Abstract
It has been recently ascribed to several inflammatory cytokines (i.e. TGF-β3, TNF-α, and IL-1) a functional role in regulating Sertoli cell blood-testis barrier (BTB) dynamics. In the testis, IL-6 inhibits meiotic DNA synthesis during the seminiferous epithelium cycle, reduces sperm motility and influences the secretion of transferrin and inhibin B by Sertoli cells. Also, it has been shown that IL-6 affects tight junction permeability in Sertoli cells, but, little is known about its role in regulating the BTB. The aim of this study was to investigate the molecular mechanisms by which IL-6 affects BTB dynamics. We show that IL-6 perturbs the integrity of the BTB, and alters the normal localization and steady-state levels of BTB integral membrane proteins. We demonstrated that IL-6 regulates the BTB by inhibiting the degradation of BTB constitutive proteins and activating ERK-MAPK pathways. Our results provide mechanistic insight into the roles of IL-6 in regulating BTB dynamics.
Adjacent Sertoli cells are separated by the blood-testis barrier (BTB) that physically divides the seminiferous epithelium into basal and adluminal compartments, thereby providing a stable microenvironment for spermatogenesis1,2,3. It consists of several different types of junctions, including tight junctions (TJs), basal ectoplasmic specializations (ES) and desmosome-gap junctions3,4,5. Recent studies have shown that several inflammatory cytokines can regulate, at least in part, BTB dynamics during spermatogenesis, which facilitates the transit of preleptotene/leptotene spermatocytes at the BTB6,7,8,9,10. For instance, TGF-β3 and TNF-α perturb BTB dynamics by accelerating clathrin-mediated endocytosis of integral membrane proteins, while interleukin-1α (IL-1α) increases the kinetics of occludin internalization and decreases its rate of degradation6,10. IL-6 impairs the Sertoli cell TJ barrier in normal rats by perturbing the MAPK14 signaling pathway11. However, the molecular mechanisms mediating the roles of IL-6 in BTB dynamics under normal physiological condition are still unknown.
IL-6 is synthesized by most testicular cells, including interstitial macrophages, Leydig cells, Sertoli cells, and germ cells12,13,14,15,16,17. In the testis, IL-6 inhibits meiotic DNA synthesis during the cycle of the seminiferous epithelium18, influences the secretion of transferrin and inhibin B by Sertoli cells19,20 and reduces sperm motility21. During testicular infection and inflammation in response to agents such as lipopolysaccharide or Ureaplasma urealyticum, the level of IL-6 expression increases22,23,24,25. In this study, the roles of IL-6 in the regulation of the Sertoli cell barrier were investigated. We demonstrated that IL-6 regulates the BTB by inhibiting the degradation of BTB constitutive proteins and activating ERK-MAPK pathways.
Results
Effects of IL-6 on the assembly and permeability of Sertoli cell TJs in vitro
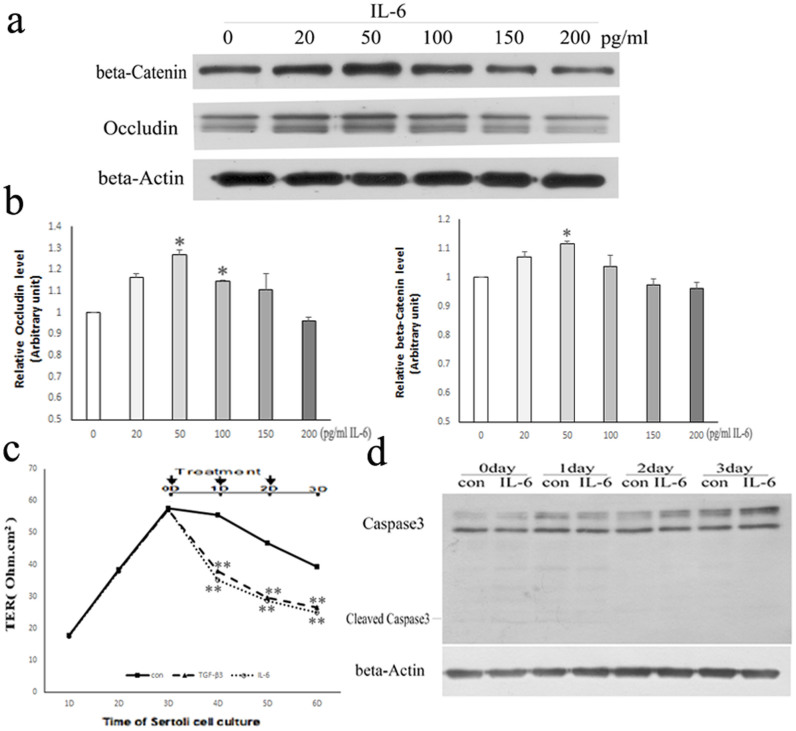
To determine whether IL-6 can affect BTB, we first investigated the expression level of BTB-constituent proteins exposed to IL-6 2 days at different concentrations (i.e. 0, 20, 50, 100,150 and 200 pg/ml), by using Western blotting assays (Fig. 1a, b). The highest expression level of Occludin and β-Catenin was detected with 50 pg/ml of IL-6 compared to other concentrations used (Fig. 1a, b). Therefore, the effect of 50 pg/ml IL-6 on BTB was most obvious, and then this concentration was used for all the experiments described in this study.
Figure 1. Effects of IL-6 on assembly of the TJ permeability barrier of cultured Sertoli cells.
(a) Western blot analysis of BTB-constituent proteins expression after treated with IL-6 2 days at different concentrations (0, 20, 50, 100, 150, 200 pg/ml). β-Actin served as the loading control. All gels had been run under the same experimental conditions. (b) Bar plots summarizing relative Occludin and β-Catenin results from several independent experiments after normalizing each data point against its corresponding actin and then against its corresponding control at 0D. 0 pg/ml IL-6 group was arbitrarily set at 1. Data points represent median ± AD (n = 3). *P < 0.05, vs. control. (c) Sertoli cells (1.0 × 106 cells/cm2), having assembled a functional TJ permeability barrier (indicated as TER) after 3 days in vitro, were treated with control, 3 ng/ml TGF-β3 or 50 pg/ml IL-6 onward of day 3, as indicated by arrows. Data represent median ± AD (n = 3). **P < 0.01 vs. control. (d) Western blot analysis of Caspase3 expression after treated with vehicle control (con) or 50 pg/ml IL-6 for increasing periods of time. β-Actin served as the loading control.
Sertoli cells were cultured at a high density (1.0 × 106 cells/cm2) on Matrigel-coated bicameral units, an experimental model that mimics the BTB in vivo26. The TER increased steadily to ~57 Ohm.cm2 over 3–4 days (Fig. 1c), indicating the assembly of intercellular junctions was complete. Treatment with recombinant rat IL-6 or TGF-β3 on day 3 disrupted the TJ barrier as reflected by a gradual decrease in TER of IL-6/TGF-β3-treated Sertoli cells compared to control-treated cells (Fig. 1c). Herein, TGF-β3 was used as a positive control in TER assay9. To determine whether the decrease of TER in the IL-6-treated cells was caused by cell viability, we checked the expression level of apoptosis gene Caspase3 using Western blotting assay. There were no response to apoptosis in Sertoli cells (no cleaved Caspase3) (Fig. 1d). These data confirm that IL-6 perturbs Sertoli cell TJ permeability in vitro11.
Effects of IL-6 on the localization of BTB-constituent proteins in cultured Sertoli cells
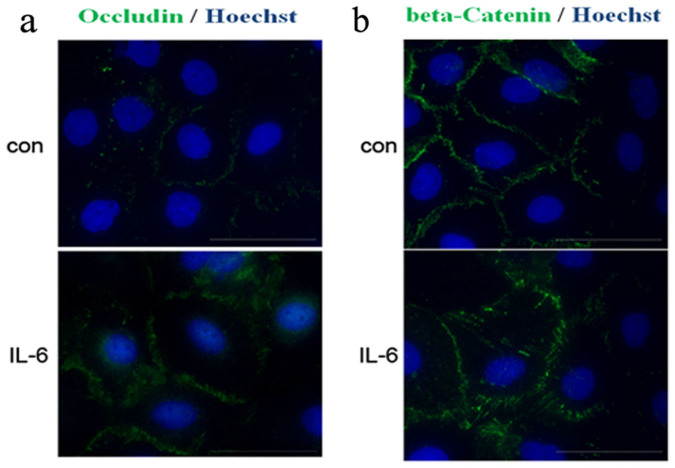
Immunocytochemistry of Sertoli cells cultured on Matrigel-coated coverslips was performed to investigate the effects of IL-6 on the localization of the TJ and basal ES proteins Occludin and β-Catenin, respectively. In control cells, fluorescent signals for Occludin (Fig. 2a) and β-Catenin (Fig. 2b) were restricted to a finite area. In contrast, in cells treated with IL-6 on day 3 and analyzed after 1 day, the labeling for both proteins was diffuse and no longer concentrated at cell-cell interfaces, indicating that IL-6 causes mislocalization of BTB-constituent proteins.
Figure 2. IL-6 caused mislocalization of BTB-constituent proteins in Sertoli cells.
Sertoli cells (4.0 × 104 cells/cm2) were treated with control (con) or 50 pg/ml IL-6 (IL-6) as described in Materials and Methods. Cells were immunostained for Occludin (green; a) or β-Catenin (green; b). Nuclei were visualized with Hoechst 33342 (blue). Scale bar = 50 μm.
Effects of IL-6 on the expression of BTB-constituent proteins in cultured Sertoli cells
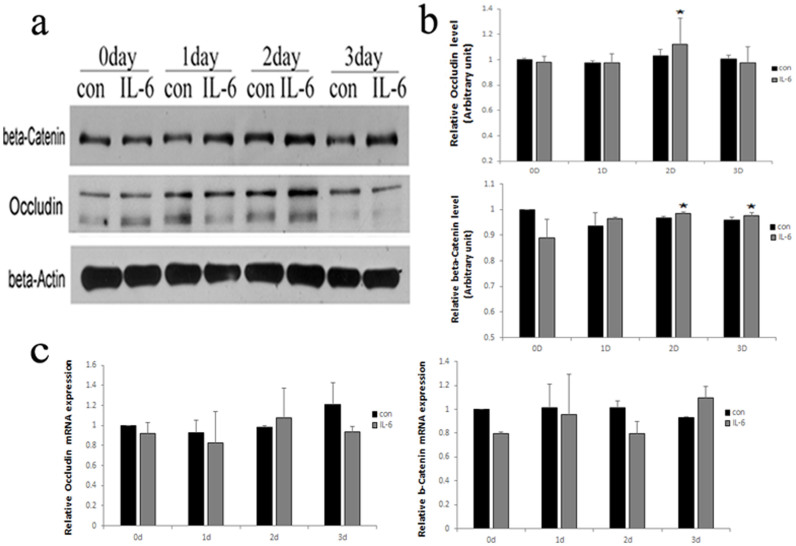
We next sought to examine whether there were quantitative changes in the expression of BTB-constituent proteins in Sertoli cells after IL-6 treatment. Immunoblotting revealed that, Occludin was increased at 2-days and β-Catenin was increased at 2 and 3 days at the protein level after treatment with IL-6 compared to controls (Fig. 3a, b). However, real-time RT-PCR analysis revealed the mRNA levels of Occludin and β-Catenin were not affected by IL-6 treatment (Fig. 3c).
Figure 3. IL-6 increased the steady-state levels of BTB-constituent proteins in vitro.
(a) Western blot analysis of BTB-constituent proteins expression after treated with vehicle control (con) or 50 pg/ml IL-6 for increasing periods of time. β-Actin served as the loading control. All gels had been run under the same experimental conditions. (b) Bar plots summarizing relative Occludin and β-Catenin results from several independent experiments after normalizing each data point against its corresponding actin time point and then against its corresponding control at 0D. Control in both experimental groups was arbitrarily set at 1. Data points represent median ± AD (n = 3). *P < 0.05, vs. control. (c) The mRNA levels of BTB components after treatment with control (con) or IL-6 for increasing periods of time. Data represent median ± AD (n = 3).
IL-6 increases the cellular levels of BTB constituents by delaying protein degradation
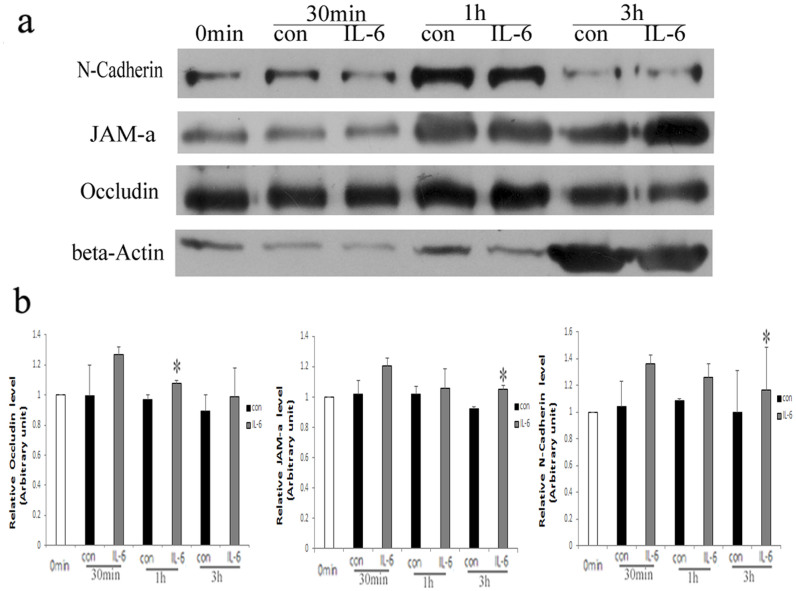
To investigate how IL-6 caused BTB-constituent protein levels to increase in light of a leaky Sertoli cell barrier (Fig. 1a, b), the kinetics of Occludin, JAM-a and N-Cadherin degradation were examined using a protein degradation assay (Fig. 4a, b). Briefly, Sertoli cell surface proteins were labeled by biotinylation, and cells were treated with IL-6 or vehicle control. Non-degraded proteins remaining on the cell surface, as well as those that had internalized after IL-6 treatment were affinity purified and detected by immunoblotting. The levels of non-degraded proteins Occludin, JAM-a, and N-Cadherin were significant increases after IL-6 treatment. The level of Occludin was significantly higher in IL-6-treated cells compared to controls over 1 hour, while the levels JAM-a, and N-Cadherin were up-regulated in IL-6-treated cells over 3 hours (Fig. 4a, b). These results indicated that IL-6 delayed the degradation of these proteins.
Figure 4. IL-6 delayed the degradation of BTB-constituent proteins.
(a) Immunoblotting for quantification of Occludin, JAM-a, and N-Cadherin that remained non-degraded after 50 pg/ml IL-6 treatment for 0 min, 30 min, 1 h and 3 h. β-Actin served as the loading control. All gels had been run under the same experimental conditions. (b) Bar plots summarizing relative Occludin, JAM-a, and N-Cadherin results from several independent experiments after normalizing each data point against its corresponding actin time point and then against its corresponding control at 0 min. Control in both experimental groups was arbitrarily set at 1. Data points represent median ± AD (n = 3). *P < 0.05, vs. control.
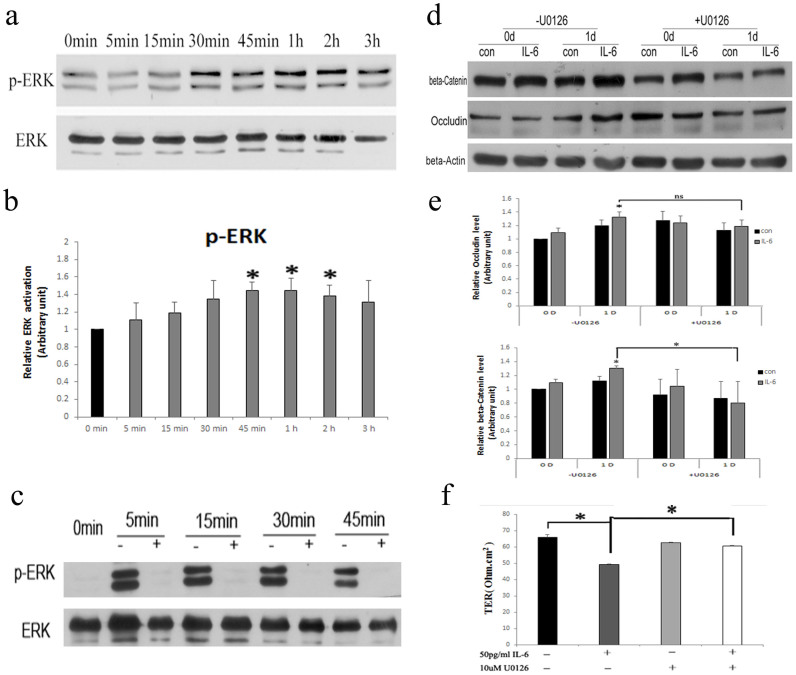
IL-6 regulates ES dynamics through the ERK-MAPK pathway
To gain insight on the molecular mechanisms that contribute to the actions of IL-6 on the BTB, signaling pathways known to be important for activity of the cytokine were studied in Sertoli cells by immunoblotting. IL-6 triggers two main signaling cascades; the SHP-2/ERK-MAPK pathway and the JAK/STAT pathway27,28. Sertoli cells were treated with IL-6 in vitro, and the time course of ERK1/2 activation was examined (Fig. 5a, b). The levels of phosphorylated proteins increased from 45 min to 2 h (Fig. 5a, b). To determine whether ERK1/2 phosphorylation affects the level of BTB-constituent proteins, cells were pre-incubated with the MEK1/2-specific inhibitor U0126 (10 μM) 30 min prior to treatment with IL-6 to block ERK1/2 phosphorylation (Fig. 5c). The levels of BTB-constituent proteins were examined after 1 day of IL-6 treatment, and this revealed that U0126 pre-treatment inhibited up-regulation of the ES protein β-Catenin in response to IL-6 (Fig. 5d, e). However, U0126 had no effect on the increase in the TJ protein Occludin stimulated by IL-6 (Fig. 5d, e). To further test whether U0126 partly inhibited IL-6-caused BTB disruption, we next measured TER across Sertoli cells in culture exposed to 50 pg/ml IL-6 for 24 h in the presence or absence of U0126. Treatment with IL-6 and U0126 reflected an increase in TER compared to only IL-6-treated Sertoli cells (Fig. 5f). Collectively, these data suggest that IL-6 regulates the dynamics of ES constituents through the ERK-MAPK pathway.
Figure 5. IL-6 regulated the dynamics of ES through the ERK-MAPK pathway.
(a) Western blot analysis of ERK and p-ERK levels after 50 pg/ml IL-6 treatment at different time points. All gels had been run under the same experimental conditions. (b) Bar plots summarizing relative ERK activation results from several independent experiments after normalizing each data point against its corresponding total ERK time point and then against its corresponding control at 0 min. Control in both experimental groups was arbitrarily set at 1. Data points represent median ± AD (n = 3). *P < 0.05, vs. control. (c) The MEK1/2-specific inhibitor 10 μM U0126 blocked the ERK-MAPK pathway. +, Present; −, absent. All gels had been run under the same experimental conditions. (d) Western blot analysis of BTB-constituent proteins after treatment with 10 μM U0126 prior to 50 pg/ml IL-6. β-Actin served as the loading control. All gels had been run under the same experimental conditions. (e) Bar plots summarizing relative Occludin and β-Catenin results from several independent experiments after normalizing each data point against its corresponding actin time point and then against its corresponding control at 0D. Control in both experimental groups was arbitrarily set at 1. Data represent the median ± AD of three independent experiments performed in triplicate. *P < 0.05, compared with absence of U0126. (f) TER across Sertoli cells in culture exposed to 50 pg/ml IL6 for 24 h in the presence or absence of 10 μM U0126. The addition of U0126 partly blocked the effect of IL6 on TER. Data represent median ± AD (n = 3). *P < 0.05, compared with absence of U0126. +, Present; −, absent.
Discussion
IL-6 plays an important role in maintaining the function of Sertoli cells and germ cells18,19,20,21. In this study, we found that IL-6 could disrupt the integrity of the Sertoli cell BTB. Furthermore, IL-6 regulated the dynamics of BTB via delaying BTB-constituent proteins degradation and the ERK-MAPK pathway. These results, when taken together with the previously reported effect of IL-6 on Sertoli cell TJs in rats with experimental autoimmune orchitis (EAO)11, provide compelling evidence that IL-6 could perturb Sertoli cell BTB integrity during testicular infection and inflammation.
When spermatozoa are released from the seminiferous epithelium, the BTB undergoes restructuring at late stage VIII to facilitate the transit of preleptotene/leptotene spermatocytes from the basal to the apical compartment29,30,31. IL-6 production under physiological conditions is lowest at stages VII-VIII of the seminiferous epithelium cycle18. However, testicular IL-6 levels are up-regulated in the setting of injury and inflammation25,32,33,34,35,11. Herein, IL-6 could affect the contractility of BTB by changing the localization and amount of BTB-constituent proteins, while the mRNA levels of BTB-constituent proteins were not changed. Moreover, a protein degradation assay revealed the kinetics of Occludin, JAM-a, and N-Cadherin degradation were delayed after IL-6 treatment, leading to their accumulation in Sertoli cells. Our data confirmed previous results11,36,37 showing that IL-6 can affect TJ permeability in Sertoli cells. Also, in other epithelial cells the effect of IL-6 on TJ permeability was recently reported38,39.
Previous studies showed that ERK is a crucial regulator of junction restructuring in the seminiferous epithelium, effects that are mediated by its downstream actions on proteases and protease inhibitors as well as actin dynamics at the apical ES40,41. Herein, IL-6 can stimulate p-ERK1/2 expression in Sertoli cells. Moreover, blocking the ERK-MAPK pathway led to restoration of the ES protein β-Catenin, but had no effect on the TJ protein Occludin. Furthermore, after blocking the ERK-MAPK pathway, Sertoli cell BTB permeability had a less disrupt. Thus, IL-6 could disassemble the ES permeability barrier via ERK-MAPK signaling pathway. Because ERK is considered as a key player in modulating cell and motility in different epithelia42, it is plausible that an altered p-ERK1/2 expression may influence the homeostasis of Sertoli cells and thus the integrity of BTB.
Since overexpression of IL-6 could disrupt the integrity of the Sertoli cell BTB (including our results) and ultimately impair spermatogenesis16,19,20,21,and IL-6 impairs the Sertoli cell TJ barrier in EAO rat by perturbing the MAPK14 signaling pathway11, our results thus will add a novel IL-6 signaling pathway in mediating testicular injury and inflammation conditions. Further studies will examine whether IL-6 could have the similar effect on BTB in rats under physiological or pathophysiological status, and whether blocking the ERK-MAPK signaling pathway can abate or prevent testicular injury and inflammation.
Methods
Animals
Male Wistar rats at 20 days of age were obtained from the Animal Center, University of Science and Technology of China (USTC), and housed at 22°C in light-controlled quarters (12 h light, 12 h dark). Rats were euthanized by CO2 asphyxiation. All the experiments on live vertebrates were performed in accordance with the relevant guidelines and regulations. This study received ethical approval from the institutional review boards of the USTC.
Isolation and culture of Sertoli cells
Primary Sertoli cell cultures were established from the testes of 20-day-old male rats as previously described43, with some modifications. Briefly, testes were decapsulated and digested with 2 mg/ml collagenase (Sigma, Type IV, USA) and 75 U/ml DNase I (Sigma, USA) in PBS at room temperature for 5 min with gentle shaking. Digested tissues were centrifuged, and the pellets were suspended in 2 mg/ml collagenase (Sigma, Type IV, USA), 75 U/ml DNase I (Sigma, USA) and 2 mg/ml hyaluronidase (Sigma, Type IV, USA) in PBS at room temperature for 5 min with vigorous shaking. After addition of DMEM/F12 medium (Invitrogen, USA), the suspension was filtered through a stainless steel filter (70 mesh) then centrifuged. The supernatant was removed and the cells were washed twice with DMEM/F12 medium (Invitrogen, USA), then resuspended in 1 ml of serum-free DMEM/F12 medium containing 15 mM HEPES, 1.2 g/l sodium bicarbonate, 10 μg/ml bovine insulin, 5 μg/ml human transferrin, 2.5 ng/ml epidermal growth factor, and 1% antibiotics. For preparation of protein lysates, Sertoli cells were cultured at 0.5 × 106 cells/cm2 on Matrigel-coated dishes (BD Biosciences, USA). For transepithelial electrical resistance (TER) measurement, cells were plated at 1.0 × 106 cells/cm2 on Matrigel-coated Millicell HA cell culture inserts (Millipore, Billerica, MA, USA). For immunofluorescence analysis, cells were plated at 4.0 × 104 cells/cm2 on Matrigel-coated glass coverslips. Cells were cultured at 35°C in a humidified atmosphere of 5% CO2 and 95% air, and the medium was replaced daily. After 48 h in culture, cells were treated with a hypotonic solution composed of 20 mM Tris-HCl, pH 7.4, at 22°C for 2 min to lyse residual germ cells as described44, and a purity of greater than 95% was typically achieved. After 3 days in vitro, resemble BTB were established between adjacent Sertoli cells26,45,46,47.
IL-6 treatment
Rat Sertoli cells isolated and cultured for 3–4 days were treated at different time points with 50 pg/ml recombinant rat IL-6 (R&D Systems, Minneapolis, MN, USA; stock dissolved in PBS, pH 7.4, containing 0.1%BSA, w/v) or control (PBS, pH 7.4, containing 0.1%BSA, w/v) diluted in DMEM/F12.
Transepithelial electrical resistance (TER)
Freshly isolated Sertoli cells were cultured at high density (1.0 × 106 cells/cm2) to promote TJ assembly that was quantified using a Millicell electrical resistance system (Millipore Corp, USA) as previously described48,49. The first TER measurement across the Sertoli cell epithelium was performed 24 h after cells were plated (i.e. day 1), and this was subsequently repeated at specific time points. Each experiment was performed in replicate and was repeated 3 times using different batches of cells. The resulting values were multiplied by the surface area of the filter (1.1 cm2) and data were presented as Ohm.cm2. The net value of electrical resistance was calculated by subtracting the background obtained by measurement of cell-free Matrigel-coated bicameral units. A functional barrier was assembled after 3 days in culture. From this time point, Sertoli cells were incubated with 50 pg/ml recombinant rat IL-6, 3 ng/ml TGF-β3 (R&D Systems, Minneapolis, MN) or control.
Western blotting
Sertoli cells were harvested and lysed for 30 min in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium dodecyl sulfate (SDS), 1% sodium deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), Complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and 5 mM sodium orthovanadate (a phosphatase inhibitor). Protein lysates were cleared by centrifugation (12,000 × g for 20 min at 4°C), separated by SDS-PAGE (Bio-Rad, Hercules, CA), then transferred to Hybond Enhanced Chemiluminescence Nitrocellulose membranes (Amersham Biosciences, Freiburg, Germany) for immunoblotting and detection using Enhanced Chemiluminescence (ECL, Kodak, Rochester, NY). The following antibodies were used: anti-β-Actin (Abcam, Ab8227-50, USA), anti-β-Catenin (Abcam, USA), anti-Occludin (Invitrogen, USA), anti-JAM-a (Invitrogen, USA), anti-N-Cadherin (BD Transduction Laboratories, USA), anti-ERK1/2 (Cell Signaling Technology, USA), anti-p-ERK1/2 (Cell Signaling Technology, USA). Protein levels were normalized to actin or ERK 1/2 and quantified using Tanon Gel image system (Tanon, Shanghai, China).
Immunofluorescence analysis
For immunostaining, cells were fixed for 20 min in 4% (w/v) paraformaldehyde, washed in PBS for 10 min three times, permeabilized with 0.25% Triton and 0.1% (w/v) Tween in PBS for 10 min, blocked with 1% BSA in PBS for 1 h. After overnight incubation at 4°C with anti-Occludin or anti-β-Catenin antibody (1:50 dilution in blocking buffer), cells were washed then incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody (1:200 dilution) for 30 min at room temperature. Nuclei were stained with Hoechst 33342 (Sigma, USA) for 2 min at room temperature. Fluorescent signals were acquired with a Nikon epifluorescence microscope (Nikon Eclipse 80i; Nikon, Tokyo, Japan).
Quantitative real-time PCR assay
For quantitative real-time PCR detection of BTB components, total RNA was extracted from cultured Sertoli cells using Trizol (Invitrogen, USA), and 500 ng samples were used to synthesize cDNA with the PrimeScript One Step RT-PCR kit Ver.2 (TaKaRa Bio Inc., Otsu, Japan) according to the manufacturers' instructions. Real-time PCR was performed in an Applied Biosystems StepOne real-time PCR system using the SYBR Premix Ex Taq II kit (TaKaRa Bio Inc., Otsu, Japan) as described previously50. The following specific primer pairs were used:
β-Actin FW: 5′-CGTTGACATCCGTAAAGAC-3′,
β-Actin RW: 5′-TAGGAGCCAGGGCAGTA-3′.
Occludin FW: 5′- CCTTGTCCGTGGATGACTTCAG-3′,
Occludin RW: 5′- CCCTTCGTGGGAGTCCTTT-3′.
β-Catenin FW: 5′- GACAAGCCACAGGACTACAAGA-3′,
β-Catenin RW: 5′- TCCGAGATCAGCAGTCTCATTC-3′.
Expression levels were normalized to β-actin, and each PCR reaction was performed in triplicate.
Protein degradation assay
A Protein degradation assay was performed as described previously6,10 with minor modifications. Briefly, Sertoli cells were seeded at 0.5 × 106 cells/cm2 on Matrigel-coated 6-well dishes. After 4 days the cells were washed twice with ice-cold PBS and surface proteins were biotinylated with 0.5 mg/ml Sulfo-NHS-SS-Biotin (Pierce, USA) in PBS, pH 7.4, 1 mM CaCl2 and 0.7 mM MgCl2, and the reaction was quenched in the same buffer containing 50 mM Tris for 15 min at 4°C. Cells were then washed twice with ice-cold PBS and incubated in DMEM/F12 with (test) or without (control) 50 pg/ml recombinant rat IL-6 for various time points. This was performed at 35°C to allow internalization of cell surface biotinylated proteins, since endocytosis does not occur at 4°C. Cells were then washed twice with ice-cold PBS and harvested in 50 mM Tris, pH 7.4, 150 mMNaCl, 2 mM EDTA, 0.1% (w/v) SDS; 1% (v/v)NonidetP-40, 1 mM PMSF, Complete EDTA-free protease inhibitor cocktail and 5 mM sodium orthovanadate. The assay does not discriminate between non-degraded proteins present on the cell surface and those in the cytosol. Protein samples (80 μg) were incubated with UltraLink Immobilized NeutrAvidin Plus (Thermo Scientific, USA) overnight at 4°C, washed three times in PBS, and biotinylated proteins were extracted in SDS sample buffer. Proteins were resolved by SDS-PAGE and immunoblotted as described above.
Statistical analysis
All experiments in this study were repeated at least three times. Data were expressed as median ± AD. The non-parametric Kruskal-Wallis test was performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA) to evaluate differences among variables. P values < 0.05 were considered statistically significant.
Author Contributions
F.S. initiated the project, conceived and designed the experiments; H.Z., Y.Y. and Z.L. were involved in preparation and isolation of Rat Sertoli cells; H.Z. and G.W. performed Western blotting; H.Z. and L.L. performed Real-Time qPCR; H.Z. carried out data analysis interpretation. All authors discussed the results and commented on the manuscript.
Funding: Studies were supported by the following grants: the National Natural Science Foundation of China (81125005), the National Basic Research Program of China (2014CB943100); the Chinese Academy of Sciences Knowledge Creative Program (KSCX2-EW-R-07).
References
- Dym M. & Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3, 308–326 (1970). [DOI] [PubMed] [Google Scholar]
- Mruk D. D. & Cheng C. Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25, 747–806 (2004). [DOI] [PubMed] [Google Scholar]
- Kopera I. A., Bilinska B., Cheng C. Y. & Mruk D. D. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci 365, 1593–1605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D. & Cheng C. Y. Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci 365, 1621–1635 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W. Y. & Lee W. M. Mechanisms of reorganization of cell-cell junctions in the testis. Front Biosci 13, 6775–6786 (2008). [DOI] [PubMed] [Google Scholar]
- Xia W., Wong E. W., Mruk D. D. & Cheng C. Y. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol 327, 48–61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Mruk D. D., Lee W. M. & Cheng C. Y. Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem 281, 16799–16813 (2006). [DOI] [PubMed] [Google Scholar]
- Li M. W. et al. Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190, 313–329 (2006). [DOI] [PubMed] [Google Scholar]
- Lui W. Y., Lee W. M. & Cheng C. Y. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 68, 1597–1612 (2003). [DOI] [PubMed] [Google Scholar]
- Lie P. P., Cheng C. Y. & Mruk D. D. Interleukin-1alpha is a regulator of the blood-testis barrier. FASEB J 25, 1244–1253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C. V. et al. Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol Reprod 87, 122 (2012). [DOI] [PubMed] [Google Scholar]
- Bryniarski K., Szczepanik M., Ptak M. & Ptak W. Modulation of testicular macrophage activity by collagenase. Folia Histochem Cytobiol 43, 37–41 (2005). [PubMed] [Google Scholar]
- Kern S., Robertson S. A., Mau V. J. & Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod 53, 1407–1416 (1995). [DOI] [PubMed] [Google Scholar]
- Boockfor F. R., Wang D., Lin T., Nagpal M. L. & Spangelo B. L. Interleukin-6 secretion from rat Leydig cells in culture. Endocrinology 134, 2150–2155 (1994). [DOI] [PubMed] [Google Scholar]
- Cudicini C., Kercret H., Touzalin A. M., Ballet F. & Jegou B. Vectorial production of interleukin 1 and interleukin 6 by rat Sertoli cells cultured in a dual culture compartment system. Endocrinology 138, 2863–2870 (1997). [DOI] [PubMed] [Google Scholar]
- Rival C., Theas M. S., Guazzone V. A. & Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol 70, 43–58 (2006). [DOI] [PubMed] [Google Scholar]
- Potashnik H. et al. Interleukin-6 expression during normal maturation of the mouse testis. Eur Cytokine Netw 16, 161–165 (2005). [PubMed] [Google Scholar]
- Hakovirta H., Syed V., Jegou B. & Parvinen M. Function of interleukin-6 as an inhibitor of meiotic DNA synthesis in the rat seminiferous epithelium. Mol Cell Endocrinol 108, 193–198 (1995). [DOI] [PubMed] [Google Scholar]
- Boockfor F. R. & Schwarz L. K. Effects of interleukin-6, interleukin-2, and tumor necrosis factor alpha on transferrin release from Sertoli cells in culture. Endocrinology 129, 256–262 (1991). [DOI] [PubMed] [Google Scholar]
- Okuma Y. et al. Regulation of activin A and inhibin B secretion by inflammatory mediators in adult rat Sertoli cell cultures. J Endocrinol 187, 125–134 (2005). [DOI] [PubMed] [Google Scholar]
- Lampiao F. & du Plessis S. S. TNF-alpha and IL-6 affect human sperm function by elevating nitric oxide production. Reprod Biomed Online 17, 628–631 (2008). [DOI] [PubMed] [Google Scholar]
- Syed V. et al. Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology 136, 3070–3078 (1995). [DOI] [PubMed] [Google Scholar]
- Cudicini C. et al. Human Leydig cells and Sertoli cells are producers of interleukins-1 and -6. J Clin Endocrinol Metab 82, 1426–1433 (1997). [DOI] [PubMed] [Google Scholar]
- Stephan J. P., Syed V. & Jegou B. Regulation of Sertoli cell IL-1 and IL-6 production in vitro. Mol Cell Endocrinol 134, 109–118 (1997). [DOI] [PubMed] [Google Scholar]
- Li R. et al. Expression of IL-1alpha, IL-6, TGF-beta, FasL and ZNF265 during sertoli cell infection by ureaplasma urealyticum. Cell Mol Immunol 6, 215–221, 10.1038/cmi.2009.29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M. K., Wong C. H., Lee W. M. & Cheng C. Y. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280, 25029–25047 (2005). [DOI] [PubMed] [Google Scholar]
- Hirano T., Nakajima K. & Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev 8, 241–252 (1997). [DOI] [PubMed] [Google Scholar]
- Mihara M., Hashizume M., Yoshida H., Suzuki M. & Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 122, 143–159 (2012). [DOI] [PubMed] [Google Scholar]
- Cheng C. Y. & Mruk D. D. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6, 380–395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y. & Mruk D. D. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 44, 245–263 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. M. & Byers S. W. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech 20, 3–33 (1992). [DOI] [PubMed] [Google Scholar]
- Ogawa Y. et al. Cadmium exposure increases susceptibility to testicular autoimmunity in mice. J Appl Toxicol (2012). [DOI] [PubMed] [Google Scholar]
- Wu H. et al. Cytokines produced by microwave-radiated Sertoli cells interfere with spermatogenesis in rat testis. Andrologia 44 Suppl 1, 590–599 (2012). [DOI] [PubMed] [Google Scholar]
- Bialas M. et al. The role of IL-6, IL-10, TNF-alpha and its receptors TNFR1 and TNFR2 in the local regulatory system of normal and impaired human spermatogenesis. Am J Reprod Immunol 62, 51–59 (2009). [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Ishikawa T., Kondo Y. & Fujisawa M. Cisplatin regulates Sertoli cell expression of transferrin and interleukins. Mol Cell Endocrinol 283, 68–75 (2008). [DOI] [PubMed] [Google Scholar]
- Schuppe H. C. et al. Chronic orchitis: a neglected cause of male infertility? Andrologia 40, 84–91 (2008). [DOI] [PubMed] [Google Scholar]
- Jacobo P., Guazzone V. A., Theas M. S. & Lustig L. Testicular autoimmunity. Autoimmun Rev 10, 201–204 (2011). [DOI] [PubMed] [Google Scholar]
- Li F. Y. & Li Y. Interleukin-6, desmosome and tight junction protein expression levels in reflux esophagitis-affected mucosa. World J Gastroentero 15, 3621–3630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Yoshinaga N. & Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 286, 31263–31271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W. Y., Lee W. M. & Cheng C. Y. Rho GTPases and spermatogenesis. Biochim Biophys Acta 1593, 121–129 (2003). [DOI] [PubMed] [Google Scholar]
- Siu M. K. & Cheng C. Y. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 26, 978–992 (2004). [DOI] [PubMed] [Google Scholar]
- Wong C. H. & Cheng C. Y. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev Biol 286, 1–15 (2005). [DOI] [PubMed] [Google Scholar]
- Chen M. et al. Effect of heat stress on expression of junction-associated molecules and upstream factors androgen receptor and Wilms' tumor 1 in monkey sertoli cells. Endocrinology 149, 4871–4882 (2008). [DOI] [PubMed] [Google Scholar]
- MICHELA, ELIO, FIORETTA, MARIO A. & STEFANINI M. Pure Sertoli Cell Cultures: A New Model for the Study of Somatic-Germ Cell Interactions. Journal of Andrology 2, 249–254 (1981). [Google Scholar]
- Byers S. W., Hadley M. A., Djakiew D. & Dym M. Growth and characterization of polarized monolayers of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl 7, 59–68 (1986). [DOI] [PubMed] [Google Scholar]
- Chung S. S., Lee W. M. & Cheng C. Y. Study on the formation of specialized inter-Sertoli cell junctions in vitro. J Cell Physiol 181, 258–272 (1999). [DOI] [PubMed] [Google Scholar]
- Yan H. H., Mruk D. D., Lee W. M. & Cheng C. Y. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 22, 1945–1959 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui W. Y., Lee W. M. & Cheng C. Y. Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology 142, 1865–1877 (2001). [DOI] [PubMed] [Google Scholar]
- Grima, Wong C. S., Zhu, Zong & Cheng C. Y. Testin Secreted by Sertoli Cells Is Associated with the Cell Surface, and Its Expression Correlates with the Disruption of Sertoli-Germ Cell Junctions but Not the Inter-Sertoli Tight Junction. J. Biol. Chem 273, 21040–21053 (1998). [DOI] [PubMed] [Google Scholar]
- Yao G. et al. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol 24, 540–551 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]