Abstract
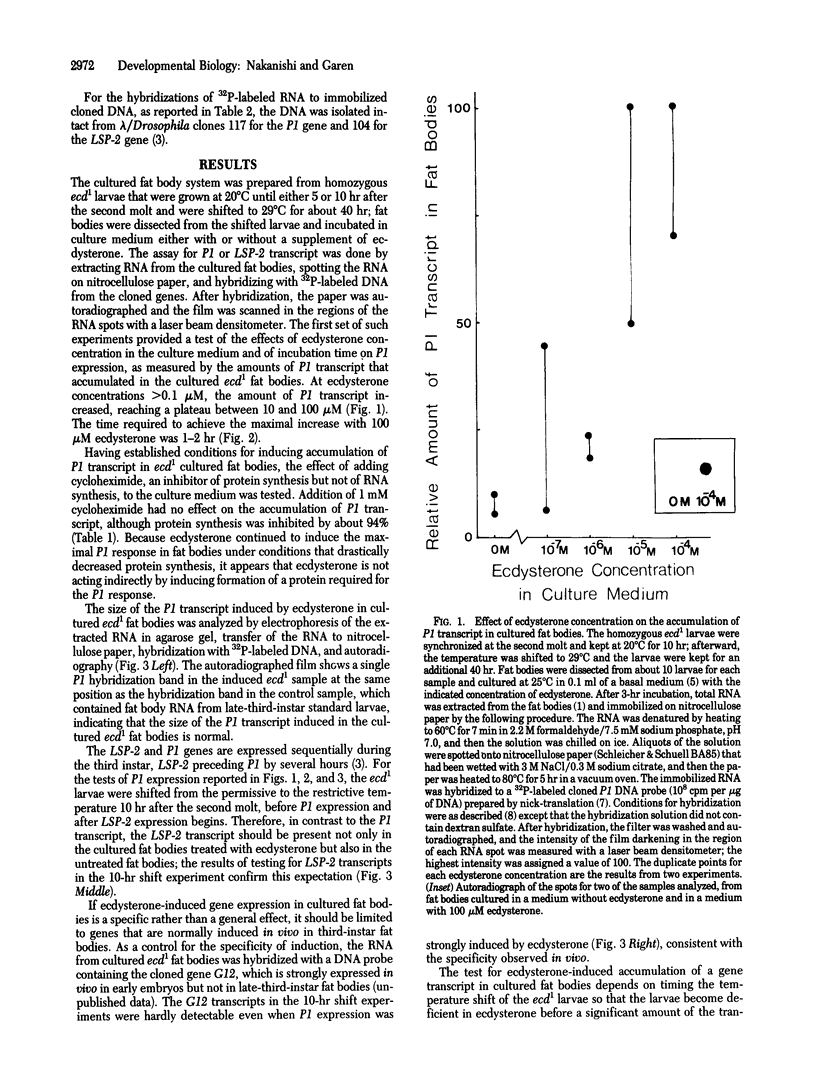
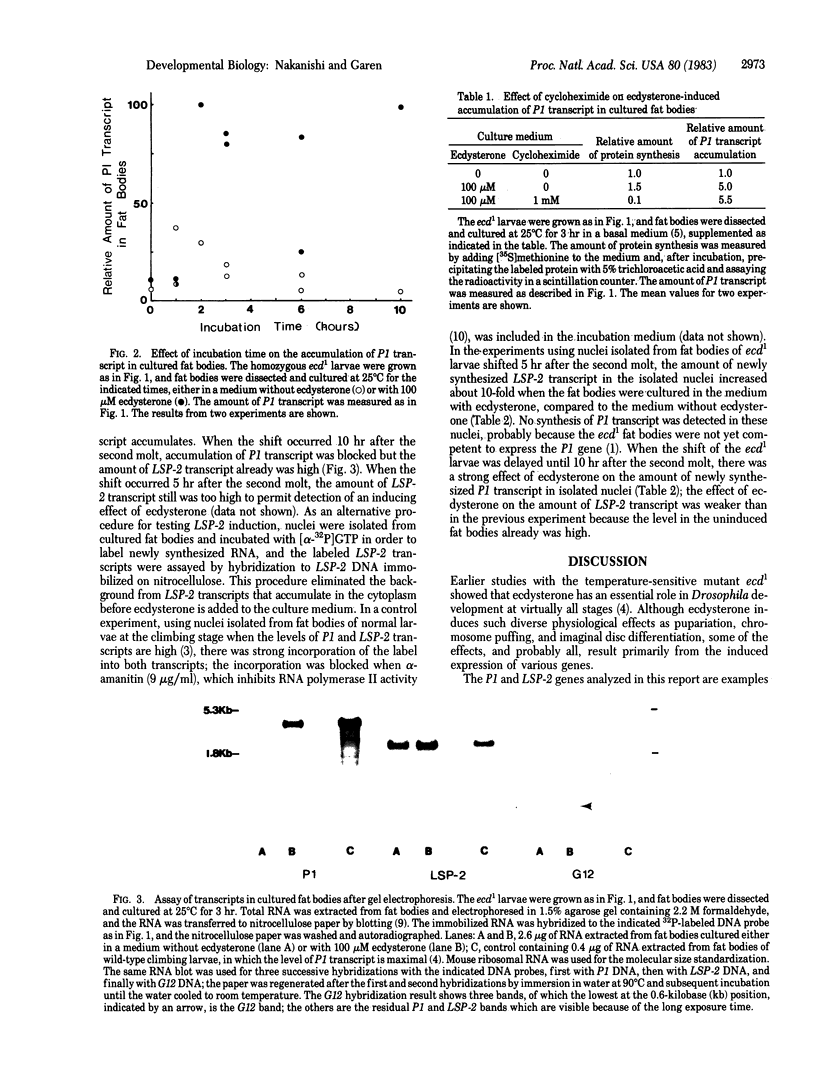
Expression of the LSP-2 and P1 genes was induced in cultured fat bodies of Drosophila third-instar larvae by supplementing the culture medium with ecdysterone. The fat bodies were isolated from ecdysterone-deficient larvae of the temperature-sensitive mutant ecd1, which were shifted from the permissive to the restrictive temperature either at the beginning of the third instar for the detection of LSP-2 induction or several hours later for the detection of P1 induction. During normal larval development, the LSP-2 gene is expressed before the P1 gene, and this order is also observed in the cultured fat bodies. Induction was demonstrated by increased amounts of LSP-2 and P1 transcripts in the ecdysterone-supplemented fat bodies. The amount of P1 transcript was determined by two methods: one involved measuring the hybridization of a labeled P1 DNA probe to total fat body RNA; the other involved labeling the newly synthesized RNA in nuclei isolated from cultured fat bodies and measuring the hybridization of the labeled RNA to P1 DNA. Only the second method was used for the LSP-2 transcript because the earlier expression of the LSP-2 gene results in a measurable accumulation of the transcript in the fat bodies before ecdysterone supplementation. The maximal level of P1 induction was reached within 2 hr after supplementation, and the induction was not affected by a concentration of cycloheximide that strongly inhibited total protein synthesis, suggesting that ecdysterone acts directly on the P1 gene rather than indirectly by inducing formation of proteins required for the subsequent induction of P1. Ecdysterone appears to function normally in the cultured fat body system because the LSP-2 and P1 genes are induced in culture in the same order as in vivo and a third gene, G12, that is not induced in fat bodies in vivo also is not induced in culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. I. Dependence upon ecdysone concentration. Dev Biol. 1973 Nov;35(1):47–61. doi: 10.1016/0012-1606(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Wagar M. A., Atkinson M. R. Calcium-dependent priming of DNA synthesis in isolated rat liver nuclei. Biochem Biophys Res Commun. 1970 Apr 24;39(2):254–259. doi: 10.1016/0006-291x(70)90786-2. [DOI] [PubMed] [Google Scholar]
- Garen A., Kauvar L., Lepesant J. A. Roles of ecdysone in Drosophila development. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts R. B., Sage B., O'Connor J. D. Ecdysone titers during postembryonic development of Drosophila melanogaster. Dev Biol. 1977 Oct 1;60(1):310–317. doi: 10.1016/0012-1606(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kejzlarova-Lepesant J., Garen A. Ecdysone-inducible functions of larval fat bodies in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5570–5574. doi: 10.1073/pnas.75.11.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Levine M., Garen A., Lepesant-Kejzlarvoa J., Rat L., Somme-Martin G. Developmentally regulated gene expression in Drosophila larval fat bodies. J Mol Appl Genet. 1982;1(5):371–383. [PubMed] [Google Scholar]
- Levine M., Garen A., Lepesant J. A., Lepesant-Kejzlarova J. Constancy of somatic DNA organization in developmentally regulated regions of the Drosophila genome. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2417–2421. doi: 10.1073/pnas.78.4.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A. Maintenance of imaginal discs of Drosophila melanogaster in chemically defined media. J Cell Biol. 1969 Jun;41(3):876–885. doi: 10.1083/jcb.41.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]




