Abstract
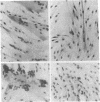
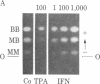
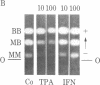
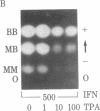
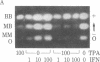
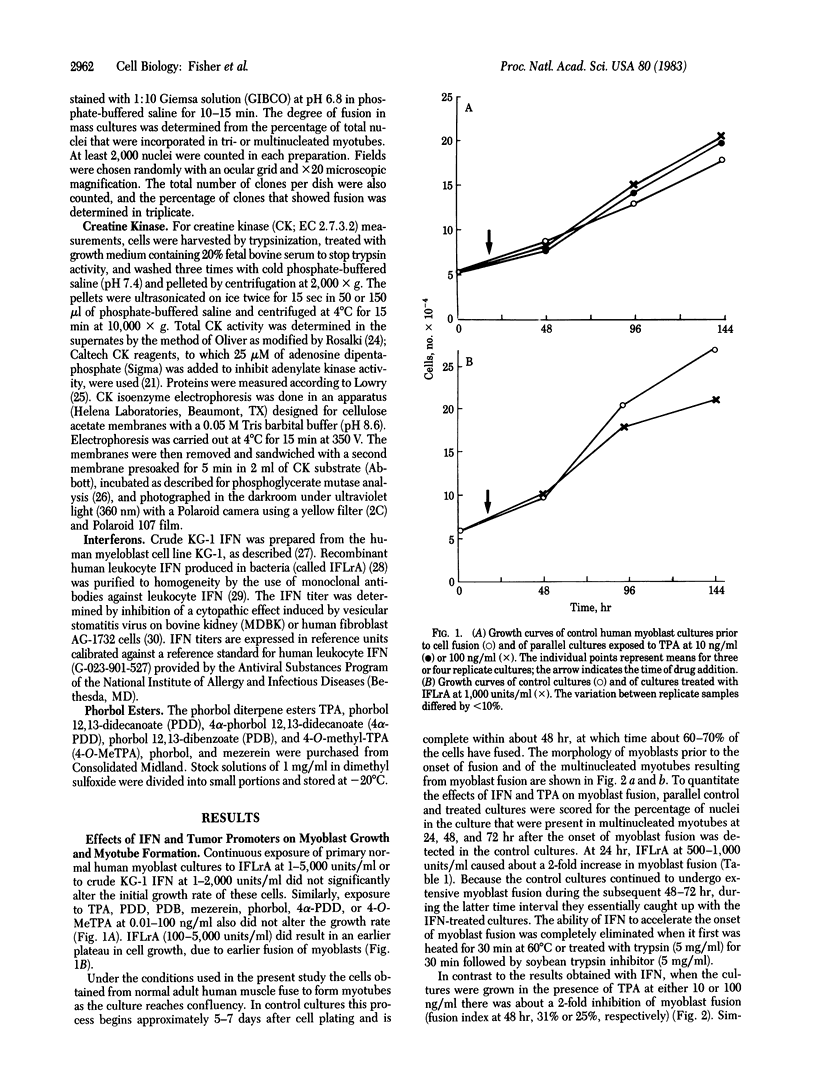
We have studied the effects of human leukocyte interferon produced in bacteria and diterpene phorbol ester tumor promoters on differentiation of normal human myoblast cultures derived from mature skeletal muscle. Interferon (100-5,000 units/ml) induced an acceleration of myotube formation and creatine kinase (CK; EC 2.7.3.2) isoenzyme transition from CK-BB to CK-MM. Heat-inactivated or trypsin-treated interferon did not affect the differentiation process. In contrast, the potent tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA), but not its inactive structural analogues phorbol and 4 alpha-phorbol 12,13-didecanoate, caused a dose-dependent (0.01-100 ng/ml) inhibition of myotube formation and CK isoenzyme transition. Neither interferon nor TPA had a significant effect on myoblast proliferation prior to fusion, and the cloning efficiencies were similar as well. Opposing effects of interferon and TPA were also demonstrated by simultaneous application of these agents to the cultures. These studies suggest that some of the antitumor effects of interferon may relate to its capacity to modulate cellular differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Baron S., Dianzani F. General considerations of the interferon system. Tex Rep Biol Med. 1977;35:1–10. [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Rochette-Egly C., Rosenfeld C., Mishal Z. Altered lipid microviscosity in lymphoblastoid cells treated with 12-O-tetradecanoyl phorbol 13-acetate, a tumor promoter. FEBS Lett. 1979 Apr 1;100(1):62–66. doi: 10.1016/0014-5793(79)81131-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Cheung H. C., Hunter E. Interferon inhibits Sendai virus-induced cell fusion: an effect on cell membrane fluidity. Proc Natl Acad Sci U S A. 1982 Feb;79(3):835–839. doi: 10.1073/pnas.79.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioé L., O'Brien T. G., Diamond L. Inhibition of adipose conversion of BALB/c 3T3 cells by interferon and 12-O-tetradecanoylphorbol-13-acetate. Cell Biol Int Rep. 1980 Mar;4(3):255–264. doi: 10.1016/0309-1651(80)90057-0. [DOI] [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Miranda A. F., Khan S., Gitlin K., Friedman R. Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy. Science. 1981 Jun 12;212(4500):1277–1279. doi: 10.1126/science.6262916. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger M., Maeda S., Pestka S. Recombinant human leukocyte interferon produced in bacteria has antiproliferative activity. J Biol Chem. 1981 Mar 10;256(5):2113–2114. [PubMed] [Google Scholar]
- Evinger M., Rubinstein M., Pestka S. Antiproliferative and antiviral activities of human leukocyte interferons. Arch Biochem Biophys. 1981 Aug;210(1):319–329. doi: 10.1016/0003-9861(81)90195-8. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Cogan U., Horowitz A. D., Schachter D., Weinstein I. B. TPA-resistance in Friend erythroleukemia cells: role of membrane lipid fluidity. Biochem Biophys Res Commun. 1981 May 15;100(1):370–376. doi: 10.1016/s0006-291x(81)80106-4. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Flamm M., Schachter D., Weinstein I. B. Tumor promoters induce membrane changes detected by fluorescence polarization. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1063–1068. doi: 10.1016/0006-291x(79)90225-0. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Miranda A. F., Mufson R. A., Weinstein L. S., Fujiki H., Sugimura T., Weinstein I. B. Effects of teleocidin and the phorbol ester tumor promoters on cell transformation, differentiation, and phospholipid metabolism. Cancer Res. 1982 Jul;42(7):2829–2835. [PubMed] [Google Scholar]
- Fisher P. B., Mufson R. A., Weinstein I. B. Interferon inhibits melanogenesis in B-16 mouse melanoma cells. Biochem Biophys Res Commun. 1981 May 29;100(2):823–830. doi: 10.1016/s0006-291x(81)80248-3. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Hobb D. S., Moschera J. A., Levy W. P., Pestka S. Purification of interferon produced in a culture of human granulocytes. Methods Enzymol. 1981;78(Pt A):472–481. doi: 10.1016/0076-6879(81)78158-8. [DOI] [PubMed] [Google Scholar]
- Horowitz A. D., Greenebaum E., Weinstein I. B. Identification of receptors for phorbol ester tumor promoters in intact mammalian cells and of an inhibitor of receptor binding in biologic fluids. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2315–2319. doi: 10.1073/pnas.78.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Grossberg S. E. Interferon inhibits the conversion of 3T3-L1 mouse fibroblasts into adipocytes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4099–4103. doi: 10.1073/pnas.77.7.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr Antiviral and cell growth inhibitory activities reside in the same glycoprotein of human fibroblast interferon. Nature. 1976 Jul 22;262(5566):302–303. doi: 10.1038/262302a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S. L., Ts'o P. O., Hollenberg M. D. The effects of interferon on epidermal growth factor action. Biochem Biophys Res Commun. 1980 Sep 16;96(1):168–174. doi: 10.1016/0006-291x(80)91196-1. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J., Keay S., Sabran J. L., Grossberg S. E. Inhibition of chicken myogenesis in vitro by partially purified interferon. Biochem Biophys Res Commun. 1982 Nov 16;109(1):92–99. doi: 10.1016/0006-291x(82)91570-4. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Filedsteel A. H., Fodge D. W. Opposing effects of tumor promoters on erythroid differentiation. Nature. 1978 Jul 20;274(5668):271–272. doi: 10.1038/274271a0. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Aguet M., Gressner I. Binding of 125I-labelled human alpha interferon to human lymphoid cells. Int J Cancer. 1981 Nov 15;28(5):575–582. doi: 10.1002/ijc.2910280508. [DOI] [PubMed] [Google Scholar]
- Mufson R. A., Fisher P. B., Weinstein I. B. Effect of phorbol ester tumor promoters on the expression of melanogenesis in B-16 melanoma cells. Cancer Res. 1979 Oct;39(10):3915–3919. [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Rossi G. B., Dolei A., Cioé L., Benedetto A., Matarese G. P., Belardelli F. Inhibition of transcription and translation of globin messenger RNA in dimethyl sulfoxide-stimulated Friend erythroleukemic cells treated with interferon. Proc Natl Acad Sci U S A. 1977 May;74(5):2036–2040. doi: 10.1073/pnas.74.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G. B., Matarese G. P., Grappelli C., Belardelli F., Benedetto A. Interferon inhibits dimethyl sulphoxide-induced erythroid differentiation of Friend leukaemia cells. Nature. 1977 May 5;267(5606):50–52. doi: 10.1038/267050a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S., Familletti P. C., Pestka S. Convenient assay for interferons. J Virol. 1981 Feb;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Control of myogenesis in vitro by Ca 2 + concentration in nutritional medium. Exp Cell Res. 1969 Nov;58(1):163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Rozengurt E., Taylor-Papadimitriou J., Burchell J. Differential effect of interferon on DNA synthesis, 2-deoxyglucose uptake and ornithine decarboxylase activity in 3T3 cells stimulated by polypeptide growth factors and tumor promotors. J Cell Physiol. 1980 Jul;104(1):1–9. doi: 10.1002/jcp.1041040102. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Hobbs D. S., Kung H., Lai C. Y., Pestka S. Purification and characterization of recombinant human leukocyte interferon (IFLrA) with monoclonal antibodies. J Biol Chem. 1981 Sep 25;256(18):9750–9754. [PubMed] [Google Scholar]
- Tomida M., Yamamoto Y., Hozumi M. Stimulation by interferon of induction of differentiation of human promyelocytic leukemia cells. Biochem Biophys Res Commun. 1982 Jan 15;104(1):30–37. doi: 10.1016/0006-291x(82)91936-2. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto Y., Hozumi M. Stimulation by interferon of induction of differentiation of mouse myeloid leukemic cells. Cancer Res. 1980 Aug;40(8 Pt 1):2919–2924. [PubMed] [Google Scholar]
- Wang E., Pfeffer L. M., Tamm I. Interferon increases the abundance of submembranous microfilaments in HeLa-S3 cells in suspension culture. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6281–6285. doi: 10.1073/pnas.78.10.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]