on NUR77, NURR1 and NOR-1 expression in gWAT
The gestational effects were studied on Sprague-Dawley rats according to the method described elsewhere42,43. Female rats were mated on the days of proestrus at approximately 10-weeks-old. The first day of pregnancy were documented by the presence of a vaginal plug with sperm after mating. gWAT was collected from pregnant rats sacrificed on days 12, 16, 19 and 21 of gestation. Non-pregnant rats were used as control.
Influence of age and gender on NUR77, NURR1 and NOR-1 expression
To analyse the effect of age and gender, male and female Sprawe-Dawley rats were studied at the following ages: 25, 45, 60 and 90 day-old as described38,44.
Effect of gonadal hormones on NUR77, NURR1 and NOR-1 expression in gWAT
In order to analyse the influence of gonadal hormones, adult male and female Sprague-Dawley rats were bilaterally orchidectomized/gonadectomized (ORX/GDX), ovarectomized (OVX) or sham operated rats as previously described42,45. Two weeks after surgery the different groups of rats were sacrificed.
Effect of pituitary axis on NUR77, NURR1 and NOR-1 expression
To elucidate the effects of the pituitary axis on NR4A family mRNA and protein levels, we performed the following experiments:
The effect of thyroid status was evaluated as described elsewhere46. Sprague-Dawley rats were rendered hypothyroid by 3 weeks of treatment with 0.1% amino-triazole (3-amino-1, 2, 4-triazole; Sigma-Aldrich, St. Louis, MO, USA) in drinking water; Aminothriazole (AMT) treatment significantly increased plasma thyroid-stimulating hormone47 levels (control rats: 3.17 ± 0.38 ng/mL; amino-triazole–treated rats: 27.17 ± 1.71 ng/mL; p < 0.05), and decreased T3/T4 levels48.
To investigate the effect of growth hormone (GH), spontaneous dwarf rats, a GH-deficient model, and Lewis wild-type age-matched rats were used and validation of this model for IGF measurements as described previously46,48.
Leptin levels measurement
Plasma leptin levels were measured by radioimmunoassay as described previously49,50, using reagents provided in commercial kits (Rat leptin radioimmunoassay, Linco Research Inc., St Charles, MO, USA). The sensitivity was 0.5 ng/ml, the coefficients of intra-assay and of inter-assay variance were 3.3 and 4.8%, respectively.
Quantitative real-time PCR (qRT-PCR) analysis
RNA was extracted using Trizol® reagent (Invitrogen) according to the manufacturer's instructions. For each RT reaction and cDNA synthesis were used 0,5 μg of total RNA.
The total volume to RT reaction was of 30 μl in the subsequent proportions: 10 μl sample, 6 μl buffer (5X first strand buffer; Invitrogen)(250 mM Tris-HCl, pH 8.3, 375 mM KCl, 15 mM MgCl2, 50 nM DDT), 6 μl dNTPs mixture 20 μM(dTTp, dCTp, dGTp & dATp) (Invitrogen), 1,5 μl MgCl2 50 mM (Invitrogen), 0.17 μl primers (Random primers, Invitrogen), 0.25 μl RNAsa (RNAseOUT®, 40 U/μl Invitrogen), 1 μl m-MLV reversotranscriptase (200 U/μl, Invitrogen) and H2O DEPC until. All reactions were carried out using the following cycling parameters: 37°C for 1 hour, 42°C for 15 min and 95°C for 5 min. Negative control reactions, containing all reagents except the sample were used to ensure specificity of the RT reaction.
For the analysis of gene expression we used real-time reverse-transcription polymerase chain reaction (RT-PCR) analyses performed in a fluorescent temperature cycler (TaqMan®; Applied Biosystems; Foster City, CA, USA) following the manufacturer's instructions44,51. Five hundred ng of total RNA were used for each RT reaction. The PCR cycling conditions included an initial denaturation at 50°C for 10 min followed by 40 cycles at 95°C for 15 sec; 60°C for 1 min. The oligonucleotide specific primers and probes used were:
HPRT (FW 5′-3′: AGCCGACCGGTTCTGTCAT; RV: GGTCATAACCTGGTTCATCATCAC; PB: CGACCCTCAGTCCCAGCGTCGTGA),
NR4A1 rat (FW: CTGTTGCTAGAGTCCGCCTTTC; RV: CAGGCCTGAGCAGAAGATGAG; PB: CCTCCGCCTGGCCTACCGATCTAA),
NR4A2 rat (FW: GCTGAAGCCATGCCTTGTG; RV: GCTGTATTCTCCCGAAGAGTGGTA; PB: TCGCCTCAAGGAGCCAGCCCC),
NR4A3 rat, mouse (FW: GAAGGAAGTTGTGCGTACAGATAGTC; RV:CTCGGACAAGGGCGTTCAT; PB: CCACTACAACAGGAGCCCTCGCAGC)
NR4A1 mouse (FW: TCTGCCTTCCTGGAACTCTTCA; RV: CAGGCCTGAGCAGAAGATGAG; PB: CCTCCGCCTGGCCTACCGATCTAA),
NR4A2 mouse (FW: GCTGAAGCCATGCCTTGTG; RV: GCTGTATTCTCCCGAAGAGTGGTA; PB: TCGCCTCAAGGAGCCAGCCCC).
Primer Express 3.0 (Applied Biosystems) program was used to primers design. The probe contains fluorophore FAM (Fluorescein-6) in 5′extreme and TAMRA in 3′.
The primers were support from Eurofins MWG Operon.
For the analysis of the data, the input value of the gene of interest was standardized to the HPRT value for the sample group and was expressed compared with the average value for the control group.
Statistical analysis
Values are plotted as the mean ± SEM for each genotype. Statistical analysis was performed using one-way ANOVA followed by a post hoc multiple comparison test (Bonferronís test). A p value less than 0.05 was considered statistically significant.
Author Contributions
S.T. and R.N. designed the experiment scheme. S.P.-S. performed experiments. S.P.-S., S.T. analyzed data. S.T., R.N. and M.L. wrote the manuscript.
Acknowledgments
This work has been supported by grants from Fondo Investigaciones Sanitarias (ST: PI12/02842), Ministerio de Economia y Competitividad (RN: RYC-2008-02219 and BFU2012-35255; MMM: BFU2010-17116), Xunta de Galicia (ML: 10PXIB208164PR and 2012-CP070; RN: EM 2012/039 and 2012-CP069), Centro de Investigación Biomédica en Red (CIBER) de Fisiopatología de la Obesidad y Nutrición. CIBERobn is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by FEDER funds. The research leading to these results has also received funding from the European Community's Seventh Framework Programme under the following grant: ML and RN: FP7/2007-2013: n° 245009: NeuroFAST.
References
- Ahima R. S. & Flier J. S. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11, 327–32 (2000). [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 580, 2917–21 (2006). [DOI] [PubMed] [Google Scholar]
- Comninos A. N., Jayasena C. N. & Dhillo W. S. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update (2013). [DOI] [PubMed] [Google Scholar]
- Philips A. et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17, 5946–51 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Fahrner T. J., Johnston M. & Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252, 1296–300 (1991). [DOI] [PubMed] [Google Scholar]
- Maruyama K. et al. The NGFI-B subfamily of the nuclear receptor superfamily (review). Int J Oncol 12, 1237–43 (1998). [DOI] [PubMed] [Google Scholar]
- McMorrow J. P. & Murphy E. P. Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans 39, 688–93 (2011). [DOI] [PubMed] [Google Scholar]
- Pearen M. A. & Muscat G. E. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24, 1891–903 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearen M. A. et al. The orphan nuclear receptor, NOR-1, is a target of beta-adrenergic signaling in skeletal muscle. Endocrinology 147, 5217–27 (2006). [DOI] [PubMed] [Google Scholar]
- Pei L. et al. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12, 1048–55 (2006). [DOI] [PubMed] [Google Scholar]
- Chao L. C. et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes 58, 2788–96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L. C. et al. Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization. J Lipid Res 53, 2610–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L. C. et al. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol 21, 2152–63 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Luo L., Luo N., Zhu X. & Garvey W. T. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem 282, 31525–33 (2007). [DOI] [PubMed] [Google Scholar]
- Veum V. L. et al. The nuclear receptors NUR77, NURR1 and NOR1 in obesity and during fat loss. Int J Obes (Lond) 36, 1195–202 (2012). [DOI] [PubMed] [Google Scholar]
- Weyrich P. et al. Common polymorphisms within the NR4A3 locus, encoding the orphan nuclear receptor Nor-1, are associated with enhanced beta-cell function in non-diabetic subjects. BMC Med Genet 10, 77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W. S., Payne V. A., O'Rahilly S. & Rochford J. J. The NR4A family of orphan nuclear receptors are not required for adipogenesis. Int J Obes (Lond) 32, 388–92 (2008). [DOI] [PubMed] [Google Scholar]
- Chao L. C., Bensinger S. J., Villanueva C. J., Wroblewski K. & Tontonoz P. Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol 22, 2596–608 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. C., Myers S. A., Eriksson N. A., Fitzsimmons R. L. & Muscat G. E. Nr4a1 siRNA expression attenuates alpha-MSH regulated gene expression in 3T3-L1 adipocytes. Mol Endocrinol 25, 291–306 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sieira S. et al. Female Nur77-deficient mice show increased susceptibility to diet-induced obesity. PLoS One 8, e53836 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J. & Gerich J. E. Effects of Growth Hormone on Insulin Action in Man: Mechanisms of Insulin Resistance, Impaired Suppression of Glucose Production, and Impaired Stimulation of Glucose Utilization. Diabetes 31, 663–669 (1982). [DOI] [PubMed] [Google Scholar]
- Duszka K. et al. Nr4a1 is required for fasting-induced down-regulation of Ppargamma2 in white adipose tissue. Mol Endocrinol 27, 135–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K. et al. Serotonin 5-HT2C receptor-independent expression of hypothalamic NOR1, a novel modulator of food intake and energy balance, in mice. Biochem Biophys Res Commun 386, 311–5 (2009). [DOI] [PubMed] [Google Scholar]
- Oita R. C., Mazzatti D. J., Lim F. L., Powell J. R. & Merry B. J. Whole-genome microarray analysis identifies up-regulation of Nr4a nuclear receptors in muscle and liver from diet-restricted rats. Mech Ageing Dev 130, 240–7 (2009). [DOI] [PubMed] [Google Scholar]
- Collins S. et al. Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol 8, 518–27 (1994). [DOI] [PubMed] [Google Scholar]
- Myers S. A., Eriksson N., Burow R., Wang S. C. & Muscat G. E. Beta-adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol Cell Endocrinol 309, 101–8 (2009). [DOI] [PubMed] [Google Scholar]
- Sone M. & Osamura R. Y. Leptin and the pituitary. Pituitary 4, 15–23 (2001). [DOI] [PubMed] [Google Scholar]
- Nogueiras R., Gualillo O., Caminos J. E., Casanueva F. F. & Dieguez C. Regulation of resistin by gonadal, thyroid hormone, and nutritional status. Obes Res 11, 408–14 (2003). [DOI] [PubMed] [Google Scholar]
- Chanoine J. P., De Waele K. & Walia P. Ghrelin and the growth hormone secretagogue receptor in growth and development. Int J Obes (Lond) 33 Suppl 1, S48–52 (2009). [DOI] [PubMed] [Google Scholar]
- Clegg D. J., Brown L. M., Woods S. C. & Benoit S. C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55, 978–87 (2006). [DOI] [PubMed] [Google Scholar]
- Marin P. et al. Androgen treatment of abdominally obese men. Obes Res 1, 245–51 (1993). [DOI] [PubMed] [Google Scholar]
- Schaffler A., Scholmerich J. & Buechler C. The role of ‘adipotropins’ and the clinical importance of a potential hypothalamic-pituitary-adipose axis. Nat Clin Pract Endocrinol Metab 2, 374–83 (2006). [DOI] [PubMed] [Google Scholar]
- Boelaert K. & Franklyn J. A. Thyroid hormone in health and disease. J Endocrinol 187, 1–15 (2005). [DOI] [PubMed] [Google Scholar]
- Dong H. et al. Hepatic gene expression changes in hypothyroid juvenile mice: characterization of a novel negative thyroid-responsive element. Endocrinology 148, 3932–40 (2007). [DOI] [PubMed] [Google Scholar]
- Garcia M. D., Casanueva F. F., Dieguez C. & Senaris R. M. Gestational profile of leptin messenger ribonucleic acid (mRNA) content in the placenta and adipose tissue in the rat, and regulation of the mRNA levels of the leptin receptor subtypes in the hypothalamus during pregnancy and lactation. Biol Reprod 62, 698–703 (2000). [DOI] [PubMed] [Google Scholar]
- Armitage J. A., Poston L. & Taylor P. D. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res 36, 73–84 (2008). [DOI] [PubMed] [Google Scholar]
- Tamas G. & Kerenyi Z. Gestational diabetes: current aspects on pathogenesis and treatment. Exp Clin Endocrinol Diabetes 109 Suppl 2, S400–11 (2001). [DOI] [PubMed] [Google Scholar]
- Nogueiras R. et al. Resistin is expressed in different rat tissues and is regulated in a tissue- and gender-specific manner. FEBS Letters 548, 21–27 (2003). [DOI] [PubMed] [Google Scholar]
- Shimada M., Tritos N. A., Lowell B. B., Flier J. S. & Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396, 670–4 (1998). [DOI] [PubMed] [Google Scholar]
- Chaffer C. L. & Morris M. J. The feeding response to melanin-concentrating hormone is attenuated by antagonism of the NPY Y(1)-receptor in the rat. Endocrinology 143, 191–7 (2002). [DOI] [PubMed] [Google Scholar]
- Gualillo O. et al. Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obesity (Silver Spring) 10, 682–687 (2002). [DOI] [PubMed] [Google Scholar]
- Nogueiras R., Gualillo O., Caminos J. E., Casanueva F. F. & Diéguez C. Regulation of Resistin by Gonadal, Thyroid Hormone, and Nutritional Status. Obesity Research 11, 408–414 (2003). [DOI] [PubMed] [Google Scholar]
- Caminos J. et al. Food intake regulating-neuropeptides are expressed and regulated through pregnancy and following food restriction in rat placenta. Reproductive Biology and Endocrinology 6, 14 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. R. et al. Regulation of visceral adipose tissue-derived serine protease inhibitor by nutritional status, metformin, gender and pituitary factors in rat white adipose tissue. J Physiol 587, 3741–50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R. et al. Regulation of Peroxisome Proliferator Activated Receptor-γ in Rat Pituitary. Journal of Neuroendocrinology 17, 292–297 (2005). [DOI] [PubMed] [Google Scholar]
- Caminos J. E., Seoane L. M., Tovar S. A., Casanueva F. F. & Dieguez C. Influence of thyroid status and growth hormone deficiency on ghrelin. European Journal of Endocrinology 147, 159–163 (2002). [DOI] [PubMed] [Google Scholar]
- Andrae J. et al. Analysis of mice lacking the heparin-binding splice isoform of platelet-derived growth factor a. Mol Cell Biol 33, 4030–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Crespo M. et al. The orexigenic effect of orexin-A revisited: dependence of an intact growth hormone axis. Endocrinology 154, 3589–98 (2013). [DOI] [PubMed] [Google Scholar]
- Lopez M. et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 55, 1327–36 (2006). [DOI] [PubMed] [Google Scholar]
- Nogueiras R. et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 53, 2552–8 (2004). [DOI] [PubMed] [Google Scholar]
- Vazquez M. J. et al. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology 149, 4534–43 (2008). [DOI] [PubMed] [Google Scholar]