Abstract
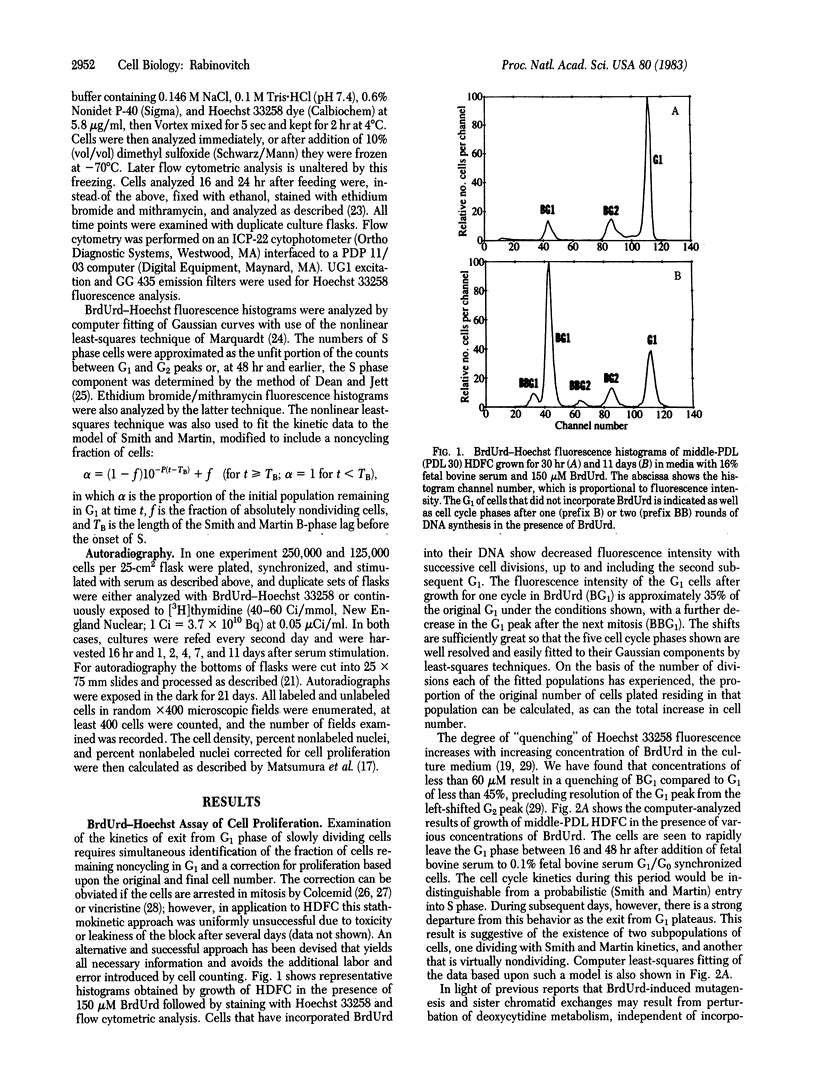
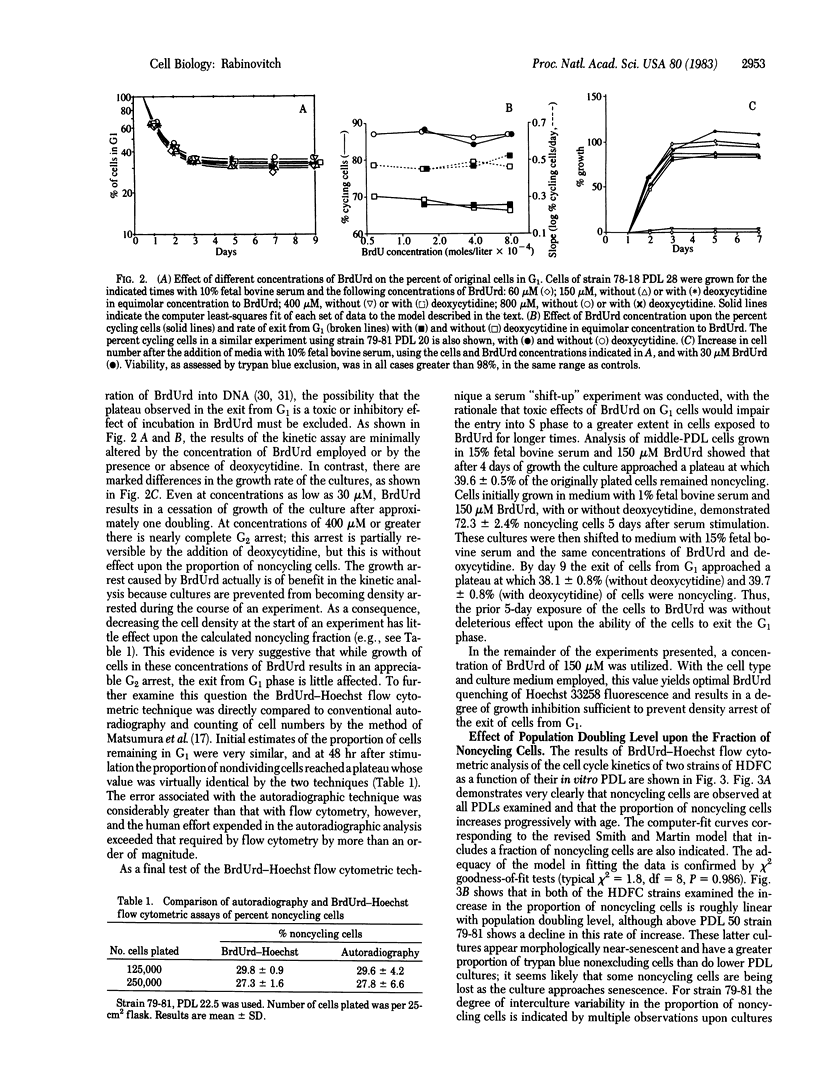
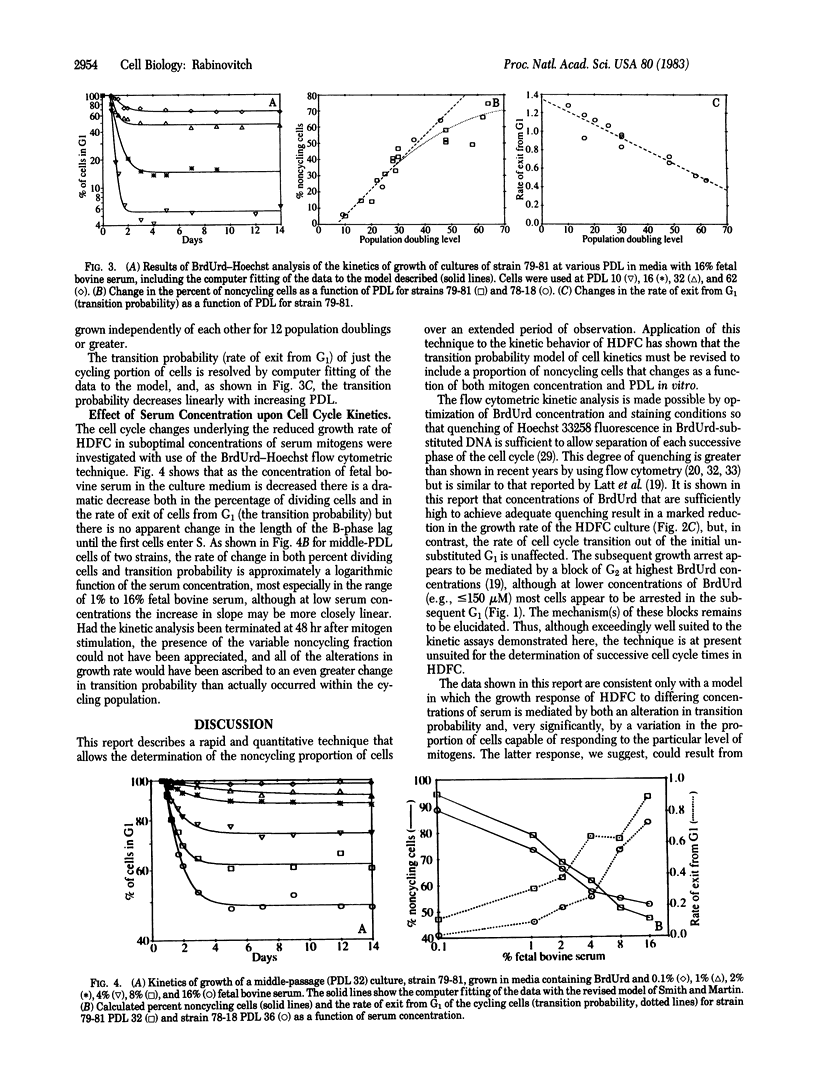
Growth of human diploid fibroblasts in the presence of 5-bromodeoxyuridine, followed by flow cytometric analysis of DNA-specific fluorescence with Hoechst 33258 dye, allows quantitation of the proportion of cells that have not cycled, as well as those in G1 and G2 of two subsequent cell cycles. This technique allows rapid and accurate quantitation of the growth fraction and G1/S transition rate of these cells. The cell cycle kinetics of human diploid fibroblasts at all population doubling levels reveal two components: cycling cells showing a probabilistic rate of G1/S transition, and a variable proportion of noncycling cells. Both the transition probability (rate of exit from G1) and the noncycling proportion of cells change systematically as a function of serum concentration and as a function of population doubling level. The data suggest the existence of an underlying heterogeneity in the population of human diploid fibroblasts with respect to the capacity to divide in the presence of a given concentration of mitogen. Models of cell cycle kinetics must be modified to include regulation of growth by changes in the fraction of cycling cells, as well as by changes in the rate of exit from G1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absher P. M., Absher R. G., Barnes W. D. Genealogies of clones of diploid fibroblasts. Cinemicrophotographic observations of cell division patterns in relation to population age. Exp Cell Res. 1974 Sep;88(1):95–104. doi: 10.1016/0014-4827(74)90622-3. [DOI] [PubMed] [Google Scholar]
- Absher P. M., Absher R. G. Clonal variation and aging of diploid fibroblasts. Cinematographic studies of cell pedigrees. Exp Cell Res. 1976 Dec;103(2):247–255. doi: 10.1016/0014-4827(76)90261-5. [DOI] [PubMed] [Google Scholar]
- Barfod I. H., Barfod N. M. Cell-production rates estimated by the use of vincristine sulphate and flow cytometry. I. An in vitro study using murine tumour cell lines. Cell Tissue Kinet. 1980 Jan;13(1):1–8. doi: 10.1111/j.1365-2184.1980.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Beck H. P. Proliferation kinetics of perturbed cell populations determined by the bromodeoxyuridine-33258 technique: radiotoxic effects of incorporated [3H]thymidine. Cytometry. 1981 Nov;2(3):170–174. doi: 10.1002/cyto.990020307. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. The kinetics of serum-induced initiation of DNA synthesis in BHK 21/C13 cells, and the influence of exogenous adenosine. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):369–377. doi: 10.1002/jcp.1040860409. [DOI] [PubMed] [Google Scholar]
- Böhmer R. M. Determination of non-proliferating cells in culture by combining flow cytometry with stathmokinetics. Cell Tissue Kinet. 1980 Sep;13(5):497–503. doi: 10.1111/j.1365-2184.1980.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Böhmer R. M. Flow cytometric cell cycle analysis using the quenching of 33258 Hoechst fluorescence by bromodeoxyuridine incorporation. Cell Tissue Kinet. 1979 Jan;12(1):101–110. [PubMed] [Google Scholar]
- Cristofalo V. J., Sharf B. B. Cellular senescence and DNA synthesis. Thymidine incorporation as a measure of population age in human diploid cells. Exp Cell Res. 1973 Feb;76(2):419–427. doi: 10.1016/0014-4827(73)90394-7. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R., Dougherty C. P., Ouellette A. M., DiFolco C. M., Latt S. A. Induction of sister chromatid exchanges by BUdR is largely independent of the BUdR content of DNA. Nature. 1980 Mar 6;284(5751):74–76. doi: 10.1038/284074a0. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R. Resistance to bromodeoxyuridine mutagenesis and toxicity in mammalian cells selected for resistance to hydroxyurea. Somatic Cell Genet. 1979 Nov;5(6):873–885. doi: 10.1007/BF01542647. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosik G. M., Barlogie B., White R. A., Göhde W., Drewinko B. A rapid automated stathmokinetic method for determination of in vitro cell cycle transit times. Cell Tissue Kinet. 1981 Mar;14(2):121–134. doi: 10.1111/j.1365-2184.1981.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Grove G. L., Cristofalo V. J. The 'transition probability model' and the regulation of proliferation of human diploid cell cultures during aging. Cell Tissue Kinet. 1976 Jul;9(4):395–399. doi: 10.1111/j.1365-2184.1976.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Grove G. L., Cristofalo V. J. Transition probability model and aging human diploid cell cultures: a reply to Smith (1977). Cell Biol Int Rep. 1978 Mar;2(2):185–188. doi: 10.1016/0309-1651(78)90040-1. [DOI] [PubMed] [Google Scholar]
- Kubbies M., Rabinovitch P. S. Flow cytometric analysis of factors which influence the BrdUrd-Hoechst quenching effect in cultivated human fibroblasts and lymphocytes. Cytometry. 1983 Jan;3(4):276–281. doi: 10.1002/cyto.990030408. [DOI] [PubMed] [Google Scholar]
- Latt S. A., George Y. S., Gray J. W. Flow cytometric analysis of bromodeoxyuridine-substituted cells stained with 33258 Hoechst. J Histochem Cytochem. 1977 Jul;25(7):927–934. doi: 10.1177/25.7.70460. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macieira-Coelho A. Are non-dividing cells present in ageing cell cultures? Nature. 1974 Mar 29;248(447):421–422. doi: 10.1038/248421a0. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A., Taboury F. A re-evaluation of the changes in proliferation in human fibroblasts during ageing in vitro. Cell Tissue Kinet. 1982 Mar;15(2):213–224. doi: 10.1111/j.1365-2184.1982.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Pfendt E. A., Hayflick L. DNA synthesis in the human diploid cell strain WI-38 during in vitro aging: an autoradiography study. J Gerontol. 1979 May;34(3):323–327. doi: 10.1093/geronj/34.3.323. [DOI] [PubMed] [Google Scholar]
- Merz G. S., Jr, Ross J. D. Viability of human diploid cells as a function of in vitro age. J Cell Physiol. 1969 Dec;74(3):219–222. doi: 10.1002/jcp.1040740302. [DOI] [PubMed] [Google Scholar]
- Mets T., Verdonk G. The theory of transition probability and the division pattern of WI--38 cells. Cell Biol Int Rep. 1978 Nov;2(6):561–564. doi: 10.1016/0309-1651(78)90064-4. [DOI] [PubMed] [Google Scholar]
- Nüsse M. Cell cycle kinetics of irradiated synchronous and asynchronous tumor cells with DNA distribution analysis and BrdUrd-Hoechst 33258-technique. Cytometry. 1981 Sep;2(2):70–79. doi: 10.1002/cyto.990020206. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Norwood T. H. Rapid kinetics of polyethylene glycol-mediated fusion. Somatic Cell Genet. 1981 May;7(3):281–287. doi: 10.1007/BF01538853. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., O'Brien K., Simpson M., Callis J. B., Hoehn H. Flow-cytogenetics. II. High-resolution ploidy measurements in human fibroblast cultures. Cytogenet Cell Genet. 1981;29(2):65–76. doi: 10.1159/000131553. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Shields R., Smith J. A. Cells regulate their proliferation through alterations in transition probability. J Cell Physiol. 1977 Jun;91(3):345–355. doi: 10.1002/jcp.1040910304. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M., Schneider E. L. Colony size distributions as a measure of in vivo and in vitro aging. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1353–1356. doi: 10.1073/pnas.75.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R. A., Jr, Huang P. C. The proportion of cells labeled with tritiated thymidine as a function of population doubling level in cultures of fetal, adult, mutant, and tumor origin. Exp Cell Res. 1976 Oct 1;102(1):31–42. doi: 10.1016/0014-4827(76)90296-2. [DOI] [PubMed] [Google Scholar]


