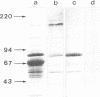
Abstract
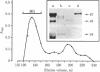
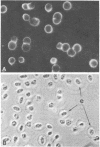
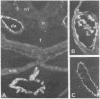
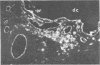
A mouse monoclonal antibody raised in response to quail immunoglobulin mu chain was found to exhibit a broad reactivity towards hemopoietic and endothelial cells in the quail (Coturnix coturnix japonica). Indirect immunofluorescence assays were performed at several stages of embryonic development and until 3 weeks after hatching, on either isolated cells or tissue sections. They revealed that the defined surface marker, referred to as MB1, (i) is expressed early on both intra- and extraembryonic hemopoietic stem cells and is transmitted to the whole progeny of these precursors, with the exception of mature erythrocytes, and (ii) is a constant feature of the endothelial cell surface throughout ontogenesis and adult life. In addition, this epitope is included in several soluble plasma components. MB1 expression was not detected in chicken tissues, and this characteristic was used to confirm its lineage restriction in quail-chicken chimeras. We stress the value of this species- and lineage-specific marker in study of the development of the hemopoietic and endothelial cell families, with special reference to their possible early common embryonic origin.
Full text
PDF
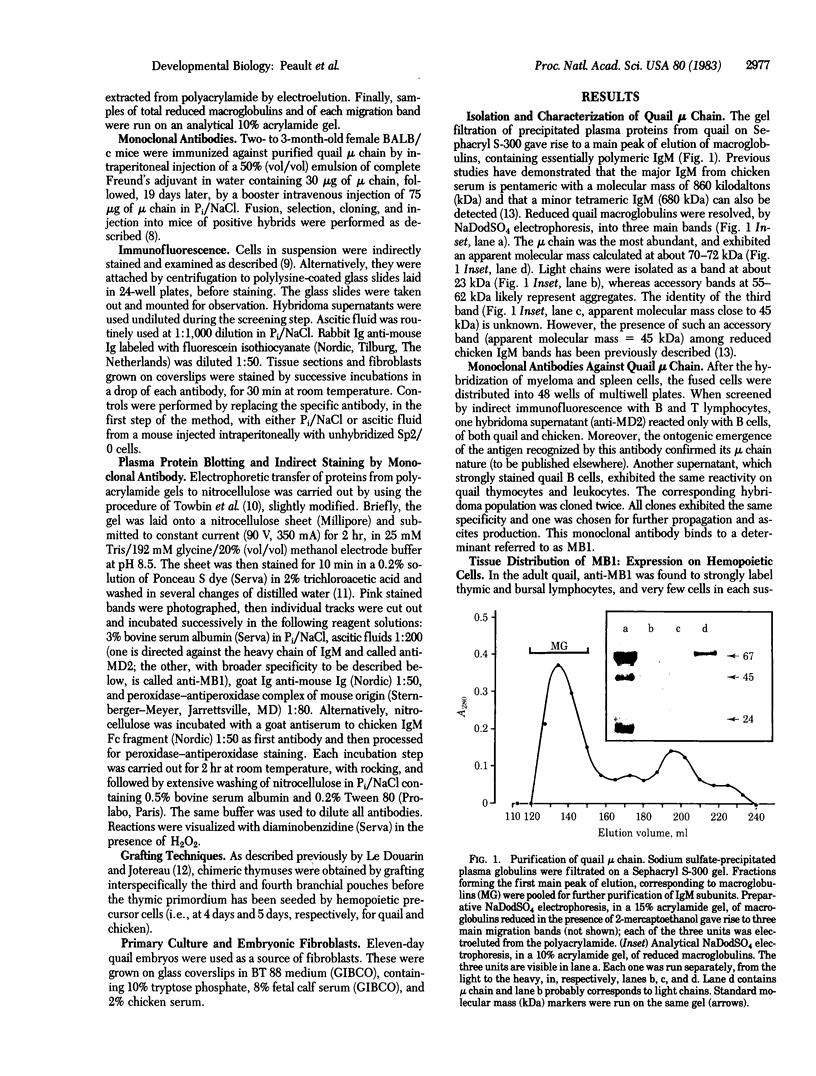
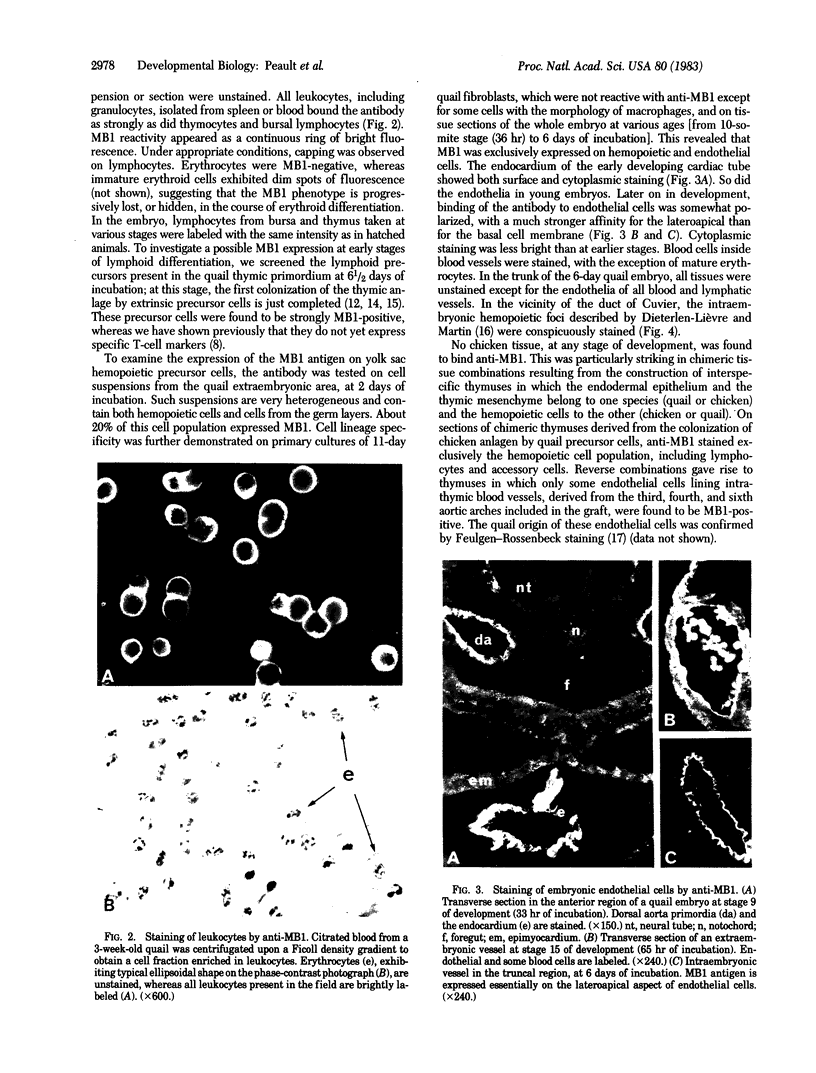


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTEIN M., PRAVERMAN A. Sur la précipitation des beta-lipoprotéines du sérum par l'héparine en présence de certains cations bivalents. C R Hebd Seances Acad Sci. 1957 Dec 23;245(26):2558–2560. [PubMed] [Google Scholar]
- Berridge M. V., Okech N. Surface antigens of murine hemopoietic stem cells. I. Cross reactivity of antisera against differentiated hemopoietic cells with bone marrow stem cells. Exp Hematol. 1979 Oct;7(9):452–468. [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen-Lièvre F., Martin C. Diffuse intraembryonic hemopoiesis in normal and chimeric avian development. Dev Biol. 1981 Nov;88(1):180–191. doi: 10.1016/0012-1606(81)90228-1. [DOI] [PubMed] [Google Scholar]
- Drews U. Cholinesterase in embryonic development. Prog Histochem Cytochem. 1975;7(3):1–52. [PubMed] [Google Scholar]
- Ivanyi J., Strudwick L., Makings C. A heterophile carbohydrate moiety common to mammalian IgM and erythrocytes detected by chicken IgM antibody. Eur J Immunol. 1977 Apr;7(4):204–209. doi: 10.1002/eji.1830070403. [DOI] [PubMed] [Google Scholar]
- Jotereau F. V., Le Douarin N. M. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J Immunol. 1982 Nov;129(5):1869–1877. [PubMed] [Google Scholar]
- Keane R. W., Lindblad P. C., Pierik L. T., Ingram V. M. Isolation and transformation of primary mesenchymal cells of the chick embryo. Cell. 1979 Aug;17(4):801–811. doi: 10.1016/0092-8674(79)90320-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Hirai H. Studies on subunit components of chicken polymeric immunoglobulins. J Immunol. 1980 Apr;124(4):1695–1704. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layton J. E. Anti-carbohydrate activity of T cell-reactive chicken anti-mouse immunoglobulin antibodies. J Immunol. 1980 Nov;125(5):1993–1997. [PubMed] [Google Scholar]
- Le Douarin N. M., Jotereau F. V. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med. 1975 Jul 1;142(1):17–40. doi: 10.1084/jem.142.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev Biol. 1973 Jan;30(1):217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Steiner L. A. Antisera to frog immunoglobulins cross-react with a periodate-sensitive cell surface determinant. Nature. 1978 Jun 29;273(5665):761–763. doi: 10.1038/273761a0. [DOI] [PubMed] [Google Scholar]
- Merler E., Gatien J., DeWilde G. Significance of immunofluorescent staining of lymphocytes with antisera to IgM immunoglobulins. Nature. 1974 Oct 18;251(5476):652–654. doi: 10.1038/251652a0. [DOI] [PubMed] [Google Scholar]
- Peault B., Coltey M., Le Douarin N. M. Tissue distribution and ontogenetic emergence of differentiation antigens on avian T cells. Eur J Immunol. 1982 Dec;12(12):1047–1050. doi: 10.1002/eji.1830121211. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Fedecka-Bruner B., Coltey M., Péault B. M., Le Douarin N. M. Biochemical characterization of avian T lymphocyte-specific antigens. Eur J Immunol. 1981 Jun;11(6):517–520. doi: 10.1002/eji.1830110614. [DOI] [PubMed] [Google Scholar]
- Sanders B. G., Dietert R. R., Kline K., Dietert M. Chicken fetal antigen: example of an antigenically complex oncodevelopmental membrane glycoprotein. Oncodev Biol Med. 1981;2(1-2):63–76. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACCHEI A. M. [The embryonal development of the Japanese quail (Coturnix coturnix japonica T. and S.)]. Arch Ital Anat Embriol. 1961;66:36–62. [PubMed] [Google Scholar]