Abstract
Human enterovirus B106 (EV-B106) is a recently identified member of enterovirus species B. In this study, we report the complete genomic characterization of an EV-B106 strain (148/YN/CHN/12) isolated from an acute flaccid paralysis patient in Yunnan Province, China. The new strain had 79.2–81.3% nucleotide and 89.1–94.8% amino acid similarity in the VP1 region with the other two EV-B106 strains from Bolivia and Pakistan. When compared with other EV serotypes, it had the highest (73.3%) VP1 nucleotide similarity with the EV-B77 prototype strain CF496-99. However, when aligned with all EV-B106 and EV-B77 sequences available from the GenBank database, two major frame shifts were observed in the VP1 coding region, which resulted in substantial (20.5%) VP1 amino acid divergence between the two serotypes. Phylogenetic analysis and similarity plot analysis revealed multiple recombination events in the genome of this strain. This is the first report of the complete genome of EV-B106.
The genus Enterovirus in the family Picornaviridae is a group of nonenveloped positive-sense RNA viruses that cause a wide range of diseases in humans and other mammals. Most human enterovirus (EV) infections are asymptomatic or result in only mild diseases such as the common cold or minor undifferentiated febrile illnesses; yet under certain conditions, EVs also cause serious human diseases such as acute flaccid paralysis (AFP); meningitis; encephalitis; myocarditis; and hand, foot, and mouth disease1,2,3,4.
The EV genome is about 7.5 kb in length. It has a single open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTRs). The 5′ UTR is about 740-nucleotides (nt) long and contains an internal ribosome-binding site, which is essential for translation initiation5,6,7. The 3′ UTR, approximately 100-nt long, forms highly conserved secondary and tertiary structures that are important for initiation of replication8,9,10. The ORF is translated into a polyprotein of 2200 amino acids (aa), which is processed by viral proteases into structural (VP4, VP2, VP3, and VP1) and non-structural (2A, 2B, 2C, 3A, 3B, 3C, and 3D) proteins11. Current EV classification is based on the molecular typing method which suggests strains with <70% VP1 nt similarity are classified as different types and the strains with >75% VP1 nt similarity are classified as members of same type. This method has been shown to correspond with serotype neutralization12,13. For the nt similarities in ‘grey zone’ of 70–75%, Brown et al. suggested that a more stringent value of 88% VP1 amino acid identity is more appropriate for routine typing14.
The genus Enterovirus consists of 12 species, 7 of which (EV-A to D, rhinovirus - A to C) are associated with human infections. EV-B consists of 61 types11,15. Molecular typing of serologically untypable strains has led to the discovery of a large number of new EV types16,17,18,19. Enterovirus B106 (EV-B106) is a newly identified member of EV-B. To date, only two partial sequences of EV-B106, from Bolivia and Pakistan, have been available in the GenBank database20.
Yunnan Province is located in southwest China, with an area of 390,000 square kilometers and a population of 45,966 million (2010 census data). It borders Vietnam, Laos, and Myanmar. In a previous study, we reported the identification and molecular epidemiology of new EV types isolated in Yunnan Province, including EV-A76, EV-B75, EV-B80, EV-B81, EV-B83, EV-B93, and EV-C9621. Here, we report the identification and genomic characterization of an EV-B106 strain (148/YN/CHN/12; hereafter referred to as strain 12148/YN) recovered from one patient with AFP in Yunnan Province, China, in 2012.
Results
Isolation, molecular typing, and VP1 sequence analysis
Strain 12148/YN was isolated on both RD and HEp-2 cells. VP1 region sequencing and BLAST analysis indicated that the type of this strain is EV-B106. The VP1-coding sequence of this strain showed 81.3% nt and 94.8% aa similarity with that of the EV-B106 Pakistan strain PAK_NIH_SP_1202. Only a 303-nt partial VP1 sequence is available for another EV-B106 strain, BOL/03-10665A from Bolivia; nevertheless, homologous comparison based on this region revealed that the Yunnan strain had 79.2% nt and 89.1% aa similarity with BOL/03-10665A. Compared with the VP1 sequences of the prototype strains of other EV-B types, strain 12148/YN had the highest similarity to the EV-B77 prototype strain CF496-99 (AY493062), with 73.3% nt and 79.5% aa identity.
Whole genome analysis
The whole genome length of strain 12148/YN was 7,420 nt. A large ORF (6,570 nt), encoding a potential polyprotein precursor of 2,192 aa, was flanked by 5′ and 3′ UTRs of 742 nt and 99 nt, respectively. The overall base compositions of the genomes were 27.5% A, 25.2% G, 23.5% C, and 23.9% U. Table 1 shows the nucleotide sequence identities of different regions of the genome between strain 12148/YN and the EV-B106 Pakistan strain (only the P1 sequence available) and prototype strains of the species EV-A, EV-B, EV-C, and EV-D. The entire list of prototype strains used in the analysis can be found as Supplementary Table S1. Strain 12148/YN had high similarity in the VP1 coding region with the Pakistan strain, confirming that the two strains belong to the same type (>75% VP1 nt sequence identity within a type). In contrast, it shared 54.9–73.1% nt sequence identity in the VP1 coding region with the other EV-B types, and less than 50% with the other species (EV-A, EV-C, EV-D).
Table 1. Pairwise nucleotide identities of strain 12148/YN with the Pakistan strain (PAK_NIH_SP_1202) and prototype strains of the species EV-A to EV-D.
| Region | PAK NIH SP1202 | EV-B | EV-A | EV-C | EV-D |
|---|---|---|---|---|---|
| 5′UTR | N/A | 79.7–89.1 | 68.8–79.0 | 54.8–69.3 | 63.5–65.1 |
| VP4 | 78.4 | 67.9–83.2 | 55.3–61.2 | 57.8–66.1 | 56.8–60.2 |
| VP2 | 82.4 | 64.7–74.6 | 47.7–52.2 | 54.7–58.7 | 50.9–54.5 |
| VP3 | 79.7 | 61.6–76.8 | 46.9–51.0 | 53.9–58.2 | 47.9–48.8 |
| VP1 | 81.3 | 54.9–73.1 | 39.3–43.3 | 44.3–49.7 | 44.6–45.6 |
| 2A | N/A | 75.5–81.7 | 55.9–61.3 | 54.4–59.1 | 51.6–53.1 |
| 2B | N/A | 74.7–83.5 | 53.5–60.6 | 49.4–57.5 | 58.5–60.9 |
| 2C | N/A | 79.6–84.1 | 60.8–64.3 | 57.5–62.6 | 63.6–64.4 |
| 3A | N/A | 73.4–82.7 | 52.8–59.5 | 54.3–60.6 | 59.5–62.1 |
| 3B | N/A | 69.6–83.3 | 53.0–63.3 | 54.5–72.7 | 65.0–65.2 |
| 3C | N/A | 75.0–84.5 | 56.1–59.5 | 59.3–63.7 | 59.7–63.9 |
| 3D | N/A | 79.2–84.7 | 61.8–64.1 | 64.8–68.9 | 65.8–66.0 |
| 3′UTR | N/A | 77.7–87.1 | 23.0–31.4 | 22.8–45.7 | 31.7–36.4 |
N/A: sequence not available for strain PAK_NIH_SP_1202.
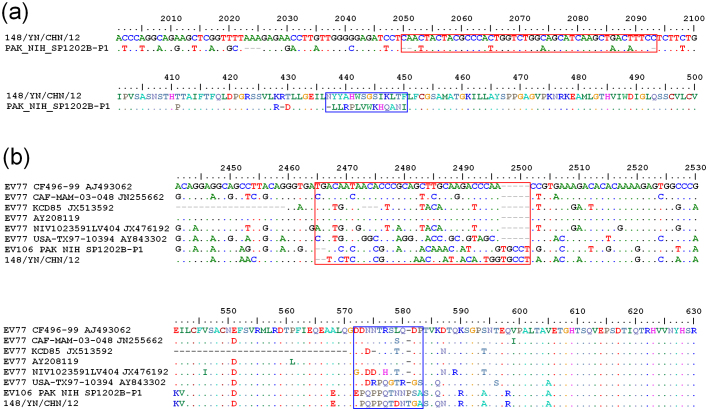
Following alignment with the P1 coding region of the EV-B106 strain PAK_NIH_SP_1202, three insertions were observed: a 3-nt “AAA” insertion at positions 2,023–2,025 (numbering according to the whole genome of strain 12148/YN), a 2-nt “CA” insertion at positions 2,050–2,051, and a 1-nt “C” insertion at position 2,093 (Fig. 1). All the insertions are located in the VP3 coding region. Because EV-B77 has the highest sequence homology with EV-B106, alignment among the EV-B106 strains 12148/YN, PAK_NIH_SP_1202, and all EV-B77 strains available in the GenBank database was conducted, and frame shifts between the two types were found (Fig. 1), including two major frame shifts observed in the VP1 coding region, which result in substantial (up to 20.5%) VP1 aa divergence between the two types.
Figure 1. Nucleotide insertions and deletions and the resulted amino acid changes in the P1 region among EV-B106 strains (a) and with EV-B77 strains (b).
Nucleotide positions are numbered according to strain 148/YN/CHN/12 (a) and EV-B77 prototype strain CF496-99 (b). The red and blue squares indicate the nucleotide frame shifts and associated amino acid changes, respectively.
Phylogenetic and recombination analysis with EV-B prototype strains
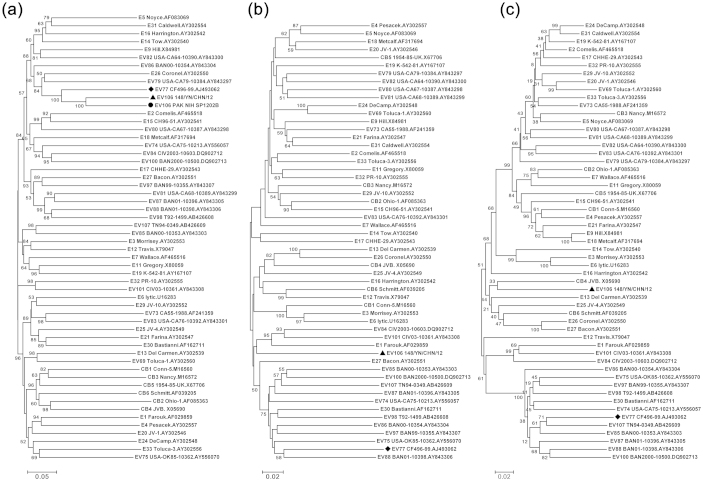
Phylogenetic analysis based on the P1, P2, and P3 coding regions of strain 12148/YN, the Pakistan strain (P1 region sequence only), and all EV-B prototype strains available in the GenBank database was conducted (Fig. 2). As expected, strain 12148/YN formed a lineage with the Pakistan strain in the tree for the P1 coding region, with a bootstrap value of 100% (Fig. 2a), which confirmed the preliminary molecular typing results. Meanwhile, the two EV-B106 strains clustered together with the closely related strain, EV-B77, having a 100% bootstrap value. However, strain 12148/YN did not cluster with any of the other EV types in the trees for the P2 and P3 coding regions (with high bootstrap support) (Fig. 2b and 2c), which reflected that one or more putative recombination events between EV-B106 and other EV-B types might have occurred.
Figure 2. Phylogenetic relationships of the EV-B106 strains and other EV-B prototype strains.
The phylogenetic trees based on nucleotide sequences for P1 (a), P2 (b), and P3 (c) coding regions were constructed from the nucleotide sequence alignment using the neighbor-joining algorithm of the MEGA 4.0 software.
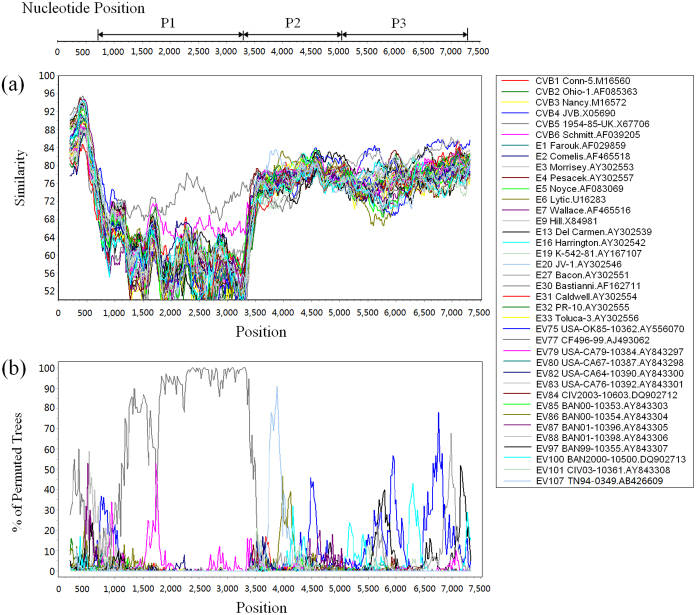
To confirm the existence of recombination events involving strain 12148/YN, the similarity plot and bootscanning analysis were conducted with the other EV-B prototype strains (Fig. 3). The results revealed recombination between strain 12148/YN and the EV-B strains at the P2 and P3 coding regions. One recombination event between 12148/YN and EV-B107 was observed in the 2B region (about nt positions 3,700–3,960).
Figure 3. Similarity plot (a) and bootscanning analysis (b) of Yunnan EV-B106 strain with EV-B prototype strains.
The analysis was conducted via Simplot v3.5.1 using a sliding window of 400 nucleotides moving in steps of 20 nucleotides. The genome of strain 148/YN/CHN/12 serves as a query sequence. One possible recombination event between 12148/YN and EV-B107 is suggested in the 2B region (about nt positions 3700–3960).
Recombination analysis with closely related strains
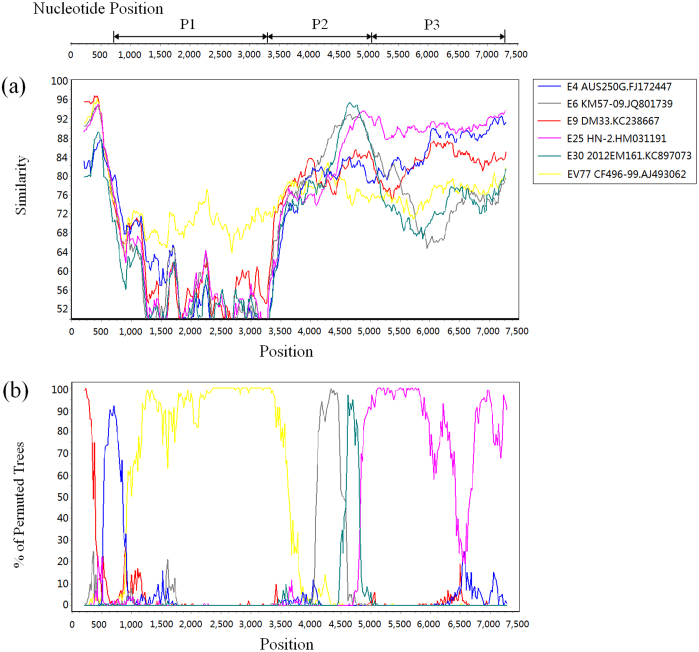
Recombination analysis was also performed for strain 12148/YN with other EV-B strains. Because of the large number of EV-B genome sequences available in the GenBank database, closely related sequences were screened using BLAST online. Since recombination events usually occur in non-structural coding regions22,23, the 5′ UTR, P2, and P3 regions of strain 12148/YN were separately analyzed using BLAST, and the sequences with the highest similarities were used in the recombination analysis. The sequences used in the study were derived from the following: E4 strain AUS250G (FJ172447) from Australia24; the E6 strain KM57/09 (JQ801739) from Yunnan Province, China; the E9 strain DM33 (KC238667) from the Netherlands25; the E25 strain HN-2 (HM031191) from Henan Province, China; and the E30 strain 2012EM161 (KC897073) from Guangdong Province, China. Because the whole genome sequence of the prototype strain of EV-B106 cannot be obtained, the prototype strain of EV-B77 (the closest type in the VP1 region) was included in the analysis. The similarity plot analysis revealed multiple recombination events in the genome sequence of strain 12148/YN, and bootscanning analysis confirmed these recombination events (Fig. 4). In the P1 coding region, 12148/YN had the highest similarity with the EV-B77 prototype strain. In the 5′ UTR region, it had high similarity with E9 strain DM33 and E4 strain AUS250G. In the P2 region, it had the highest similarity with E6 strain KM57/09, followed by E30 strain 2012EM161. In nt positions 4,940–7,280 (the partial P2 and entire P3 coding region), it had the highest similarity with E25 strain HN-2 (HM031191).
Figure 4. Similarity plot (a) and bootscanning analysis (b) of the Yunnan EV-B106 strain with closely related strains.
The analysis was conducted via Simplot v3.5.1 using a sliding window of 400 nucleotides moving in steps of 20 nucleotides. The genome of strain 148/YN/CHN/12 serves as a query sequence. Several possible recombination events are suggested in P2 and P3 coding regions.
Discussion
There is no specific EV surveillance system in place for the mainland of China, although AFP surveillance, established as part of the Global Polio Eradication Initiative, has been conducted since 1994. As non-polio enteroviruses (NPEVs) can be isolated via the AFP surveillance, investigation of these viruses can provide valuable information on the molecular epidemiology of local EVs. Since the introduction of the molecular typing method, a considerable number of new EV types have been identified26,27. In addition, many newer EV types have been reported from retrospective investigations of the NPEVs from AFP surveillance conducted in the provinces of Yunnan and Shandong21,28,29. Here, for the first time, we report the whole genome sequence of a recently identified new type, EV-B106, which was also obtained from AFP surveillance.
Although the EV-B106 strain was isolated from stool samples of a patient with AFP during the course of onset, we could not collect supplementary data to conclude that EV-B106 was the causative agent in this case, because the virus was not isolated from the sites of pathology in the patient. Further surveillance data, such as sero-epidemiology, on this virus in populations in the future might provide valuable information.
Yunnan is a frontier province in China and borders three countries: Vietnam, Laos, and Myanmar. The EV-B106 virus in this study was isolated in Honghe Prefecture, which is a region that borders Vietnam. For some villages near the border, daily communications across the border are very frequent, and surveillance for importation of medical pathogens should be of great concern.
EVs evolve rapidly (around 1–2% per nt site per year), with most of the diversity in the form of nucleotide substitutions, deletions, or insertions30. Frame shifts in the VP3 coding region were found between the Yunnan EV-B106 strain (12148/YN) and the Pakistan EV-B106 strain. Taking the high divergence between the two EV-B106 strains (81.3% nt similarity in VP1 region) into consideration, the divergence and frame shifts between them indicate that the type has been circulating independently for a long time. Because most EV infections are subclinical, it is difficult to isolate the viruses in the initial stage of transmission.
Frame shifts were also found between the EV-B106 and EV-B77 strains. The VP1 nt similarity between EV-B77 and EV-B106 is 73.3%, which is not sufficient to distinguish strain 12148/YN from the existing types. However, two major frame shifts observed in the VP1 region result in 20.5% (Yunnan strain) and 16.5% (Pakistan strain) aa divergence from the EV-B77 prototype strain, which is great enough to separate the Yunnan strain from the EV-B77 strains14, and classify it as a new type, EV-B106. EV-B77 has been reported to be isolated from children with gastroenteritis or AFP in USA, India, Kosovo, and Central African Republic, respectively19,31,32,33,34. The complete VP1 nucleotide sequences of EV-B77 were found to diverge by >29% from all prototypes19. However, the sequence data on EV-B77 was also in scarce in GenBank database, more genomic sequences are needed to further clarify the genetic relationship between EV-B77 and EV-B106.
In the P1 region, continuous accumulation of nt substitutions and natural selection are well-known mechanisms of genome evolution35. The VP1 sequence is correlated with the types and can be used for classification of EVs. Conversely, in the P2 and P3 non-capsid regions, nt and aa sequences are relatively conserved within an EV species36,37, and intra- and inter-serotypic recombination have usually been observed in these regions38,39,40. In our study, the Yunnan EV-B106 strain is no exception―evidence of recombination with other EV-B strains in the P2 and P3 regions was found. Although similarity plot and bootscanning analysis suggested a recombination event between the Yunnan EV-B106 strain and EV-B107 prototype strains in the non-structural region, the percent support values were not high (<85%). Since the recombination frequency of EV is very high and many prototype strains have been isolated several decades ago, it is often difficult (or impossible) to detect exact recombination partners if currently circulating strains are compared to the prototype strains. Therefore, the recombination analysis between EV-B106 strain 12148/YN and more closely related isolates were performed. In another approach, we screened some strains sharing high similarity with Yunnan EV-B106 in the 5′ UTR, P2, and P3 regions by using BLAST, and performed a similar analysis (Fig. 4). Recombination events were observed between strain 12148/YN and the selected strains in the non-structural coding region with relatively higher percent support value (>90%). These selected strains were isolated from Australia, Finland, the Guangdong Province, and the Henan Province in China, and these regions are all geographically remote from the Yunnan Province. Hence, it is reasonable to conclude that long-term transmission of these viruses has taken place, so as to provide the spatial and temporal circumstances for recombination to occur.
In conclusion, this is the first report on the whole genome sequence of a recently described EV type, EV-B106. The extremely rare isolation rate suggests that it has not been a prevalent type in China, or even in the world, and the great genetic divergence among EV-B106 strains indicates that this type is not a newly emergent virus, but has circulated in the world for many years.
Methods
Virus isolation
The EV-B106 strain 12148/YN was isolated from an 18-month-old boy in the Honghe Prefecture of the Yunnan Province of China in July 2012. The patient initially had upper respiratory tract infection, and then developed asymmetric paralysis in his lower limbs. No residual paralysis was observed at the 60-day follow-up.
Stool samples from the child were collected and processed according to standard procedures recommended by the World Health Organization (WHO)41. Human rhabdomyosarcoma (RD) and human laryngeal epidermoid carcinoma (HEp-2) cell lines were used for virus isolation. Both cell lines were gifts from the WHO Global Poliovirus Specialized Laboratory in the USA and were all originally purchased from the American Type Culture Collection. A total of 200 μl of chloroform-treated stool solution was added to each of the cell culture tubes. To ensure no cross contamination had occurred, cell tubes of normal RD and HEp-2 cells served as negative controls. Infected cell cultures were harvested and used for further examination after a complete cytopathic effect had been obtained.
Amplification and sequencing
The viral RNA was extracted from the infected cell culture using a QIAamp Viral RNA Mini Kit (Qiagen, USA). Primer pairs 008-013 and 187-011, previously described by Oberste et al., were used for amplifying the entire VP1 coding region12,13. The reverse transcription–polymerase chain reaction (RT-PCR) was performed with the Access One-step RT-PCR Kit (Promega, USA) according to the manufacturer's instructions. Amplicons were purified and then sequenced using a BigDye Terminator v3.0 Cycle Sequencing Kit, and were analyzed using an ABI 3130 Genetic Analyzer (Applied Biosystems). The VP1 sequences obtained were compared with sequences available in the GenBank database by using the Basic Local Alignment Search Tool (BLAST) from the National Center for Biotechnology Information. Virus isolates showing >75% nucleotide sequence identity with a known EV type were designated as the relative type13. The RT-PCR amplification on the rest of the genome was performed using six pairs of primers. Positive products were purified and sequenced as described above.
Phylogenetic and recombination analysis
Alignment of the whole genome sequences of strain 12148/YN and other EV types was performed using the BioEdit software (version 7.0.5.3)42. The phylogenetic trees were constructed with MEGA 4.043 using the neighbor-joining method with a Kimura two-parameter model. Bootstrapping was performed with 1000 duplicates and bootstrap values greater than 80% were considered statistically significant for grouping. The SimPlot 3.5.1 program was used for similarity plot and bootscanning analysis44,45, with a 400 nt window moving in 20 nt steps and a Jukes–Cantor correction.
Nucleotide sequence accession number
The complete genome sequence of the EV-B106 strain 12148/YN described in this study was deposited in the GenBank database under the accession number KF990476.
Author Contributions
J.T., Z.T., Z.D. and W.X. conceived the study and drafted the paper, J.T., Z.T. and Y.Z. gathered and analyzed the data, and J.Z., B.T., Z.Z. and L.Z. helped to interpret results and contributed to the writing.
Supplementary Material
Supplementary Table S1
Acknowledgments
This study was supported by the National Natural Science Foundation of China (project no. 81160198) (http://www.nsfc.gov.cn).
References
- Khetsuriani N., Lamonte-Fowlkes A., Oberst S. & Pallansch M. A. Centers for Disease Control and Prevention. Enterovirus surveillance--United States, 1970–2005. MMWR Surveill. Summ. 55, 1–20 (2006). [PubMed] [Google Scholar]
- Faustini A. et al. An outbreak of aseptic meningitis due to echovirus 30 associated with attending school and swimming in pools. Int. J. Infect. Dis. 10, 291–297 (2006). [DOI] [PubMed] [Google Scholar]
- Starlin R. et al. Acute flaccid paralysis syndrome associated with echovirus 19, managed with pleconaril and intravenous immunoglobulin. Clin. Infect. Dis. 33, 730–732 (2001). [DOI] [PubMed] [Google Scholar]
- Zhu Z. et al. Molecular epidemiological analysis of echovirus 19 isolated from an outbreak associated with hand, foot, and mouth disease (HFMD) in Shandong Province of China. Biomed. Environ. Sci. 20, 321–328 (2007). [PubMed] [Google Scholar]
- Pelletier J. & Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 334, 320–325 (1988). [DOI] [PubMed] [Google Scholar]
- Molla A., Jang S. K., Paul A. V., Reuer Q. & Wimmer E. Cardioviral internal ribosomal entry site is functional in a genetically engineered dicistronic poliovirus. Nature. 356, 255–257 (1992). [DOI] [PubMed] [Google Scholar]
- Chen C. Y. & Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268, 415–417 (1995). [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Maslova S. V., Sinyakov A. N. & Agol V. I. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic. Acids. Res. 20, 1739–1745 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V. et al. Cis-element, oriR, involved in the initiation of (−) strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary (‘kissing’) interactions. EMBO J. 15, 5428–5436 (1996). [PMC free article] [PubMed] [Google Scholar]
- Mirmomeni M. H., Hughes P. J. & Stanway G. An RNA tertiary structure in the 39 untranslated region of enteroviruses is necessary for efficient replication. J. Virol. 71, 2363–2370 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. Fields Virology, 5th ed. (Lippincott Williams & Wilkins, Philadelphia, 2007). [Google Scholar]
- Oberste M. S. et al. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J. Clin. Microbiol. 38, 1170–1174 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Kilptrick D. R. & Pallansch M. A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73, 1941–1948 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A. et al. Resolving ambiguities in genetic typing of human enterovirus species C clinical isolates and identification of enterovirus 96, 99 and 102. J. Gen. Virol. 90, 1713–1723 (2009). [DOI] [PubMed] [Google Scholar]
- Picornaviridae. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. (Elsevier, San Diego, 2011). [Google Scholar]
- Oberste M. S. et al. Molecular identification of 13 new enterovirus types, EV79–88, EV97, and EV100–101, members of the species Human Eneterovirus B. Virus Res. 128, 34–42 (2007). [DOI] [PubMed] [Google Scholar]
- Oberste M. S. et al. Molecular identification and characterization of two proposed new enterovirus serotypes, EV74 and EV75. J. Gen. Virol. 85, 3205–3212 (2004). [DOI] [PubMed] [Google Scholar]
- Oberste M. S. et al. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A. J. Gen. Virol. 86, 445–451 (2005). [DOI] [PubMed] [Google Scholar]
- Norder H. et al. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. J. Gen. Virol. 84, 827–836 (2003). [DOI] [PubMed] [Google Scholar]
- Shaukat S. et al. Characterization of a novel enterovirus serotype and an enterovirus EV-B93 isolated from acute flaccid paralysis patients. PLoS ONE 8, e80040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingjun T. et al. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan province, the People's Republic of China. J. Med. Virol. 80, 670–679 (2008). [DOI] [PubMed] [Google Scholar]
- Andersson P., Edman K. & Lindberg A. M. Molecular analysis of the echovirus 18 prototype: evidence of interserotypic recombination with echovirus 9. Virus Res. 85, 71–83 (2002). [DOI] [PubMed] [Google Scholar]
- Huang T. et al. Evidence of recombination and genetic diversity in human rhinoviruses in children with acute respiratory infection. PLoS ONE 4, e6355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey P. G., Davis J. S., Harnett G. B., Williams S. H. & Speers D. J. Meningitis and a febrile vomiting illness caused by echovirus type 4, Northern Territory, Australia. Emerg. Infect. Dis. 16, 63–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paananen A. et al. A single amino acid substitution in viral VP1 protein alters the lytic potential of clone-derived variants of echovirus 9 DM strain in human pancreaticislets. J. Med. Virol. 85, 1267–1273 (2013). [DOI] [PubMed] [Google Scholar]
- Sun Q. et al. Transmission of human enterovirus 85 recombinants containing new unknown serotype HEV-B donor sequences in Xinjiang Uighur autonomous region, China. PLoS ONE 8, e55480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Isolation and characterization of a Chinese strain of human enterovirus 74 from a healthy child in the Tibet Autonomous Region of China. Arch. Virol. 157, 1593–1598 (2012). [DOI] [PubMed] [Google Scholar]
- Tao Z. et al. Isolation and genomic characterization of three enterovirus 90 strains in Shandong, China. Arch. Virol. 158, 479–483 (2013). [DOI] [PubMed] [Google Scholar]
- Tao Z. et al. Complete genome sequence of enterovirus 87 isolated from a child with acute flaccid paralysis in China in 2000. Virus Genes 47, 156–159 (2013). [DOI] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Patterson M. A. & Pallansch M. A. The complete genome sequence for an American isolate of enterovirus 77. Arch. Virol. 152, 1587–1591 (2007). [DOI] [PubMed] [Google Scholar]
- Bailly J. L., Cardoso M. C., Labbé A. & Peigue-Lafeuille H. Isolation and identification of an enterovirus 77 recovered from a refugee child from Kosovo, and characterization of the complete virus genome. Virus Res. 99, 147–155 (2004). [DOI] [PubMed] [Google Scholar]
- Rao D. C. et al. Non-polio enteroviruses and their association with acute diarrhea in children in India. Infect. Genet. Evol. 17, 153–161 (2013). [DOI] [PubMed] [Google Scholar]
- Bessaud M. et al. Molecular characterization of human enteroviruses in the Central African Republic: uncovering wide diversity and identification of a new human enterovirus A71 genogroup. J. Clin. Microbiol. 50, 1650–1658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmivandana R., Yergolkar P., Gopalkrishna V. & Chitambar S. D. Characterization of the non-polio enterovirus infections associated with acute flaccid paralysis in South-Western India. PLoS ONE 8, e61650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K. & Pallansch M. A. Evidence of frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J. Virol. 78, 855–867 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B., Oberste M. S., Maher K. & Pallansch M. A. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J. Virol. 77, 8973–8984 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Penaranda S., Maher K. & Pallansch M. A. Complete genome sequences of all members of the species Human enterovirus A. J. Gen. Virol. 85, 1597–1607 (2004). [DOI] [PubMed] [Google Scholar]
- Lukashev et al. Recombination in circulating enteroviruses. J. Virol. 77, 10423–10431 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprisan G. et al. Natural genetic recombination between co-circulating heterotypic enteroviruses. J. Gen. Virol. 83, 2193–2200 (2002). [DOI] [PubMed] [Google Scholar]
- Santti J., Hyypiä T., Kinnunen L. & Salminen M. Evidence of recombination among enteroviruses. J. Virol. 73, 8741–8749 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolation and identification of polioviruses. WHO Polio laboratory manual, 4th edn. (World Health Organization, Geneva, 2004). [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. 41, 95–98 (1999). [Google Scholar]
- Tamura K., Dudley J., Nei M. & Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007). [DOI] [PubMed] [Google Scholar]
- Lole K. S. et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73, 152–160 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen M. O., Carr J. K., Burke D. S. & McCutchan F. E. Identification of breakpoints in Intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res. Hum. Retroviruses 11, 1423–1425 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1